Abstract
Kinesin-1 dimerizes via the coiled-coil neck domain. In contrast to animal kinesins, neck dimerization of the fungal kinesin-1 NcKin requires additional residues from the hinge. Using chimeric constructs containing or lacking fungal-specific elements, the proximal part of the hinge was shown to stabilize the neck coiled-coil conformation in a complex manner. The conserved fungal kinesin hinge residue W384 caused neck coiled-coil formation in a chimeric NcKin construct, including parts of the human kinesin-1 stalk. The stabilizing effect was retained in a NcKinW384F mutant, suggesting important π -stacking interactions. Without the stalk, W384 was not sufficient to induce coiled-coil formation, indicating that W384 is part of a cluster of several residues required for neck coiled-coil folding. A W384-less chimera of NcKin and human kinesin possessed a non–coiled-coil neck conformation and showed inhibited activity that could be reactivated when artificial interstrand disulfide bonds were used to stabilize the neck coiled-coil conformation. On the basis of yeast two-hybrid data, we propose that the proximal hinge can bind kinesin's cargo-free tail domain and causes inactivation of kinesin by disrupting the neck coiled-coil conformation.
INTRODUCTION
Kinesin motors use the energy derived from ATP hydrolysis to move unidirectionally along microtubules. The best-studied kinesin motors belong to the class of kinesin-1, previously referred to as conventional kinesins (Dagenbach and Endow, 2004). They were purified originally from squid and bovine brain (Brady, 1985; Vale et al., 1985) and are now known to be present in many organisms ranging from lower eukaryotes to vertebrates (Hirokawa, 1998; Kirchner et al., 1999; Klopfenstein et al., 2002; Kollmar and Glockner, 2003; Schoch et al., 2003).
All kinesin-1 motors characterized so far move processively in 8-nm steps toward the microtubule plus end. They are composed of two identical heavy chains that are organized into distinct domains referred to as head (or motor domain), neck, stalk, and tail (Figure 1). The motor heads are responsible for ATP hydrolysis and microtubule binding and thus represent the catalytic core of the motor. Between the conserved motor core and the coiled-coil neck a structural element of ∼10 amino acids in length is found; the neck-linker that is thought to amplify the conformational changes in the head and thus is essential for the generation of movement (Rice et al., 1999; Case et al., 2000). The neck domain that follows assures head-head coordination and is important for processive movement, which clearly depends on dimerization (Jiang et al., 1997; Hancock and Howard, 1998, 1999). The so-called hand-overhand model of kinesin motility assumes that the two heads of a dimer take alternating steps to bind at sites spaced 8 nm apart along the microtubule axis. To ensure processive movement, at least one head of the motor has to be bound tightly to the microtubule and the catalytic cycles of both heads have to be kept out of phase (Hackney, 1994, 1995). This requires a structural connection between the heads, which apparently is mediated by the coiled-coil neck domain. Truncation, deletion, and insertion studies (Grummt et al., 1998; Romberg et al., 1998; Kallipolitou et al., 2001; Hackney et al., 2003) showed that kinesin's processive movement clearly depends on the intact neck domain, but here a significant difference between animal and fungal kinesins has been observed. In animal kinesins, the presence of the neck domain is sufficient for dimerization and processive motility (Berliner et al., 1995; Jiang et al., 1997), whereas in fungal kinesins, constructs that include the entire neck, are unable to dimerize. In the best characterized fungal kinesin-1, NcKin from Neurospora crassa, only constructs exceeding the neck domain by at least 16 amino acid residues are dimeric and move processively at wild-type velocity on streptavidin-coated coverslips when biotinylated at an artificial, C-terminal cysteine (Kallipolitou et al., 2001). These additional residues are part of the hinge and are not predicted to form a coiled-coil. Not surprisingly, therefore, deletions or other changes of the hinge region also severely affect motility in fungal kinesins (Grummt et al., 1998).
Figure 1.
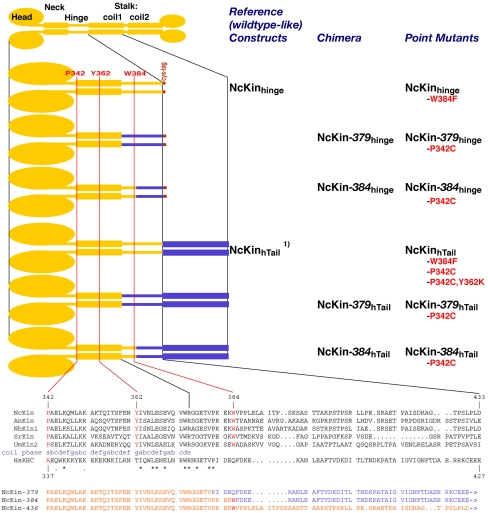
Summary of wild-type and mutant NcKin constructs. The domain organization of kinesin-1 is indicated at the top of the figure, and below are enlarged schemes and primary sequences of relevant regions. Parts from NcKin are orange, and human kinesin blue; domains are not drawn in scale. The positions of the residues W384, Y362, and P342 that have been mutated in this study are indicated in red. The construct names indicate the origin of the kinesin used (NcKin), followed by the fusion point (italic number) if it is a chimera; then the length of the construct in subscript (hinge/hTail), finally any exchanged amino acid residue(s), if applicable. Constructs used for two-hybrid assays were essentially comparable to these protein backgrounds, and differed only in some details (Material and Methods). The short constructs based on NcKinhinge were used for kinetic and dimerization assays and tested in cross-linking studies as P342C mutants. The longer NcKinhTail-based constructs stuck readily to glass surfaces and were used for multiple motor gliding assays, as well as kinetic and, in the P342C mutant background, cross-linking studies. The primary sequences at the bottom show a sequence alignment of wild-type kin-esin-1s (NcKin, N. crassa; AnKin, Aspergillus nidulans; NhKin1, Nectria hematococca; SrKin, Syncephalastrum racemosum; UmKin2, Ustilago maydis; HsKHC, Homo sapiens), and the amino acid sequences of chimera NcKin-379 and -384. The phase of the neck coiled-coil is indicated (abcdefg positions), the similarity between the fungal and the human sequences by asterisks for amino acid identity in all cases, and dots for similarity. 1) The NcKinhTail construct can also be regarded as chimera NcKin-433. It behaved indistinguishable from full-length wild-type NcKinhTail. For reasons of readability and by analogy to the NcKinhinge construct, it is referred to as NcKinhTail wild-type-like reference construct in this article.
Only little structural information is available on the hinge domain. Sequence analysis reveals very poor conservation of the hinge region as a whole among kinesin-1 motors, but the N-terminal parts show clear similarities. Remarkably, all fungal kinesins contain a conserved tryptophan residue (W384 in NcKin) that is not present in animal kinesin-1 (Figure 1). Three-dimensional structural information on the hinge conformation is scarce and mainly based on one NMR analysis of a synthetic peptide comprising the C-terminal part of the neck and the first 15 amino acid residues of the hinge of rat kinesin. It confirms that the hinge consists of short nonhelical secondary elements that break the neck coiled-coil structure (Seeberger et al., 2000). Remarkably, neck and hinge are linked via a highly flexible motif that allows for a large torsional freedom. This may help kinesin motors that are bound to a cargo in arbitrary orientations to align and move along a microtubule in a concerted manner (Hunt and Howard, 1993), but other functions, such as a contribution to neck dimerization, cannot be excluded.
