Abstract
Purpose
CA 19-9 is the only established tumor marker in pancreatic cancer (PC); the prognostic role of other serum markers like CEA, CRP, LDH or bilirubin has not yet been defined.
Methods
We pooled pre-treatment data on CA 19-9, CEA, CRP, LDH and bilirubin levels from two German multicenter randomized phase II trials together with prospective patient data from one high-volume German Cancer Center. Marker levels were assessed locally before the start of palliative first-line therapy for advanced PC and serially during treatment (for CA 19-9 only). Clinical and biomarker data (overall 12 variables) were correlated with the efficacy endpoints time-to-progression (TTP) and overall survival (OS) by using uni- and multivariate Cox models.
Results
Data from 291 patients were included in this pooled analysis; 253 patients (87 %) received treatment within prospective clinical trials. Median TTP in the study cohort was 5.1 months and median OS 9.0 months. In univariate analysis, pre-treatment CA 19-9 (HR 1.55), LDH (HR 2.04) and CEA (HR 1.89) levels were significantly associated with TTP. Regarding OS, baseline CA 19-9 (HR 1.46), LDH (HR 2.07), CRP (HR 1.69) and bilirubin (HR 1.62) were significant prognostic factors. Within multivariate analyses, pre-treatment log [CA 19-9] (as continuous variable for TTP) and log [bilirubin] as well as log [CRP] (for OS) had an independent prognostic value. A CA 19-9 decline of ≥25 % during the first two chemotherapy cycles was predictive for TTP and OS, independent of the applied CA 19-9 assay.
Conclusion
Baseline CA 19-9 and CA 19-9 kinetics during first-line chemotherapy are prognostic in advanced PC. Besides that finding other serum markers like CRP, LDH and bilirubin can also provide prognostic information on TTP and OS.
Keywords: Gemcitabine, Pancreatic cancer, Prognostic factor
Introduction
The diagnosis of pancreatic cancer (PC) is still associated with a dismal prognosis: only 10–15 % of patients initially present with resectable disease and can undergo curative-intent surgery. However, with a 5-year survival rate of only 5 %, most of these patients suffer from relapse and are treated with systemic chemotherapy or chemoradiotherapy in the course of their disease (Vincent et al. 2011). Since 1997, gemcitabine has been the standard chemotherapy agent for advanced PC (Heinemann et al. 2012). The addition of the tyrosine kinase inhibitor erlotinib has shown a clinically moderate but statistically significant prolongation of survival for metastatic disease (Moore et al. 2007). The combination of 5-fluorouracil, folinic acid, oxaliplatin and irinotecan (“FOLFIRINOX”) has recently been described to almost double the survival time, but this rather intensive combination scheme can only be applied to relatively young and medically fit patients without relevant co-morbidities and normal bilirubin values, thus appealing to only a minority of patients (Conroy et al. 2011). To date, however, there is no assured biomarker which might predict the efficacy of one of these specific treatment regimens.
Several serum tumor markers have been investigated in PC with CA 19-9 being the one with the highest evidence to date (Boeck et al. 2006; Duffy et al. 2010). CA 19-9 is also the most commonly used PC tumor marker in clinical routine, as many studies in patients with resectable and advanced disease proved CA 19-9 to be a useful tool for the evaluation of treatment response and for the prediction of prognosis (Hess et al. 2008; Reni et al. 2009; Boeck et al. 2010; Humphris et al. 2012). Two important research issues, however, still remain unclear: first, if a CA 19-9 decline during chemotherapy is predictive for efficacy endpoints (and specifically which cutoff to use for a definition of a clinically relevant CA 19-9 decline) and if the application of unique CA 19-9 assay is required to generate valid and comparable data (Boeck et al. 2006; Duffy et al. 2010). Early evidence suggests that also other serum markers like CEA and LDH levels might have a prognostic relevance in patients with advanced PC receiving palliative treatment (Stocken et al. 2008; van Cutsem et al. 2009; Duffy et al. 2010). Furthermore, there is increasing evidence that an acute phase response plays an important role in the pathobiology and prognosis of PC and that the serum C-reactive protein (CRP) concentration thus might also serve as a potential biomarker in this disease (van Cutsem et al. 2009; Moses et al. 2009; Pine et al. 2009).
