Abstract
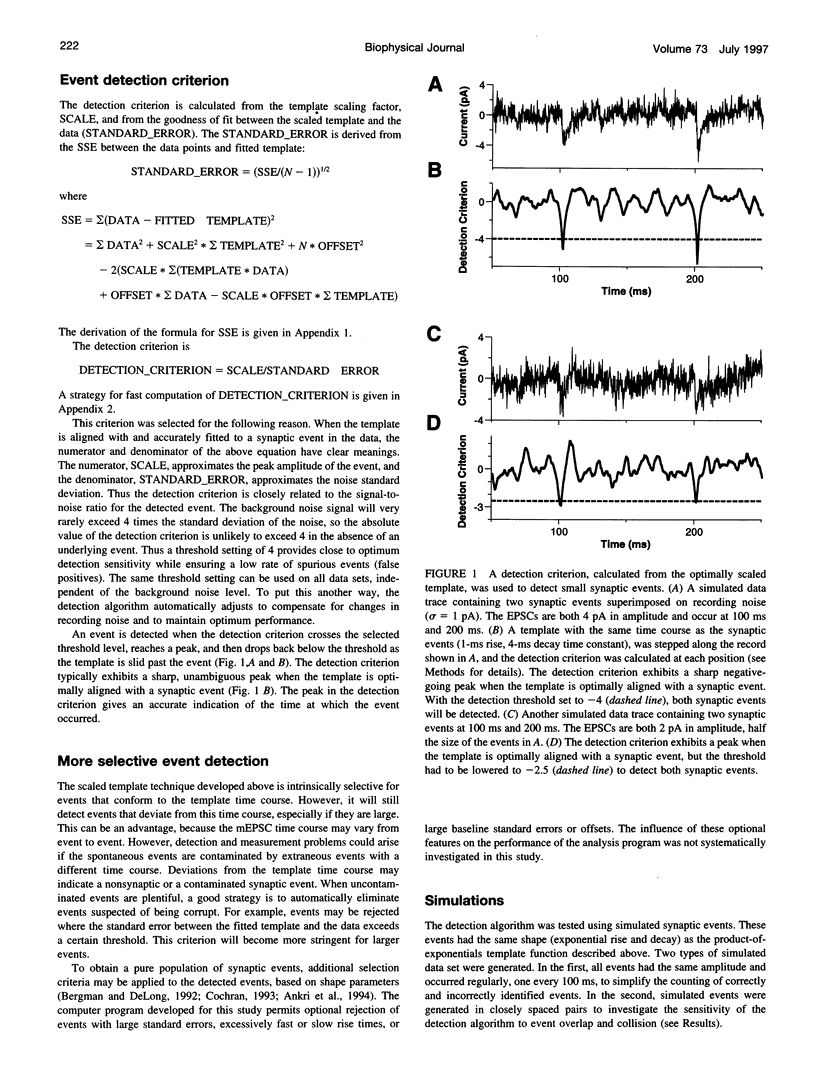
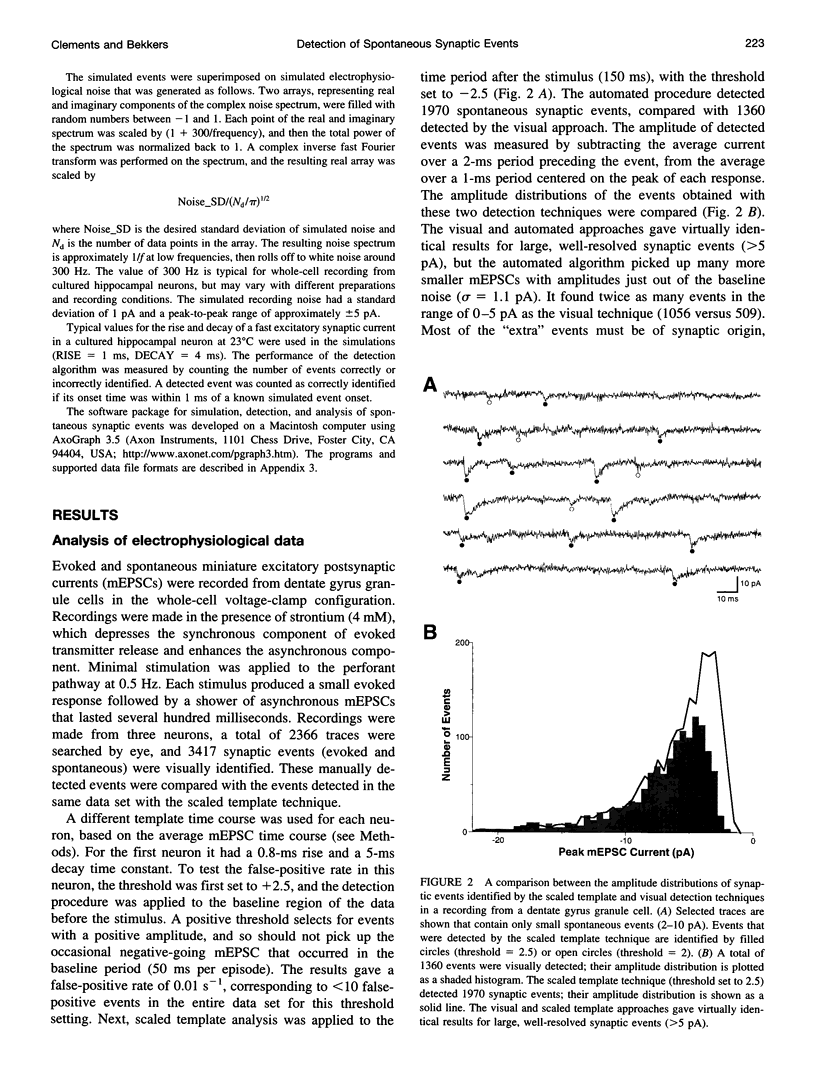
Spontaneous synaptic events can be difficult to detect when their amplitudes are close to the background noise level. Here we report a sensitive new technique for automatic detection of small asynchronous events. A waveform with the time course of a typical synaptic event (a template) is slid along the current or voltage trace and optimally scaled to fit the data at each position. A detection criterion is calculated based on the optimum scaling factor and the quality of the fit. An event is detected when this criterion crosses a threshold level. The algorithm automatically compensates for changes in recording noise. The sensitivity and selectivity of the method were tested using real and simulated data, and the influence of the template parameter settings was investigated. Its performance was comparable to that obtained by visual event detection, and it was more sensitive than previously described threshold detection techniques. Under typical recording conditions, all fast synaptic events with amplitudes of at least three times the noise standard deviation (3 sigma) could be detected, as could 75% of events with amplitudes of 2 sigma. The scaled template technique is implemented within a commercial data analysis application and can be applied to many standard electrophysiological data file formats.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Ghani M. A., Valiante T. A., Pennefather P. S. Sr2+ and quantal events at excitatory synapses between mouse hippocampal neurons in culture. J Physiol. 1996 Aug 15;495(Pt 1):113–125. doi: 10.1113/jphysiol.1996.sp021578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankri N., Legendre P., Faber D. S., Korn H. Automatic detection of spontaneous synaptic responses in central neurons. J Neurosci Methods. 1994 Apr;52(1):87–100. doi: 10.1016/0165-0270(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Vidovic M., Ymer S. Differential effects of histamine on the N-methyl-D-aspartate channel in hippocampal slices and cultures. Neuroscience. 1996 Jun;72(3):669–677. doi: 10.1016/0306-4522(95)00586-2. [DOI] [PubMed] [Google Scholar]
- Bergman H., DeLong M. R. A personal computer-based spike detector and sorter: implementation and evaluation. J Neurosci Methods. 1992 Mar;41(3):187–197. doi: 10.1016/0165-0270(92)90084-q. [DOI] [PubMed] [Google Scholar]