To trace such possible functions, fungal kinesin-1 motors offer unique possibilities. The N. crassa kinesin NcKin and its homologues from other filamentous fungi clearly group with other kinesin-1 (conventional kinesin) motor proteins (Steinberg and Schliwa, 1995). However, they show a number of sequence elements, in particular in the neck and hinge regions adjacent to the motor core that are conserved among the fungal group of kinesin-1 motors but not in animal kinesin-1s (Figure 1). One conspicuous residue is tyrosine Y362 in the center of the neck coiled-coil domain. We showed that it is required for proper folding of the α-helical coiled-coil, but also for down-regulation of the motor activity, presumably in the inactivated, cargo-free state (Schafer et al., 2003). To explain how this residue inhibits the motor activity under repressing conditions, but allows full function under activating conditions, we hypothesized that the neck can switch between an α-helical coiled-coil conformation (active) and another, unknown conformation that allows interaction between Y362 and the core motor domain (inactivated). To further investigate the nature of this presumed switch, we are asking here whether one or more of the other conserved sequence elements in neck and hinge domains are responsible for altering the conformational state of the neck domain and which functional influence they have.
MATERIALS AND METHODS
Cloning of Constructs
The NcKinhTail construct contains amino acid residues 1–436NcKin from N. crassa conventional kinesin and a portion of the human kinesin tail (residues 432–546HsKin), which promotes unspecific attachment of kinesin to surfaces in gliding assays. It was generated by standard PCR and cloning techniques and was shown to be indistinguishable from wild-type NcKin in gliding and steady state ATPase assays (Hahlen, unpublished results).
The chimeric constructs NcKin-379hTail and NcKin-384hTail contained NcKin sequences up to amino acid residue 379 or 384, respectively, followed by human kinesin sequences (Navone et al., 1992) up to residue 546 (Figure 1). Human kinesin-coding sequences were transferred to NcKin plasmids by PCR on a pT7-based vector that contained the human kinesin gene pK546_N2K2 with modified restriction sites (Kallipolitou, unpublished results). To facilitate cloning, silent mutations were introduced into the NcKin expression vector that 1) knocked out an endogenous KpnI site, 2) created a new one between the regions encoding neck and hinge domains, and 3) a NgoMIV restriction site between hinge and stalk. The vector, pNK433(C307A) also 4) contained the functionally unimportant, mutated cysteine residue C307A (Hahlen, unpublished results). A reverse primer at the 3′ end of the human kinesin gene with a downstream PstI site was used to generate PCR products of human kinesin in combination with forward primers that annealed at the homologous region of the introduced NcKin-KpnI site. For NcKin-379hTail the forward primer fully matched the human kinesin sequence, for NcKin-384hTail it encoded amino acid residues 378–384 from NcKin and the rest of human kinesin. Constructs lacking the human stalk (NcKinhinge) were based on the respective NcKinhTail pendants and were cloned by replacing the NgoMIV-PstI fragment with a short oligonucleotide dimer that encoded a cysteine tag (PSIVHRKCF; Funatsu et al., 1995) with stop codon and a downstream PstI site.
To introduce cross-links between the neck domains of the two polypeptide chains of a kinesin dimer, proline 342 was exchanged to cysteine, because this residue is expected to be in the first a-position of the α-helical neck coiled-coil (Schafer et al., 2003). Such an arrangement is known to form interstrand disulfide bonds readily (Zhou et al., 1993). P342 was mutated using a PCR-based mutagenesis protocol (Stratagene QuickChange, La Jolla, CA). The chimeric constructs were cloned into this background via the internal BamHI and NgoMIV restriction sites. The Stratagene Quickchange protocol was also used to generate the W384F point mutation. All plasmids were verified by sequencing.
Expression and Purification of Kinesin Constructs
Expression and purification of NcKin constructs was accomplished as described (Schafer et al., 2003). Briefly, 4 g of Escherichia coli cells were suspended in buffer A (20 mM Na-phosphate, pH 7.4, 50 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 1 mM dithiothreitol [DTT], 10 μM ATP, protease inhibitor mix (Roche Diagnostics, Penzberg, Germany), sonified, and centrifuged (35 min at 100,000 × g). The supernatant was loaded on a 5-ml HiTrap SP Sepharose column (Amersham Pharmacia Biotech, Piscataway, NJ) and eluted without DTT in a manual NaCl step gradient. The peak fractions were pooled, frozen in liquid N2 with 10% glycerol, and stored at –70°C. Protein concentrations were evaluated by a Bradford assay.
Size Determination
To characterize the oligomerization state of NcKin constructs, sedimentation coefficients Sw,20 and Stokes' radii (rstokes) of the constructs were determined by comparison with standard proteins.
Sw,20 coefficients were determined by centrifugation of 5–10 μM protein solutions on 5–18% sucrose gradient cushions in buffer B (20 mM Na-phosphate, pH 7.4, 200 mM NaCl, 5 mM MgCl2, 10 μM ATP, 1 mM DTT) using the Beckman rotor SW 50.1 (Berkeley, CA) at ∼20,000 × g for 16 h at 4°C. As internal standards aldolase, bovine serum albumin (BSA), and carboanhydrase (Sw,20 = 7.4, 4.3, and 3.2, Roche Diagnostics) were included. The gradient was fractionated and analyzed on SDS-polyacrylamide gels. The position of the protein peaks were used to determine the Sw,20 values (NIH-Image program, Kaleidagraph software, Reading, PA).
The proteins' Stokes radii (rstokes) were determined by gel filtration analysis. Protein solutions (8–15 μM) were loaded on a Sephadex 200 column (Amersham Pharmacia Biotechnol), equilibrated with buffer B. The elution volumes of the samples were compared with standard proteins (ferritin: 5.9 nm rstokes, aldolase: 4.5 nm, BSA: 3.5 nm, carboanhydrase: 2.4 nm, cytochrome c: 1.6 nm; Roche Diagnostics and Sigma Chemical, St. Louis, MO). Stokes radii were determined from a plot of elution volumes versus standard sizes (Andrews, 1970). The molecular weights (Mr) of kinesin constructs were calculated according to Cantor and Schimmel (1980):
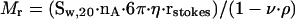 |
where nA is the Avogadro number, η viscosity (10–3 N/m–1s–1), ν is the specific volume of the protein (0.725 cm3/g), and ρ is the density of the medium (1.0 g/cm3).
