Abstract
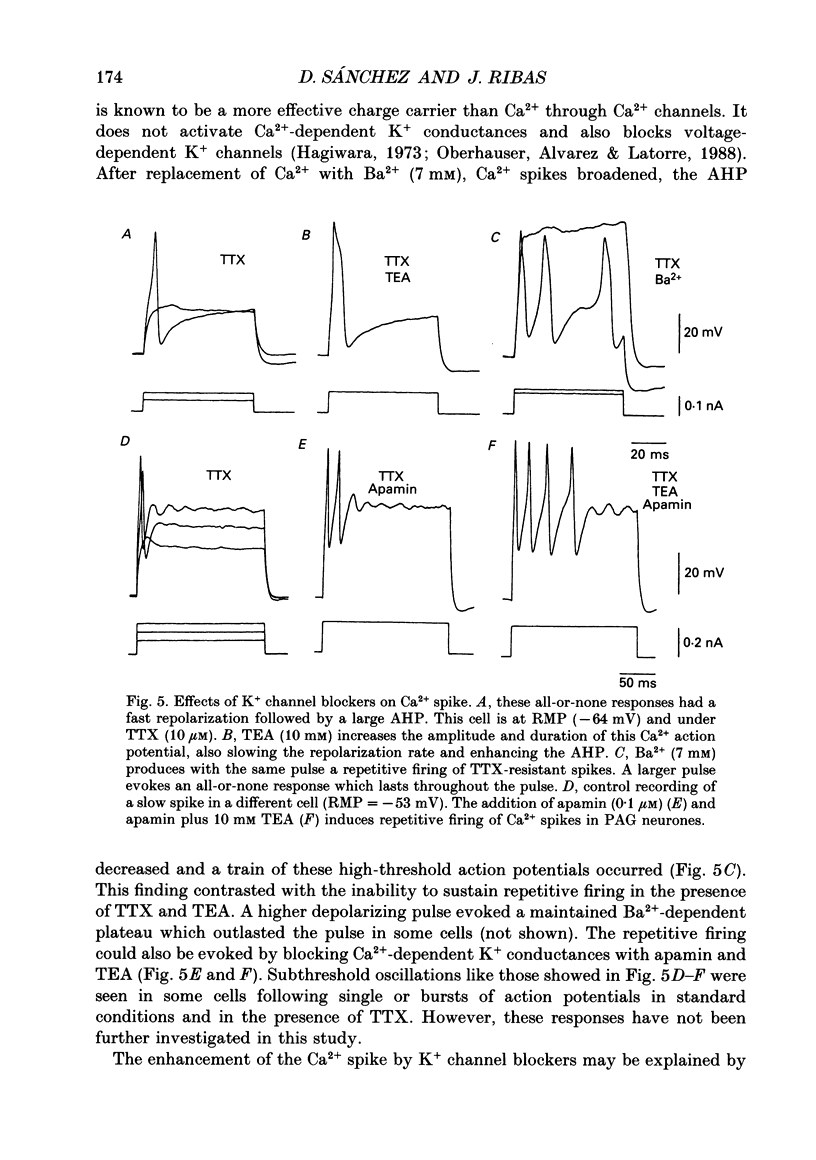
1. Action potentials of neurones of the ventral part of the guinea-pig periaqueductal grey (PAG) were studied by intracellular recording in a mesencephalic slice preparation maintained in vitro. 2. Fast spikes spontaneously fired last 2.8 +/- 0.6 ms (mean +/- S.D.) and have an amplitude of 72.3 +/- 5.3 mV (n = 28). The neurones could be antidromically activated from the neighbouring white matter and these spikes show an initial segment component that triggers the soma-dendritic spike. These two components were dissociated by hyperpolarization. Action potentials are Na+ dependent and a Ca2+ conductance is responsible for the hump on the falling phase. Hyperpolarization makes the hump disappear and a faster rate of rise and fall are seen. Accommodation of the firing threshold is observed in response to depolarizing ramps, which is eliminated with hyperpolarization. 3. High-threshold Ca2+ spikes are evoked in either Na(+)-free solution or in the presence of tetrodotoxin (TTX). These presumed dendritic action potentials display a fast repolarization and a large after-hyperpolarization (AHP) that prevent repetitive firing. This AHP is mainly generated by Ca(2+)-dependent K+ conductances. 4. The repolarization of fast action potentials depends on the activation of K+ conductances as well as a Na+ inactivation process. A fast-activated tetraethyl-ammonium (TEA)-sensitive K+ conductance, that could be Ca2+ dependent, and a K+ conductance blocked by apamin seem to be involved in the repolarization. 5. Each fast action potential is followed by a pronounced AHP with two components, an initial fast and a slow decaying phase. Membrane hyperpolarization around -60 mV eliminated the first component and the AHP acquired a plateau-like shape. At -90 mV the AHP was nullified. The slow phase was Ca2+ dependent and an apamin-sensitive K+ conductance is involved in its generation. This conductance may be active during the early part of the AHP, but a fast-activated TEA-sensitive K+ conductance and other voltage-dependent K+ conductances might also be present. A Ca2+ conductance is hypothesized to account for the fast depolarizing change after the AHP peak. 6. A delayed return to the baseline is observed after hyperpolarizing pulses. It is generated by the activation of a transient voltage-dependent K+ conductance that is inactive at resting membrane potential (RMP, around -50 mV). This transient hyperpolarization is abolished by Ca2+ channel blockers and insensitive to high external concentrations of 4-aminopyridine, TEA and Cs+.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins P. T., Surmeier D. J., Kitai S. T. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990 Mar 15;344(6263):240–242. doi: 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G., López-Barneo J. Ionic basis of the differential neuronal activity of guinea-pig septal nucleus studied in vitro. J Physiol. 1988 Feb;396:399–415. doi: 10.1113/jphysiol.1988.sp016969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. The interactions between potassium and sodium currents in generating action potentials in the rat sympathetic neurone. J Physiol. 1988 Mar;397:127–147. doi: 10.1113/jphysiol.1988.sp016992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W., Brown D. A. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett. 1987 Nov 23;82(2):185–190. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Randle J. C., Renaud L. P. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol. 1985 Dec;54(6):1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A. W., Morton C. R. Tonic descending inhibition and spinal nociceptive transmission. Prog Brain Res. 1988;77:193–211. doi: 10.1016/s0079-6123(08)62786-7. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Grantyn A., Schierwagen A. Passive membrane properties, afterpotentials and repetitive firing of superior colliculus neurons studied in the anesthetized cat. Exp Brain Res. 1983;50(2-3):377–391. doi: 10.1007/BF00239204. [DOI] [PubMed] [Google Scholar]