- Carlson C. G., Krieger J. W. A baseline detection method for analyzing transient electrophysiological events. J Neurosci Methods. 1996 Aug;67(2):211–220. [PubMed] [Google Scholar]
- Cocatre-Zilgien J. H., Delcomyn F. A slope-based approach to spike discrimination in digitized data. J Neurosci Methods. 1990 Aug;33(2-3):241–249. doi: 10.1016/0165-0270(90)90028-e. [DOI] [PubMed] [Google Scholar]
- Cochran S. L. Algorithms for detection and measurement of spontaneous events. J Neurosci Methods. 1993 Oct;50(1):105–121. doi: 10.1016/0165-0270(93)90061-u. [DOI] [PubMed] [Google Scholar]
- Liao D., Jones A., Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992 Dec;9(6):1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Liu H. H., Kim Y. I. A computer system for real-time data acquisition and analysis of biopotentials and quantal content at the neuromuscular junction. Comput Programs Biomed. 1983 Jun;16(3):161–173. doi: 10.1016/0010-468x(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Morales F. R., Boxer P. A., Jervey J. P., Chase M. H. A computerized system for the detection and analysis of spontaneously occurring synaptic potentials. J Neurosci Methods. 1985 Mar;13(1):19–35. doi: 10.1016/0165-0270(85)90041-x. [DOI] [PubMed] [Google Scholar]
- Oghalai J. S., Street W. N., Rhode W. S. A neural network-based spike discriminator. J Neurosci Methods. 1994 Sep;54(1):9–22. doi: 10.1016/0165-0270(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Salganicoff M., Sarna M., Sax L., Gerstein G. L. Unsupervised waveform classification for multi-neuron recordings: a real-time, software-based system. I. Algorithms and implementation. J Neurosci Methods. 1988 Oct;25(3):181–187. doi: 10.1016/0165-0270(88)90132-x. [DOI] [PubMed] [Google Scholar]
- Yamada S., Kage H., Nakashima M., Shiono S., Maeda M. Data processing for multi-channel optical recording: action potential detection by neural network. J Neurosci Methods. 1992 Jun;43(1):23–33. doi: 10.1016/0165-0270(92)90063-j. [DOI] [PubMed] [Google Scholar]
- Yang X. W., Shamma S. A. A totally automated system for the detection and classification of neural spikes. IEEE Trans Biomed Eng. 1988 Oct;35(10):806–816. doi: 10.1109/10.7287. [DOI] [PubMed] [Google Scholar]