Gliding Assays
To bind kinesin molecules on glass coverslips, NcKinhTail constructs containing part of the human kinesin tail region (amino acid residues 432–546 in the human kinesin sequence) were used (Hahlen, unpublished results). After 1–3 min of incubation of 1.5 μg kinesin (20–30 μmol) on an 18 × 18-mm2 glass coverslip, the assay was started by adding motility buffer (10 mM MgCl2, 10 mM ATP, 10–20 mM KCl, and microtubules in BRB80+ [Pipes·KOH, 80 mM, 5 mM Mg-acetate, 0.5 mM EGTA, final pH 6.8 with 0.25–1 g/l Casein]) to a volume of 10 μl (Kallipolitou et al., 2001). Motility was monitored in a Zeiss Axiophot using video-enhanced phase-contrast microscopy (Thornwood, NY; Steinberg and Schliwa, 1996). To determine the gliding velocities, at least 20 microtubules were measured and averaged.
Steady State ATPase Activity
Microtubule-stimulated steady state ATPase rates were measured in a photometric assay that couples ATP hydrolysis to NADH oxidation by phosphoenolpyruvate, pyruvate kinase, and l-lactate dehydrogenase (Kallipolitou et al., 2001).
Chemical Cross-linking
Reversible disulfide bonds were formed between the two cysteines of dimeric NcKinhTailP342C by incubation with 5,5′dithio-bis-(2-nitrobenzoic acid) (DTNB) or air (Tomishige and Vale, 2000; Hahlen, unpublished results). DTNB, 200 μM, was added to 10–30 μM kinesin containing the P342C mutation and incubated for 5 min. To verify the cross-link, aliquots were analyzed on SDS gels without reducing agents. To visualize air-oxidation, untreated kinesin constructs were mixed with sample buffer.
Disulfide bonds were broken by reduction with DTT. DTT, 2–5 mM, was added to 10–30 μM kinesin, incubated for 1–3 h, and then mixed with SDS sample buffer. For recovery after DTNB incubation (“rescue”), samples were incubated with at least 5 mM DTT for 4 h. SDS-PAGE was performed with all samples followed by Coomassie Blue staining.
To verify the behavior of the most sensitive mutant, NcKin-379hTailP342C, 5 mM DTT was present in a control preparation. To minimize unspecific oxidation, this preparation was assayed immediately after purification. Oxidation was achieved by removal of DTT on a Sephadex G25 gel filtration column and air exposure or DTNB incubation.
To perform ATPase activity or gliding assays under reducing or oxidizing conditions, kinesin samples were treated as described above, starting the assays immediately after DTT or DTNB incubation. In gliding assays DTT or DTNB was present in the motility buffer.
Yeast Two-hybrid Assay
To test the interaction of kinesin's tail with the neck and/or hinge regions, the Matchmaker III system was used (Clontech/BD Biosciences, San Jose, CA). As bait construct, the NcKin tail domain from amino acid 725–928 was cloned into the plasmid pGBKT7 using the internal NcoI restriction site. N-terminal kinesin fragments were cloned into the prey vector pGADT7 by PCR on the plasmid pT12.1 (Henningsen and Schliwa, 1997; Seiler et al., 1999), yielding pNK343GADT7 (encoding the core motor domain, residues 1–343, NcKincore), pNK378GADT7 (1–378, NcKinneck), pNK400GADT7 (1–400, NcKinhinge; due to cloning difficulties, this construct ends 33 codons earlier than NcKinhinge from Figure 1), and pNK480GADT7 (1–480, NcKinTail; this construct contains the NcKin counterpart of human kinesin's stalk). DNA fragments encoding chimeric constructs were PCR-amplified from their respective expression plasmids, cut in restriction digestions, and used to replace the SacII/NgoMIV fragment of pNK480GADT7, encoding NcKinTailW384F, NcKin-379Tail, and NcKin-384Tail. A second version of the NcKinTail prey construct was cloned this way (pNK480(C307A)GADT7), because all chimeric constructs were based on a modified expression vector with altered restriction sites and codon usage (pNK433(C307A), see above). The C307A point mutation did not change the behavior in the two-hybrid assay.
Bait and prey plasmids were cotransformed into the yeast strain AH109 using the PEG/LiAc method as described by BD Biosciences. Double transformants were selected on LW–plates. The interaction was screened on LWHA–plates where only colonies of strains with strongly interacting gene products grow or on LW-/X-Gal plates where interactions are indicated by blue colony coloration. These tests were performed on three independent transformants. To quantify the interaction, five independent yeast transformants were tested in an enzymatic assay for β-galactosidase activity using chlorophenol red-β-galactopyranoside (CPRG, Clontech Matchmaker manual) as a substrate. The activity was calculated as described by the manufacturer, which involves corrections for variable cell densities and assay times:
 |
RESULTS
Conservation and Importance of W384
To investigate the influence of the proximal part of NcKin's hinge domain on neck coiled-coil formation, we constructed a series of mutants with altered hinges. Previous work had shown that Neurospora kinesin offers unique possibilities for such studies because, in contrast to animal kinesins, the presence of the hinge turned out to be a prerequisite for neck coiled-coil formation (Kallipolitou et al., 2001). A C-terminal deletion series had shown that truncated NcKin variants up to amino acid 383 are monomeric, but constructs comprising 391 or more residues are dimeric, demonstrating that the hinge residues 384–391 are crucial for neck coiled-coil formation. One of these truncated kinesins, NcKinhinge, ending at the C-terminal end of the hinge after residue D433 and before the stalk coiled-coil, turned out to be a very useful construct with wild-type properties (stable dimer, gliding velocity typically 2.0–2.5 μm/s, steady state ATPase rate 60–80 s–1). It is well expressed in E. coli and rather stable in vitro. We therefore used NcKinhinge as the basis for our mutagenesis. We initially mutated a fungal-specific tryptophan, W384, located nine residues downstream of the end of the neck coiled-coil, into a phenylalanine. The exchange W→ F is the mildest possible natural side-chain substitution. The resulting mutant, NcKinhingeW384F was monomeric and showed a decelerated ATPase rate (21 ± 6 vs. 66 ± 21 s–1 for wild type, Table 1). This slow ATPase rate is typical for constructs that are inhibited by the residue Y362 in the neck domain (Schafer et al., 2003) and therefore suggests that the absence of W384 mimics the inactive state of NcKin where the coiled-coil conformation of the neck is disrupted.
Table 1.
Motile and kinetic characteristics and oligomerization state of relevant constructs
| Molecular masses (kDa)
|
|||||
|---|---|---|---|---|---|
| Construct | Gliding velocity (μm/s) | Steady state ATPase (s-1) | Derived | Predicted | Oligomerization state |
| NcKinhTail | 2.69 ± 0.28 | 71 ± 14 | —a | ||
| NcKinhinge | n/d | 66 ± 21 | 83.4 | 48.2 | Dimer |
| NcKinhTailW384F | 2.27 ± 0.23 | 41 ± 1a | —a | ||
| NcKinhingeW384F | n/d | 21 ± 6a | 51.2 | 47.2 | Monomer |
| NcKin-379hTail | 0.76 ± 0.19 | 27 ± 4 | —a | ||
| NcKin-379hinge | n/d | 13 ± 3 | 50.7 | 48.2 | Monomer |
| NcKin-384hTail | 2.16 ± 0.3 | 56 ± 2 | —a | ||
| NcKin-384hinge | n/d | 16 ± 3 | 50.5 | 48.3 | Monomer |
The values are averages from at least two independent measurements, standard deviations are given for each value. n/d, not determined.