- Gutnick M. J., Yarom Y. Low threshold calcium spikes, intrinsic neuronal oscillation and rhythm generation in the CNS. J Neurosci Methods. 1989 May;28(1-2):93–99. doi: 10.1016/0165-0270(89)90014-9. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Jahnsen H. Electrophysiological characteristics of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986 Mar;372:129–147. doi: 10.1113/jphysiol.1986.sp016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodkowski J. S., Viana F., Dick T. E., Berger A. J. Repetitive firing properties of phrenic motoneurons in the cat. J Neurophysiol. 1988 Aug;60(2):687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987 Aug;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J., Llinás R. Electrophysiology of mammalian tectal neurons in vitro. I. Transient ionic conductances. J Neurophysiol. 1988 Sep;60(3):853–868. doi: 10.1152/jn.1988.60.3.853. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Sugiyama K. A modulatory action of divalent cations on transient outward current in cultured rat sensory neurones. J Physiol. 1988 Feb;396:417–433. doi: 10.1113/jphysiol.1988.sp016970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser A., Alvarez O., Latorre R. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane. J Gen Physiol. 1988 Jul;92(1):67–86. doi: 10.1085/jgp.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Segal M., Rogawski M. A., Barker J. L. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984 Feb;4(2):604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez D., Armengol J. A., Ribas J. The Study of Passive Membrane Properties and Morphology Reveals Neuronal Differences Along the Sagittal Axis of the Ventral Periaqueductal Grey Matter. Eur J Neurosci. 1990;2(12):1135–1143. doi: 10.1111/j.1460-9568.1990.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Sánchez D., Ganfornina M. D., Ribas J. Periaqueductal gray neurons' activity in a mesencephalic slice preparation. Brain Res. 1988 Jul 5;455(1):166–169. doi: 10.1016/0006-8993(88)90128-x. [DOI] [PubMed] [Google Scholar]
- Walton K., Fulton B. P. Ionic mechanisms underlying the firing properties of rat neonatal motoneurons studied in vitro. Neuroscience. 1986 Nov;19(3):669–683. doi: 10.1016/0306-4522(86)90291-5. [DOI] [PubMed] [Google Scholar]
- Wilcox K. S., Gutnick M. J., Christoph G. R. Electrophysiological properties of neurons in the lateral habenula nucleus: an in vitro study. J Neurophysiol. 1988 Jan;59(1):212–225. doi: 10.1152/jn.1988.59.1.212. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Shefner S. A., Nishi S., Egan T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984 Sep;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 1978 Dec 29;159(2):385–390. doi: 10.1016/0006-8993(78)90544-9. [DOI] [PubMed] [Google Scholar]
- Yarom Y., Llinás R. Long-term modifiability of anomalous and delayed rectification in guinea pig inferior olivary neurons. J Neurosci. 1987 Apr;7(4):1166–1177. doi: 10.1523/JNEUROSCI.07-04-01166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom Y., Sugimori M., Llinás R. Ionic currents and firing patterns of mammalian vagal motoneurons in vitro. Neuroscience. 1985 Dec;16(4):719–737. doi: 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Polosa C., Nishi S. Afterhyperpolarization mechanisms in cat sympathetic preganglionic neuron in vitro. J Neurophysiol. 1986 Jun;55(6):1234–1246. doi: 10.1152/jn.1986.55.6.1234. [DOI] [PubMed] [Google Scholar]
- Zbicz K. L., Weight F. F. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985 Apr;53(4):1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]
- Zhang L., Krnjević K. Apamin depresses selectively the after-hyperpolarization of cat spinal motoneurons. Neurosci Lett. 1987 Feb 10;74(1):58–62. doi: 10.1016/0304-3940(87)90051-6. [DOI] [PubMed] [Google Scholar]


