Abstract
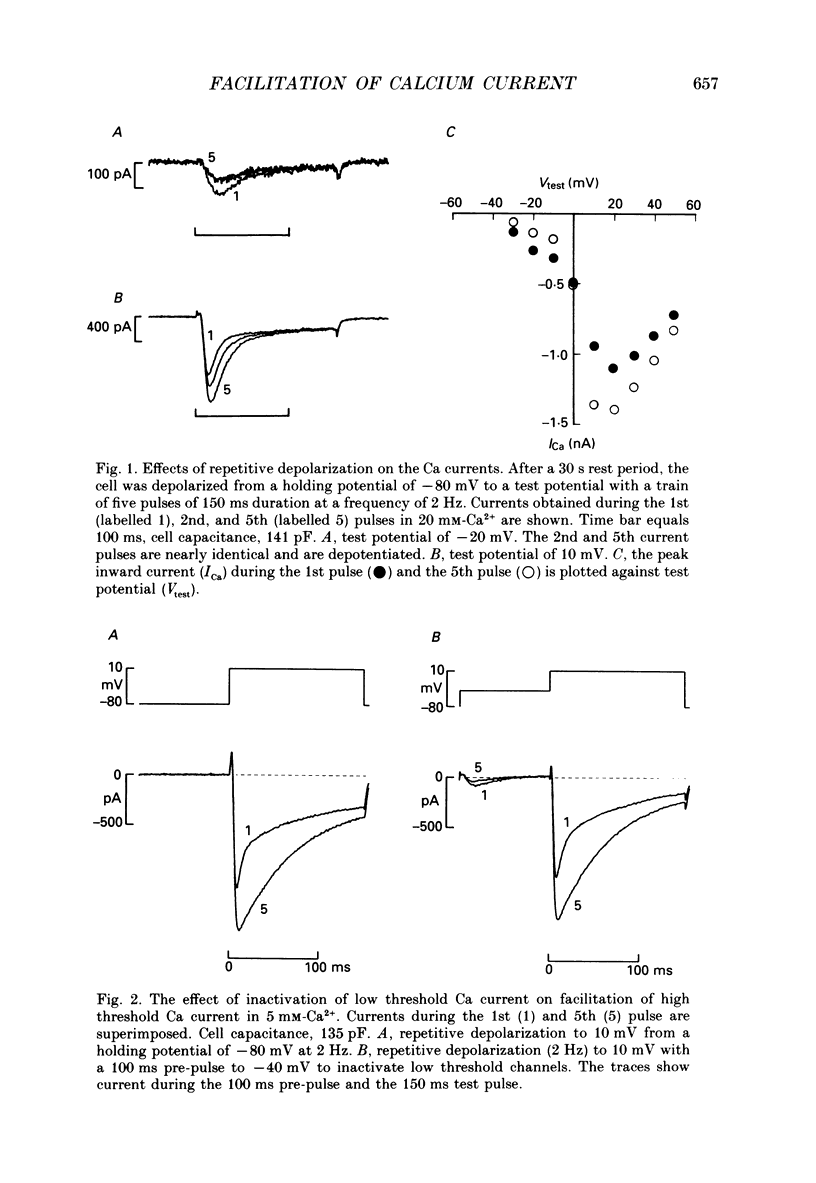
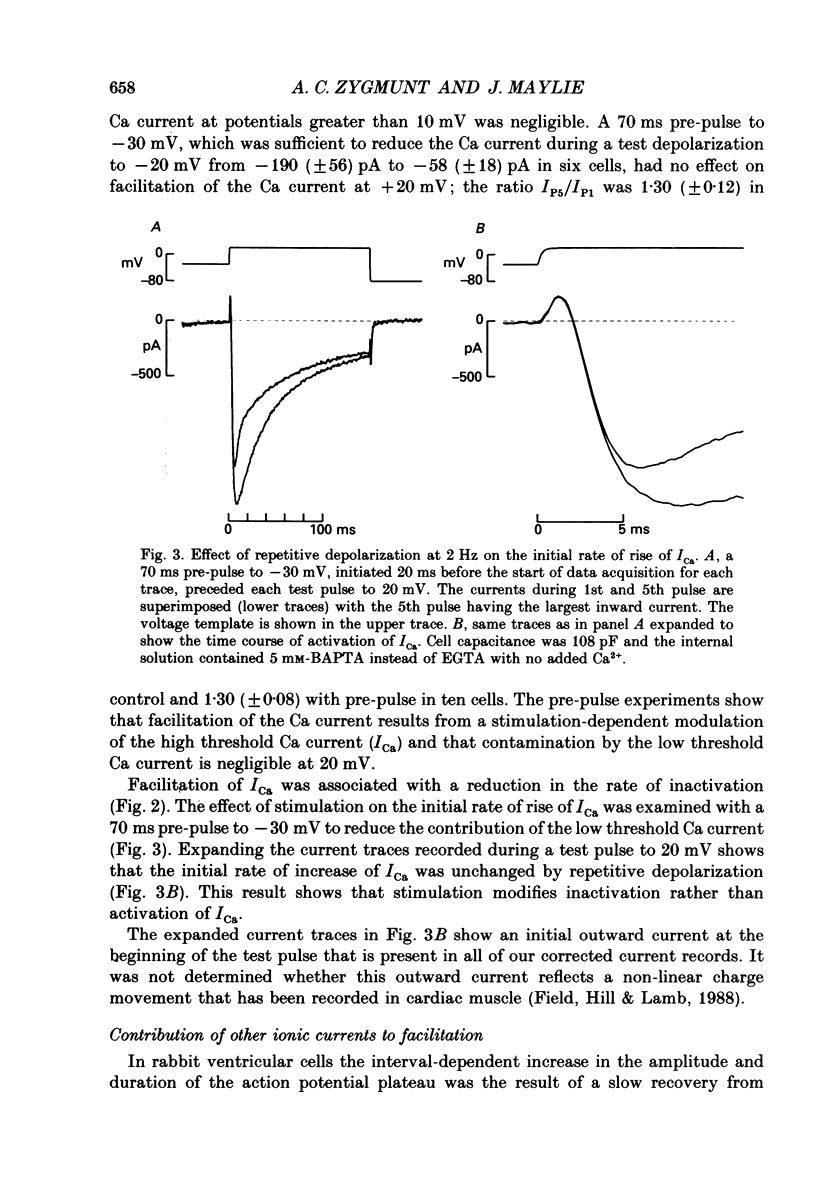
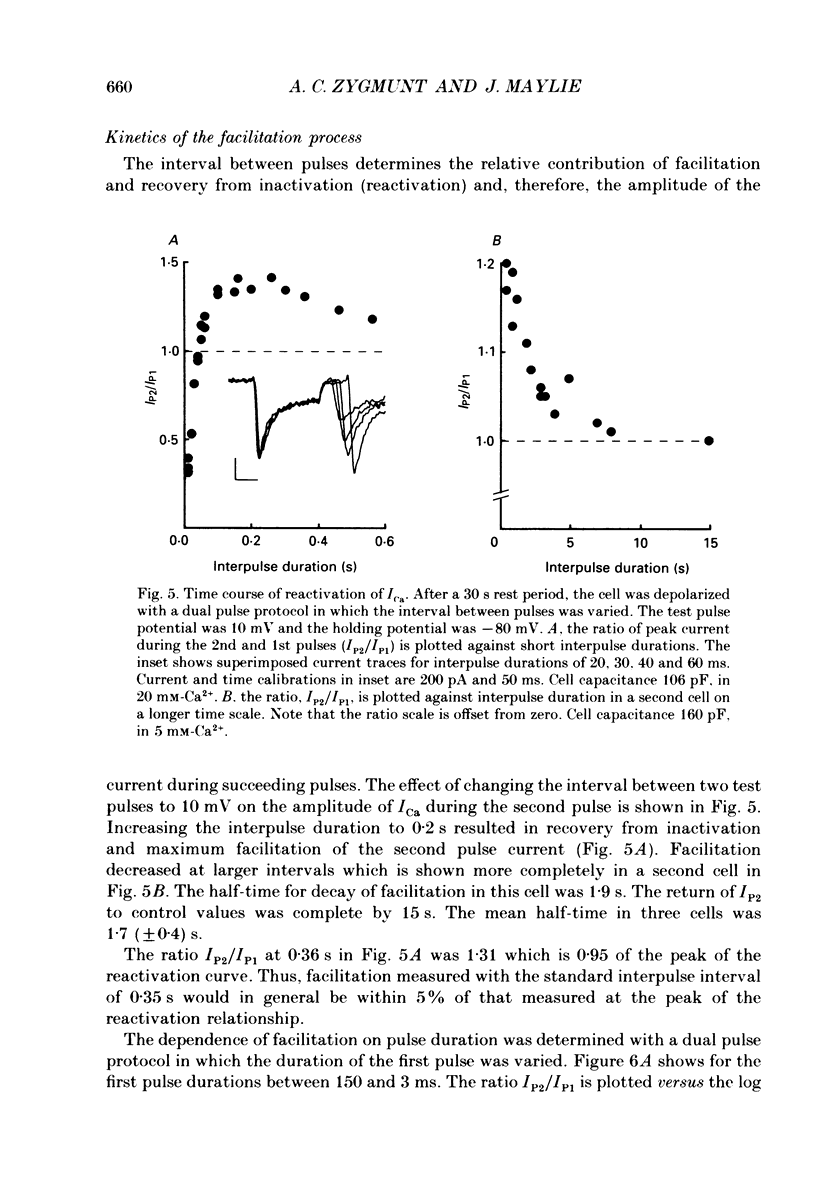
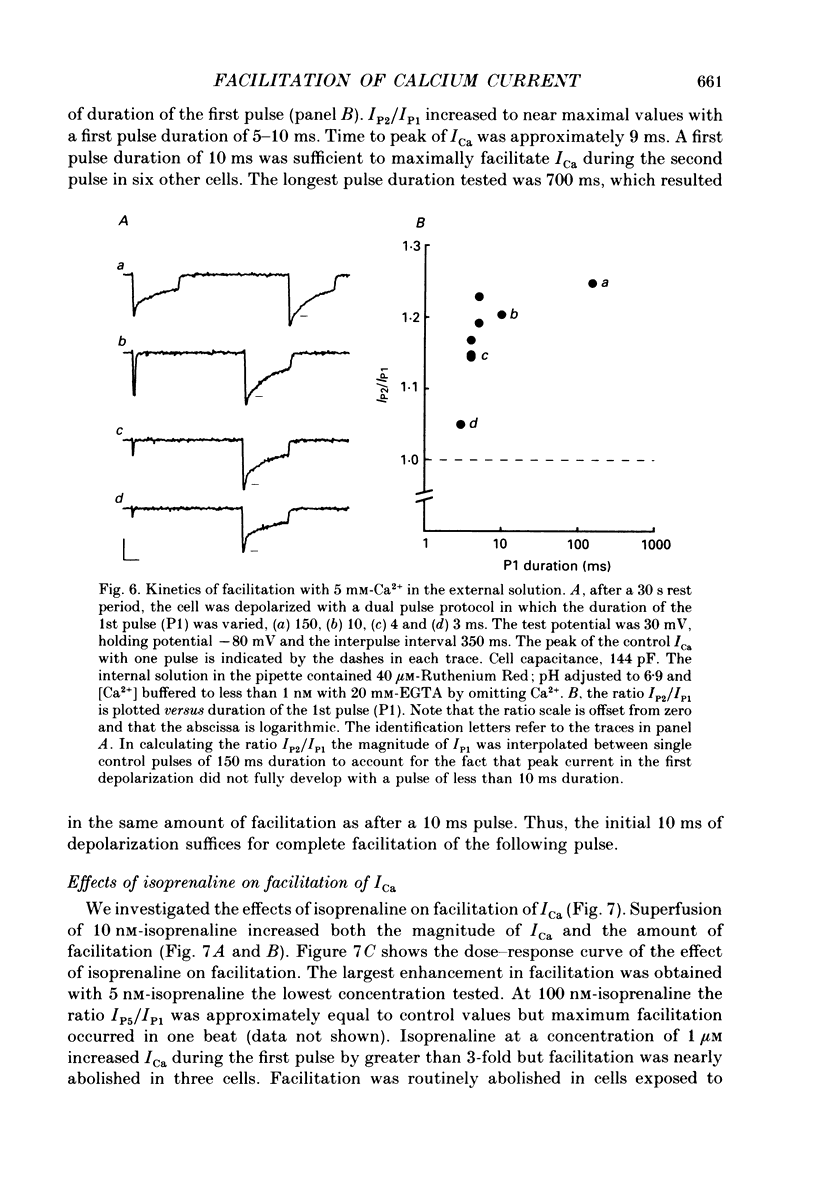
1. Stimulation-dependent modulation of Ca currents was examined in guinea-pig ventricular myocytes at room temperature. Whole-cell recordings of Ca currents were made under conditions which minimized ionic fluxes through other channels. 2. Stimulation from rest at a rate of 2 Hz resulted in a decrease of the low threshold Ca current within one pulse and facilitation of the high threshold Ca current within five pulses. Facilitation was associated with a reduction in the rate of inactivation. 3. Pulse durations as short as 10 ms facilitated the high threshold Ca current in subsequent pulses. Facilitation produced by a single pulse decayed with a half-time of several seconds. 4. Substitution of Ba2+ or Sr2+ for external Ca2+ reduced the rate of inactivation of the high threshold Ca current and abolished facilitation of the current. 5. Facilitation persisted with 40 microM-Ruthenium Red added to the internal solution or 0.2-2 microM-ryanodine added to the bath solution to reduce Ca2+ release from the sarcoplasmic reticulum. 6. Facilitation was modulated by isoprenaline. Low concentrations of isoprenaline (5-10 nM) increased the amount of facilitation. Isoprenaline (1 microM) increased the Ca current approximately 3-fold, however, facilitation was nearly abolished. 7. Caffeine (0.5 and 1 mM) affected the Ca current and facilitation in a manner similar to 1 microM-isoprenaline. It increased the Ca currents approximately 2.5-fold and facilitation was not observed. 