—, dimerization via hTail-domain in all constructs.
To test the possible influence of the neighboring residues, we also constructed chimeric mutants consisting of NcKin head and neck domain, and variable amounts of the hinge domain. These N-terminal parts were fused to the rest of the human kinesin hinge as indicated in Figure 1. As expected, a chimera comprising NcKin 1–379 in front of the human hinge, and thus lacking the fungal-specific W384 (NcKin-379hinge), failed to dimerize (Table 1). As the W384 F point mutant, the NcKin-379hinge chimera showed a substantially decreased ATPase activity (27 s–1), again consistent with the neck domain being in an unfolded state, as explained above. Together, the observations on NcKinhingeW384F and NcKin-379hinge were in full agreement with previous results from domain mapping studies that identified the region between amino acid 383 and 391 as a crucial element for NcKin dimerization. NcKin variants were monomeric when truncated before residue 384, but dimeric after 391 (Kallipolitou et al., 2001). The novel data on the W384F point mutant and the NcKin-379 chimera therefore suggested that W384 might be chiefly responsible for coiled-coil formation and thus dimerization of NcKin.
However, the chimera NcKin-384hinge turned out to be a monomer as well, although it includes the important residue W384 (Table 1). As other monomeric mutants, it exhibited a slow ATP turnover (16 ± 3 s–1). Therefore, W384 is not sufficient on its own to induce dimer formation and wild-type-like ATPase behavior in the NcKinhinge protein background. Still, W384 seems to enhance or induce coiled-coil conformation of the neck domain, as indicated in a longer construct background.
The Human Kinesin Stalk Suppresses the Strict Requirement for W384
Further insights into the role of W384 and its vicinity were gained when we used constructs longer than 433 amino acids. To test NcKin mutants in microscopic gliding assays, we added part of the human kinesin stalk domain after the hinge (NcKinhTail constructs, Figure 1; the part used for glass attachment did not comprise the tail region responsible for tail inhibition). The stalk portion causes unspecific adsorption to microscopic glass coverslips and thus facilitates multiple motor gliding assays. According to previous studies, we expected that mutants with a disrupted coiled-coil show slow and nonprocessive motility in these assays (Schafer et al., 2003). This was true for the NcKin-379hTail chimera, which showed about one third of the wild-type gliding velocity (0.8 μm/s), and thus closely resembled the NcKin-Y362K point mutant with a disrupted neck coiled-coil (Table 1; Schafer et al., 2003). Unexpectedly, the NcKinhTail W384F point mutant and the NcKin-384hTail chimera both moved microtubules at wild-type velocity (2.3 and 2.2 μm/s), suggesting that the additional stalk sequence rescued the coiled-coil neck conformation. If this were true, W384 would behave like an element of a hydrophobic staple (Tripet and Hodges, 2002) in the NcKinhTail background. However, the simple standard prove for neck coiled-coil formation— gel filtration/sucrose density centrifugation to test dimerization—is meaningless in this protein background, because the human stalk portion forms a coiled-coil and thus leads to a dimeric molecule regardless of the structural state of the neck domain (Vale and Fletterick, 1997). We therefore established an assay that probes neck coiled-coil formation in molecules that are dimeric due to the presence of additional domains.
Structural Information on the NcKin Neck Detected by Disulfide Bonds
To gain evidence for the structural integrity of kinesin's neck coiled-coil, we studied the distances of mutant neck domains between both subunits by introducing cysteine residues at the first coiled-coil a position (P342C mutations) and testing the capability of forming an intersubunit disulfide bridge. The formation of disulfide bonds requires close proximity of two –SH groups (∼2 Å) and has been shown to occur readily between opposing a positions of an intact coiled-coil (Zhou et al., 1993). To detect disulfide cross-links, we treated proteins with DTT (negative control), air, and DTNB and tried to revert the DTNB oxidation by adding excess DTT (rescue; Tomishige and Vale, 2000). Proteins incubated under these conditions were analyzed on nonreducing SDS gels, where uncrosslinked proteins appear at the calculated size of a single polypeptide, but proteins with intersubunit cross-links at multiples of this size.
Two negative controls were used in the cross-linking experiments: first, wild-type NcKinhTail without introduced cysteines and second NcKinhTail P342C, Y362K, which had been shown to contain a disrupted neck coiled-coil despite of being dimeric (Table 2; Schafer et al., 2003). As expected, both constructs were unaffected by oxidation and remained uncrosslinked under all conditions (Figure 2). By contrast, the NcKinhTailP342C positive control showed bands at 205 kDa under untreated (i.e., air) and DTNB conditions that disappeared under DTT conditions, indicating formation of intersubunit disulfide bridges. The observed size of 205 kDa was higher than expected for a dimer, but gel filtration and sucrose density centrifugation experiments revealed a mass of 129 kDa, as expected for a dimer (Table 2). These results proved the feasibility of the method and agreed well with previous observations on synthetic peptides (Deluca et al., 2003; Schafer et al., 2003).
Table 2.
Characterization of P342C mutants
| Gliding velocity (μm/s)a
|
Steady state ATPase (s-1)
|
Molecular masses (kDa)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Construct | Oxidized | Reduced | Oxidized | Reduced | Rescued | Derived | Predicted | Oligomerization state |
| NcKinhTailP342C | 2.12 ± 0.13 | 2.53 ± 0.16 | 66 ± 1 | 64 ± 5 | 63 ± 5 | 128.8b | 60.9b | Dimerb |
| NcKinhTailP342C,Y362K | n/d | n/d | n/d | n/d | n/d | 106.9c | 60.8c | Dimerc |
| NcKin-379hTailP342C | 2.12 ± 0.32 | 2.04 ± 0.28 | 66 ± 3 | 30 ± 17 | 24 ± 9 | n/d | n/d | n/d |
| 2.11 ± 0.53d | 1.77 ± 0.38d | 37d | 15d | |||||
| NcKin-384hTailP342C | 2.05 ± 0.17 | 2.12 ± 0.17 | n/d | n/d | n/d | n/d | n/d | n/d |
Gliding velocities were determined by measuring and averaging at least 20 microtubules, standard deviations are given for each value. n/d, not determined.
Oligomerization of NcKinhTailP342C was tested in the oxidized state to verify dimer formation.
Oligomerization of NcKinhTailP342C,Y362K was tested to verify dimerization via the hTail domain.
Freshly measured after preparation in the presence of 5 mM DTT; to perform measurements in the oxidized state the DTT was removed via gel filtration.
Figure 2.
Cross-linking of P342C mutants. The mutants are fractionated on a nonreducing SDS-gel under different redox-conditions: –, no reagent, oxidation by air; ox, +0.2 mM DTNB; red, +2–5 mM DTT; res, +0.2 mM DTNB 5′, 5 mM DTT 4 h. Under oxidizing conditions (air, DTNB) a bandshift to ∼200 kDa is visible for P342C and the chimeras NcKin-379hTailP342C and NcKin-384hTailP342C to a nearly complete extent, indicating an interchain cross-link (see also text). The cross-link is reversible as it is seen by the appearance of a 60-kDa band (corresponding to one kinesin polypeptide chain) instead of the 200-kDa band under reducing conditions (DTT). No cross-link occurred with the negative control without P342C and with the double mutant P342C,Y362K.
The chimeric mutants, however, behaved somewhat unexpectedly in this assay. Both the NcKin-384hTail P342C and the -379hTailP342C chimera showed formation of intersubunit cross-links in oxidizing environments, but only the former was expected to do so. As its cysteine-less parent, NcKin-384hTailP342C behaved like constructs with an intact neck coiled-coil in motility assays (2.1 μm/s; Table 2). Notably, the P342C point mutation did not affect the gliding velocity in the NcKinhTail background (2.1 μm/s). The NcKin-379hTailP342C chimera also caused a similarly fast gliding velocity (2.1 μm/s), and SDS-PAGE on nonreducing gels confirmed that the neck of this mutant was cross-linkable. However, the cysteine-less NcKin-379hTail parent showed slow motility and slow catalytic activity, reminiscent of constructs with a disrupted coiled-coil (Table 1; Kallipolitou et al., 2001; Schafer et al., 2003). The most intuitive interpretation, that the P342C mutation stabilized the coiled-coil conformation even in the reduced, disulfide-free situation, is unlikely because both the short NcKin-379hingeP342C and the NcKin-384hingeP342C chimera are monomeric in the presence of DTT (Supplementary Table S1).
Therefore, most likely the reduced activity of the NcKin-379hTail parent construct is due to a disrupted neck coiled-coil structure. However, coiled-coil and non–coiled-coil structures seem to be very close to thermodynamic equilibrium. Support for this came from ATPase measurements on a control preparation of NcKin-379hTailP342C performed in the presence of 5 mM DTT to exclude unintended autooxidation during protein purification. ATPase activity was tested immediately after the purification and without adding glycerol and in the presence of high levels of DTT (at least 5 mM). Under these reducing conditions the steady-state ATP turnover rate decreased to 15 s–1 (even lower than 27 s–1 for the NcKin-379hTail parent) and was accelerated 2.5-fold to 37 s–1 upon removal of DTT by gel filtration. This result suggests that 1) NcKin-379hTailP342C oxidizes very easily, and has to be kept under high DTT concentrations to prevent disulfide bond formation. If kept reduced properly, 2) the ATPase is slow but can be accelerated by oxidation, which is most easily explained by the reformation of the previously disrupted neck coiled-coil conformation.
Unlike in ATPase assays, the NcKin-379hTailP342C mutant was as fast as wild-type NcKin in gliding assays under all conditions, even in the control preparation in the presence of 5 mM DTT (Table 2). However, multiple motor gliding assays are somewhat deceiving because the gross action that is measured does not necessarily average the behavior over the entire population, but may be dominated by a small fraction of very active motors. To test this possibility, we mixed NcKin-379hTail (0.8 μm/s) with wild-type kinesin (app. 2.5 μm/s) in variable amounts and measured the resulting gliding velocities (Supplementary Material). The results of these experiments suggested that a fraction of <20% of motor with intact neck coiled-coil was able to mask the mutant effect and to produce wild-type gliding velocity. We therefore think that the apparently undisturbed gliding velocity of NcKin-379hTailP342C under reducing conditions is an experimental artifact that is due to a small fraction (5–10%) of oxidized mutant motor protein that is already present in the recombinant E. coli protein.
Together, the results described above suggest a complex interplay between the single amino acid residue W384, its immediate neighborhood, and the more distant neck and tail coiled-coil structures. 1) The replacement of the indole ring of W384 with a phenyl group (F384) leads to the disruption of the α-helical neck coiled-coil structure in the otherwise rock-solid NcKinhinge dimer background. 2) The strict requirement of W384 for neck coiled-coil formation is suppressed when dimerization is induced by kinesin's stalk, and phenylalanine can replace tryptophan in this case. 3) Still, W384 and its close neighbors facilitate neck coiled-coil formation, because the NcKin-379hTail chimera retains the non–coiled-coil neck structure, whereas the NcKin-384hTail contains a functional neck coiled-coil. The fact that NcKinhTailW384F also forms an active neck conformation (despite the lack of W384) may indicate that either the W→F exchange is mild because it retains an aromatic ring or that elements from the close vicinity (residues 385–391; Kallipolitou et al., 2001) contribute to the effect of W384. Thus, W384 is neither absolutely necessary nor completely sufficient for neck coiled-coil formation under all circumstances, but seems to cause its formation when present in its proper environment.
What may be the physiological role of this proximal part of the hinge domain? Previous studies strongly argue for intramolecular regulation where the tail folds back on the head/neck/hinge region to inactivate the motor when no cargo is bound (Coy et al., 1999; Friedman and Vale, 1999; Kirchner et al., 1999; Hackney and Stock, 2000; Seiler et al., 2000). To test whether the proximal hinge plays a role in this regulation mechanism, we studied the tail–neck/hinge interaction using yeast two-hybrid assays.
The Hinge near W384 May Be a Target of Motor Regulation by the Tail
To study the neck/hinge–tail interaction in a yeast two-hybrid assay, part of the tail domain (amino acids 725–928) was used as the bait construct and cotransformed into yeast with prey plasmids encoding different regions of the head/neck/hinge domains (Figure 3). The strongest interaction was found in wild-type prey constructs that that encoded NcKinTail, (amino acids 1–480NcKin; cf. Material and Methods for details). Yeast transformants with these preys even grew under the most restrictive conditions on LWHA–plates. No interaction was seen when a shorter tail construct (amino acid 725–844) was used, consistent with the tail interaction site being close to the globular C-terminus (Kirchner et al., 1999; Seiler et al., 2000). All shorter NcKin prey constructs, as well as all kinesin chimera and point mutants were negative under these stringent growth conditions.
Figure 3.