8. We conclude that stimulation-dependent facilitation of the high threshold Ca current is mediated by calcium and hypothesize that calcium affects a site near the Ca channel that modifies the rate of inactivation. The common actions of caffeine and high concentrations of isoprenaline suggest that calcium modulates a phosphorylation step.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S., Marshall M. W. Effects of intracellular ruthenium red on excitation-contraction coupling in intact frog skeletal muscle fibres. J Physiol. 1989 Jan;408:617–635. doi: 10.1113/jphysiol.1989.sp017480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. M., Lederer W. J. Changes in the calcium current of rat heart ventricular myocytes during development. J Physiol. 1988 Dec;406:115–146. doi: 10.1113/jphysiol.1988.sp017372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Mechanism of the use dependence of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:461–475. doi: 10.1113/jphysiol.1988.sp017342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Noble D., Spindler A. J. Use-dependent reduction and facilitation of Ca2+ current in guinea-pig myocytes. J Physiol. 1988 Nov;405:439–460. doi: 10.1113/jphysiol.1988.sp017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. C., Hill C., Lamb G. D. Asymmetric charge movement and calcium currents in ventricular myocytes of neonatal rat. J Physiol. 1988 Dec;406:277–297. doi: 10.1113/jphysiol.1988.sp017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Charnet P., Pye J. M., Nargeot J. Augmentation of cardiac calcium current by flash photolysis of intracellular caged-Ca2+ molecules. Nature. 1989 Sep 7;341(6237):65–68. doi: 10.1038/341065a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Mechanism of increased amplitude and duration of the plateau with sudden shortening of diastolic intervals in rabbit ventricular cells. Circ Res. 1987 Jan;60(1):14–26. doi: 10.1161/01.res.60.1.14. [DOI] [PubMed] [Google Scholar]