Interaction between the kinesin tail and the hinge domain. The interaction between the NcKin tail and hinge domains was tested in a yeast two-hybrid assay. The kinesin tail from amino acid 725–928 was used as bait, different N-terminal kinesin constructs as preys. The interactions were detected by the activation of the LacZ reporter gene, which leads to the expression of β-galactosidase. The β-galactosidase expression level was assayed enzymatically with CPRG as a substrate (bar chart, n = 5–15 independent transformants; error bars, SD) and by blue coloration of the colonies on X-α-Gal containing plates (inset). The longest kinesin prey construct, NcKinTail, showed the strongest signal on X-α-Gal LW–selection plates, in CPRG assays, and grew on LWHA-plates. The next shorter construct, NcKinhinge, was still positive on X-α-Gal plates and in the enzymatic assay, but failed to grow on LWHA-plates. All shorter prey constructs, as well as the point mutant NcKinTailW384F and the chimera NcKin-379Tail, were highly variable on X-α-Gal plates but were clearly negative in the enzymatic assay. The NcKin-384Tail chimera was also negative, suggesting additional structural requirements for a robust interaction.
The NcKinTail prey construct and the truncated NcKinhinge were also positive on LW–plates containing X-α-Gal, suggesting a robust tail interaction in constructs that contain the wild-type hinge domain (Figure 3). The shorter NcKincore and NcKinneck constructs were clearly negative on X-α-Gal plates; the point mutant NcKinTailW384F, as well as the chimeric NcKin-379Tail and -384Tail constructs were highly variable in this assay. Therefore, five independent transformants of each bait-prey pair were compared in parallel in an enzymatic assay, using CPRG as a substrate. The result confirmed the observations of the previous assays and revealed a 3–5-fold higher β-galactosidase activity with the NcKinTail and NcKinneck prey constructs than with the other mutants (Figure 3). In this assay, the W384F point mutant and both chimeras were clearly negative.
These findings indicate that the proximal region of the hinge, from amino acid 378 to amino acid 400, which includes W384, may be a target site for tail-mediated inhibition of motor activity. The fact that the NcKin-384 chimera was negative in all two-hybrid assays indicates that residue W384 alone is not sufficient for a strong interaction but requires additional motifs further C-terminal.
DISCUSSION
Mutagenesis of the hinge of NcKin reveals a complicated mesh of interdependencies that is required for formation of the neck coiled-coil structure. Previous studies demonstrated that the C-terminally truncated NcKin383 is monomeric and shows low ATPase activity, whereas NcKin391, only seven residues longer and including the fungal conserved W384, is a dimer with high ATPase activity (Kallipolitou et al., 2001). The present study addresses the functional and physiological role of the proximal hinge region and shows how the conspicuous W384 residue and its neighbors might contribute to its function.
Sequence Requirements for Folding of the NcKin Neck Domain
The study of two chimeras, one retaining W384 (NcKin-384) and one lacking it (NcKin-379) shows that only the former displays normal gliding and ATPase activities. In addition to W384, the mutants differ in residues K381, E382, and K382, and based on the sequence alignments, both lysine residues might also contribute to the observed differences, although neither of these residues is strictly conserved in fungal kinesins (Figure 1). Cross-linking assays that probe the integrity of the neck coiled-coil indicate that only the chimera that comprises W384 is able to form an α-helical neck coiled-coil in the NcKinhTail background. Data on the W384F point mutant NcKinhTailW384F that does not contain W384 but still moves quickly and forms a functional neck domain seem to contradict this interpretation. However, the side chain of the introduced F384 still contains an aromatic ring and thus might still be able to contribute to possible π -stacking interactions (as e.g., observed by Zheng et al., 2004). Such stacking interactions have been found to increase the stability of the human kinesin neck, but at a completely different place (Tripet and Hodges, 2002). Alternatively, additional residues C-terminal of W384 may contribute to the stabilizing effect of the hinge on the neck coiled-coil and be sufficient for neck coiled-coil formation in constructs that are dimerized via the stalk (NcKinhTail), but insufficient in those that lack this region (NcKinhinge). We suspect that both stacking interactions and neighboring residues of W384 contribute to the stabilizing effect of the hinge domain.
Possible Mechanism of Neck Coiled-coil Stabilization
As far as known, all kinesin-1 neck domains are able to form coiled-coils. Formally, coiled-coils are stabilized by the hydrophobic interface between the strands, and the exclusion of water leads to a gain in entropy that drives dimerization. However, the sequences and spectroscopic data on kinesin-1 neck peptides show that these coils are far from being optimal. Therefore, even subtle mutations in the NcKin neck/hinge region apparently lead to less favorable entropy changes and prevent kinesin from dimerizing. However, these unfavorable thermodynamic changes can be overcome in some cases by the presence of the stalk coiled-coil that provides a positive offset for the entropy change. How do these general considerations apply to the case of NcKin?
There are several obvious deviations from the perfect heptad pattern that leads to an α-helical coiled-coil structure. Particularly, the N-terminal half of the human kinesin neck is known to contain several nonideal, hydrophilic or bulky residues in the a and d heptad positions that at first glance seem to exclude a coiled-coil conformation (Morii et al., 1997; Tripet et al., 1997; Thormahlen et al., 1998). However, a closer look reveals the presence of a novel set of so-called helix-capping motifs outside the coiled-coil that are able to compensate the destabilizing effect of the nonideal residues (Tripet and Hodges, 2002). Here, a tryptophan residue was found to be part of a “molecular sandwich” or “hydrophobic staple” that stabilizes the coiled-coil conformation. The term “sandwich” was used to describe layered aromatic amino acid side chains that are stabilized by hydrophobic or π -stacking interactions.
In fungi a typical subgroup of kinesin-1 motors evolved that shows obvious sequence dissimilarities to animal kinesin-1s in the N-terminal part of the neck, but is very similar toward the C-terminal end of the neck. Therefore the stabilizing effect of the proximal part of the fungal-specific hinge most likely involves interactions with the N-terminus or central part of the neck. Is such a long-distance interaction likely to occur?
NMR spectroscopy on rat kinesin hinge peptides show that the N-terminal portion of the hinge consists of short, well-defined secondary structure elements (Seeberger et al., 2000) that are likely to occur also in fungal kinesins because of substantial sequence similarities at the neck-hinge junction (Figure 1). According to the NMR data, the glycine-rich neck/hinge junction is highly flexible and allows the hinge to explore a large volume in space almost freely. In the C-terminal region of the neck-hinge peptides, several residues downstream of the neck coiled-coil, a relatively stiff motif (E374-Q381 in rat kinesin) is found that includes the counterpart of the NcKin W384 residue, Q381rat (Figure 4). Apparently, the three-dimensional structure of kinesin's hinge allows interactions between hinge and neck coiled-coil.
Figure 4.
Possible role of neck/hinge dynamics for the regulation of fungal kinesins. On state: The neck is in a two-stranded coiled-coil state due to stabilization via W384 and adjacent residues (XXX). The hinge region is folded backward to enable interaction of W384 with the neck domain, requiring large spatial freedom that is achieved by the highly flexible neck-hinge junction (Seeberger et al., 2000). Dimerization of the neck provides mechanochemical coupling of the two heads and thus effective and processive movement, powered by fast ATP hydrolysis in the head domain. Off state: In absence of cargo, the tail domain in the full-length protein folds back (compact conformer) and captures W384 and its close vicinity (XXX). This greatly destabilizes the coiled-coil structure, and the neck domain transits in an unfolded state. The lack of head-head coupling stops processive movement of the motor. The ATPase activity is down-regulated because of the inhibitory interaction of Y362 with the motor core.
Multiple Structural States of the NcKin Neck Domain
One of the most intriguing findings of this study is the evidence for multiple structural states of the NcKin neck domain. In our study we used three approaches to tackle the coiled-coil conformation of the NcKin neck: 1) kinetic evidence, based on previous experience (Schafer et al., 2003), 2) dimerization assays on truncated NcKinhinge motors, and 3) the cross-linking behavior of a mutant with introduced cysteine residues (NcKinhTailP342C). These three lines of evidence together give a rather complex, but informative picture on kinesin mechanism and regulation.
Kinesin-1 type motors (conventional kinesins) seem to move by a hand-overhand mechanism that produces fast, processive, stepwise motility. This mechanism relies on the coordinated action of two coupled motor heads, which, in turn, requires the mechanical coupling of the two heads via the coiled-coil neck domain. In one extreme model, the neck coiled-coil is static and does not need to “breathe” or “melt” during the stepping process (Romberg et al., 1998; Tomishige and Vale, 2000). Our motility data on cross-linked kinesin mutants support this simplistic model, because the position of the introduced disulfide bond (P342C) does not permit dissociation of the neck coiled-coil but still allows fast motility. Still, our experiments also indicate that the neck domain may assume conformations other than an α-helical coiled-coil. The solution is that the hand-overhand model only describes the mechanical cycle of kinesin, but does not exclude that the neck refolds for regulatory purposes.
In the present study, the NcKin-379hTail chimera and the NcKinhTailP342C,Y362K mutant obviously deviate from wild-type NcKin, and both do not form a coiled-coil neck, as indicated by their motility properties and their behavior in dimerization assays in the NcKinhinge background (cf. Schafer et al., 2003). However, unlike the P342,Y362K double mutant, the NcKin-379hTailP342C chimera can be cross-linked, indicating different structural states of the neck domain in these mutants. The strict conservation of Y362 among fungal kinesins and the fact that its absence cannot be suppressed indicates that the structure of the neck in NcKin-Y362K mutants may not be physiological. By contrast, the easy oxidation of reduced NcKin-379hTailP342C that leads to a functional conformation suggests that its inactive, non–coiled-coil structure is not just “denatured” but represents a down-regulated state. Together, these observations suggest that stabilizing and destabilizing entropic effects of specific sequence elements are in such a close balance in NcKin that it is conceivable that different neck conformations occur and are easily induced by external influences.
Physiological Role of Different Neck Conformations
Our present data fit well in a model that attributes the switch between the coiled-coil and the non–coiled-coil neck conformation to the action of the proximal hinge region around W384. According to this model, the proximal part of the hinge would either interact with the tail domain, leading to the inhibitory, non–coiled-coil conformation of the neck, or with the neck, leading to the active coiled-coil structure (Figure 4).
The regulatory influence of the tail domain on the motor head has been described in several studies on animal and fungal kinesin-1 motors (Friedman and Vale, 1999; Seiler et al., 1999; Stock et al., 1999). Most likely, the ADP release is inhibited in the folded kinesin state where the conserved IAK tail sequence motif signals the absence of cargo to the motor domain (Hackney and Stock, 2000). In fungal kinesins, the inhibitory effect of the neck is largely due to the fungal-specific residue Y362 whose exchange leads to constitutively fast and active kinesins (Schafer et al., 2003). However, the exact binding target of the tail and details of the inhibitory mechanism have not been described so far. Our yeast two-hybrid assays show that the tail domain interacts with the N-terminal part of the hinge domain (379–400), suggesting that it may no longer be free to interact with the neck domain and to stabilize its coiled-coil conformation. This scenario predicts a conformation where hinge and tail interact, and the neck assumes a non–coiled-coil conformation that inhibits the motor domain via Y362 (Figure 4). The tail binding, however, seems not to rely solely on W384 because the yeast two-hybrid interaction was absent also in chimera that comprised W384. Apparently, the near environment is required for tail binding as well, and might supply stabilizing interactions or impose structural constraints.
Supplementary Material
Acknowledgments
We thank Sven Leier for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 413 and SPP 1068) and the Fonds der Chemischen Industrie.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–11–0957) on May 18, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Andrews, P. (1970). Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem. Anal. 18, 1–53. [PubMed] [Google Scholar]
- Berliner, E., Young, E. C., Anderson, K., Mahtani, H. K., and Gelles, J. (1995). Failure of a single-headed kinesin to track parallel to microtubule protofilaments [see comments]. Nature 373, 718–721. [DOI] [PubMed] [Google Scholar]
- Brady, S. T. (1985). A novel brain ATPase with properties expected for the fast axonal transport motor. Nature 317, 73–75. [DOI] [PubMed] [Google Scholar]
- Cantor, C. R., and Schimmel, P. R. (1980). Techniques for the study of biological structure and function. In: Biophysical Chemistry, Vol. 2, San Francisco: W. H. Freeman.