- Horne P., Triggle D. J., Venter J. C. Nitrendipine and isoproterenol induce phosphorylation of a 42,000 dalton protein that co-migrates with the affinity labeled calcium channel regulatory subunit. Biochem Biophys Res Commun. 1984 Jun 29;121(3):890–898. doi: 10.1016/0006-291x(84)90761-7. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Borsotto M., Lazdunski M. Phosphorylation and dephosphorylation of dihydropyridine-sensitive voltage-dependent Ca2+ channel in skeletal muscle membranes by cAMP- and Ca2+-dependent processes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3733–3737. doi: 10.1073/pnas.83.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Rothlein J., Smith S. J. Facilitation of Ca2+-channel currents in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5871–5875. doi: 10.1073/pnas.81.18.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto Y., Yatani A., Reeves J. P., Codina J., Birnbaumer L., Brown A. M. Alpha-subunit of Gs directly activates cardiac calcium channels in lipid bilayers. Am J Physiol. 1988 Oct;255(4 Pt 2):H722–H728. doi: 10.1152/ajpheart.1988.255.4.H722. [DOI] [PubMed] [Google Scholar]
- Kaibara M., Kameyama M. Inhibition of the calcium channel by intracellular protons in single ventricular myocytes of the guinea-pig. J Physiol. 