- Case, R. B., Rice, S., Hart, C. L., Ly, B., and Vale, R. D. (2000). Role of the kinesin neck linker and catalytic core in microtubule-based motility [see comments]. Curr. Biol. 10, 157–160. [DOI] [PubMed] [Google Scholar]
- Coy, D. L., Hancock, W. O., Wagenbach, M., and Howard, J. (1999). Kinesin's tail domain is an inhibitory regulator of the motor domain [see comments]. Nat. Cell Biol. 1, 288–292. [DOI] [PubMed] [Google Scholar]
- Dagenbach, E. M., and Endow, S. A. (2004). A new kinesin tree. J. Cell Sci. 117, 3–7. [DOI] [PubMed] [Google Scholar]
- Deluca, D., Woehlke, G., and Moroder, L. (2003). Synthesis and conformational characterization of peptides related to the neck domain of a fungal kinesin. J. Pept. Sci. 9, 203–211. [DOI] [PubMed] [Google Scholar]
- Friedman, D. S., and Vale, R. D. (1999). Single-molecule analysis of kinesin motility reveals regulation by the cargo-binding tail domain. Nat. Cell Biol. 1, 293–297. [DOI] [PubMed] [Google Scholar]
- Funatsu, T., Harada, Y., Tokunaga, M., Saito, K., and Yanagida, T. (1995). Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 374, 555–559. [DOI] [PubMed] [Google Scholar]
- Grummt, M., Woehlke, G., Henningsen, U., Fuchs, S., Schleicher, M., and Schliwa, M. (1998). Importance of a flexible hinge near the motor domain in kinesin-driven motility. EMBO J. 17, 5536–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney, D. (1994). Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl. Acad. Sci. USA 91, 6865–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney, D. D. (1995). Highly processive microtubule-stimulated ATP hydrolysis by dimeric kinesin head domains. Nature 377, 448–450. [DOI] [PubMed] [Google Scholar]
- Hackney, D. D., and Stock, M. F. (2000). Kinesin's IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat. Cell Biol. 2, 257–260. [DOI] [PubMed] [Google Scholar]
- Hackney, D. D., Stock, M. F., Moore, J., and Patterson, R. A. (2003). Modulation of kinesin half-site ADP release and kinetic processivity by a spacer between the head groups. Biochemistry 42, 12011–12018. [DOI] [PubMed] [Google Scholar]
- Hancock, W. O., and Howard, J. (1998). Processivity of the motor protein kinesin requires two heads. J. Cell Biol. 140, 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, W. O., and Howard, J. (1999). Kinesin's processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl. Acad. Sci. USA 96, 13147–13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen, U., and Schliwa, M. (1997). Reversal in the direction of movement of a molecular motor [see comments]. Nature 389, 93–96. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N. (1998). Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519–526. [DOI] [PubMed] [Google Scholar]
- Hunt, A. J., and Howard, J. (1993). Kinesin swivels to permit microtubule movement in any direction. Proc. Natl. Acad. Sci. USA 90, 11653–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, W., Stock, M. F., Li, X., and Hackney, D. D. (1997). Influence of the kinesin neck domain on dimerization and ATPase kinetics. J. Biol. Chem. 272, 7626–7632. [DOI] [PubMed] [Google Scholar]
- Kallipolitou, A., Deluca, D., Majdic, U., Lakamper, S., Cross, R., Meyhofer, E., Moroder, L., Schliwa, M., and Woehlke, G. (2001). Unusual properties of the fungal conventional kinesin neck domain from Neurospora crassa. EMBO J. 20, 6226–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner, J., Woehlke, G., and Schliwa, M. (1999). Universal and unique features of kinesin motors: insights from a comparison of fungal and animal conventional kinesins. Biol. Chem. 380, 915–921. [DOI] [PubMed] [Google Scholar]
- Klopfenstein, D. R., Holleran, E. A., and Vale, R. D. (2002). Kinesin motors and microtubule-based organelle transport in Dictyostelium discoideum. J. Muscle Res. Cell Motil. 23, 631–638. [DOI] [PubMed] [Google Scholar]
- Kollmar, M., and Glockner, G. (2003). Identification and phylogenetic analysis of Dictyostelium discoideum kinesin proteins. BMC Genom. 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii, H., Takenawa, T., Arisaka, F., and Shimizu, T. (1997). Identification of kinesin neck region as a stable alpha-helical coiled coil and its thermodynamic characterization. Biochemistry 36, 1933–1942. [DOI] [PubMed] [Google Scholar]
- Navone, F., Niclas, J., Hom-Booher, N., Sparks, L., Bernstein, H. D., McCaffrey, G., and Vale, R. D. (1992). Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J. Cell Biol. 117, 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, S. et al. (1999). A structural change in the kinesin motor protein that drives motility. Nature 402, 778–784. [DOI] [PubMed] [Google Scholar]
- Romberg, L., Pierce, D. W., and Vale, R. D. (1998). Role of the kinesin neck region in processive microtubule-based motility. J. Cell Biol. 140, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, F., Deluca, D., Majdic, U., Kirchner, J., Schliwa, M., Moroder, L., and Woehlke, G. (2003). A conserved tyrosine in the neck of a fungal kinesin regulates the catalytic motor core. EMBO J. 22, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch, C. L., Aist, J. R., Yoder, O. C., and Gillian Turgeon, B. (2003). A complete inventory of fungal kinesins in representative filamentous ascomycetes. Fungal Genet. Biol. 39, 1–15. [DOI] [PubMed] [Google Scholar]
- Seeberger, C., Mandelkow, E., and Meyer, B. (2000). Conformational preferences of a synthetic 30mer peptide from the interface between the neck and stalk regions of kinesin. Biochemistry 39, 12558–12567. [DOI] [PubMed] [Google Scholar]
- Seiler, S., Kirchner, J., Horn, C., Kallipolitou, A., Woehlke, G., and Schliwa, M. (2000). Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nat. Cell Biol. 2, 333–338. [DOI] [PubMed] [Google Scholar]
- Seiler, S., Plamann, M., and Schliwa, M. (1999). Kinesin and dynein mutants provide novel insights into the roles of vesicle traffic during cell morphogenesis in Neurospora. Curr. Biol. 9, 779–785. [DOI] [PubMed] [Google Scholar]
- Steinberg, G., and Schliwa, M. (1995). The Neurospora organelle motor: a distant relative of conventional kinesin with unconventional properties. Mol. Biol. Cell 6, 1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, G., and Schliwa, M. (1996). Characterization of the biophysical and motility properties of kinesin from the fungus Neurospora crassa. J. Biol. Chem. 271, 7516–7521. [DOI] [PubMed] [Google Scholar]
- Stock, M. F., Guerrero, J., Cobb, B., Eggers, C. T., Huang, T. G., Li, X., and Hackney, D. D. (1999). Formation of the compact confomer of kinesin requires a COOH-terminal heavy chain domain and inhibits microtubule-stimulated ATPase activity. J. Biol. Chem. 274, 14617–14623. [DOI] [PubMed] [Google Scholar]
- Thormahlen, M., Marx, A., Sack, S., and Mandelkow, E. (1998). The coiled-coil helix in the neck of kinesin. J. Struct. Biol. 122, 30–41. [DOI] [PubMed] [Google Scholar]
- Tomishige, M., and Vale, R. D. (2000). Controlling kinesin by reversible disulfide cross-linking. Identifying the motility-producing conformational change. J. Cell Biol. 151, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripet, B., and Hodges, R. S. (2002). Helix capping interactions stabilize the N-terminus of the kinesin neck coiled-coil. J. Struct. Biol. 137, 220–235. [DOI] [PubMed] [Google Scholar]
- Tripet, B., Vale, R. D., and Hodges, R. S. (1997). Demonstration of coiled-coil interactions within the kinesin neck region using synthetic peptides. Implications for motor activity. J. Biol. Chem. 272, 8946–8956. [DOI] [PubMed] [Google Scholar]
- Vale, R. D., and Fletterick, R. J. (1997). The design plan of kinesin motors. Annu. Rev. Cell Dev. Biol. 13, 745–777. [DOI] [PubMed] [Google Scholar]
- Vale, R. D., Reese, T. S., and Sheetz, M. P. (1985). Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L., Goddard, J. P., Baumann, U., and Reymond, J. L. (2004). Expression improvement and mechanistic study of the retro-Diels-Alderase catalytic antibody 10F11 by site-directed mutagenesis. J. Mol. Biol. 341, 807–814. [DOI] [PubMed] [Google Scholar]
- Zhou, N. E., Kay, C. M., and Hodges, R. S. (1993). Disulfide bond contribution to protein stability: positional effects of substitution in the hydrophobic core of the two-stranded alpha-helical coiled-coil. Biochemistry 32, 3178–3187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.