1988 Sep;403:621–640. doi: 10.1113/jphysiol.1988.sp017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Fill M., Knudson C. M., Campbell K. P., Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988 Oct 7;242(4875):99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- Maylie J., Morad M. A transient outward current related to calcium release and development of tension in elephant seal atrial fibres. J Physiol. 1984 Dec;357:267–292. doi: 10.1113/jphysiol.1984.sp015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I. N., Francini A. O., Scales D. J., Waterhouse A. L., Casida J. E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J Biol Chem. 1986 Jul 5;261(19):8643–8648. [PubMed] [Google Scholar]
- Reichardt L. F., Kelly R. B. A molecular description of nerve terminal function. Annu Rev Biochem. 1983;52:871–926. doi: 10.1146/annurev.bi.52.070183.004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardt G., Waas W., Kranzhöfer R., Mayer E., Schömig A. Adenosine inhibits exocytotic release of endogenous noradrenaline in rat heart: a protective mechanism in early myocardial ischemia. Circ Res. 1987 Jul;61(1):117–123. doi: 10.1161/01.res.61.1.117. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., Morad M. Regulation of Ca2+ current in frog ventricular myocytes by the holding potential, c-AMP and frequency. Pflugers Arch. 1989 Oct;415(1):1–11. doi: 10.1007/BF00373135. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Calcium current restitution in mammalian ventricular myocytes is modulated by intracellular calcium. Circ Res. 1988 Aug;63(2):468–482. doi: 10.1161/01.res.63.2.468. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Hoffman B. F. Two components of transient outward current in canine ventricular myocytes. Circ Res. 1989 Apr;64(4):633–647. doi: 10.1161/01.res.64.4.633. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]