The primary aim of this large retrospective pooled analysis was to define the prognostic role of several clinical and pre-treatment laboratory factors (like CA 19-9, CEA, CRP, LDH and bilirubin) with regard to time-to-progression (TTP) and overall survival (OS) in a homogeneous patient population with advanced PC receiving first-line palliative chemotherapy (mainly within a prospective clinical trial). We furthermore investigated the impact of CA 19-9 kinetics during treatment on outcome, separately analyzed for the applied CA 19-9 assay (standardized CA 19-9 Elecsys® assay vs. any assay).
Patients and methods
Patient population and treatment
Patients included in this pooled analysis were recruited from two previously published German randomized phase II trials (Boeck et al. 2008; Kullmann et al. 2009), and additionally, also, patients with advanced PC treated between July 2002 and June 2008 at the outpatient clinic of the “Pancreas Center” at the Department of Internal Medicine III, Ludwig-Maximilians-University of Munich (LMU) were considered eligible. All patients had histologically or cytologically proven exocrine PC and were treated with palliative chemotherapy (or chemoradiotherapy) for locally advanced or metastatic disease. Previous treatment with chemo- or chemoradiotherapy was not allowed except adjuvant treatment after curative-intent surgical tumor resection. Patients with endocrine pancreatic tumors or secondary malignancies were excluded. As reported previously, patients treated within the Ro96 trial received gemcitabine/capecitabine, gemcitabine/oxaliplatin or capecitabine/oxaliplatin, respectively (Boeck et al. 2008). Patients included into the GEMOXCET study were treated with either gemcitabine/oxaliplatin or gemcitabine/oxaliplatin plus cetuximab (Kullmann et al. 2009). Patients treated outside clinical trials at the “Pancreas Center” at the Department of Internal Medicine III received—based on the decision of the treating medical oncologist SB and VH—standard gemcitabine or gemcitabine-based chemotherapy until disease progression, unacceptable toxicity or patient refusal.
Measurement of laboratory parameters
The baseline concentrations of CA 19-9, CEA, LDH, hemoglobin and bilirubin were measured routinely in each included patient before treatment initiation. As described previously, a subgroup of patients whose CA 19-9 values had been measured by a unique method (Elecsys®, Roche Diagnostics, Germany) was generated based on patients treated at the LMU and within the GEMOXCET trial (Kullmann et al. 2009; Boeck et al. 2010). Data on pre-treatment CRP levels were only available from LMU patients. Serial measurements were conducted for CA 19-9 every three to 4 weeks during treatment. All serum markers were measured prospectively in each participating patient.
Study design and statistical analyses
This tumor marker study was designed, conducted and analyzed according to the 2005 REMARK guidelines (“REporting recommendations for tumor MARKer prognostic studies”) as appropriate (McShane et al. 2005). The pre-defined primary endpoint for the current retrospective pooled analysis was to define the prognostic value of 12 clinical and laboratory parameters with regard to TTP and OS in patients with advanced PC undergoing palliative chemotherapy (or chemoradiotherapy). TTP was defined as the time interval between the initiation of first-line therapy until disease progression ascertained by imaging; OS was calculated from the time point of initiation of first-line therapy until death from any cause. The observations were censored if disease progression was apparent through clinical deterioration without imaging for TTP, and for OS for patients being alive at a pre-defined time point (October 15, 2008). The applied cutoffs (in order to generate dichotomized variables) for the assessed laboratory values were derived either from previously published reports, from the reference level of the validated assay or based on the median values of the respective subgroups. Additionally, we also analyzed the assessed laboratory values as continuous variables by transforming them into the natural logarithm (log [variable]). Survival times were estimated by using the Kaplan–Meier method; differences were calculated by using the log-rank test on a significance level of 0.05 (two sided). Parameters that reached statistical significance in univariate analysis were included in multivariate Cox regression models using backward elimination.
In order to evaluate the prognostic significance of serial CA 19-9 measurements during treatment, the lowest CA 19-9 measurement (=nadir) in addition to the baseline measurement within the first two cycles of chemotherapy (between day 28 ± 8 and day 56 ± 8) was taken into account. The 16 patients who underwent chemoradiotherapy were excluded from this analysis due to the heterogeneity of the treatment modality. For the definition of a biochemical response to therapy, two cutoffs of a 25 and 50 % decline during treatment as proposed by other authors were chosen (Boeck et al. 2006). In order to evaluate the impact of the respective method of CA 19-9 measurement on the outcome results, an additional subgroup analysis including only values assessed with the unique assay (Elecsys®, Roche Diagnostics, Germany) was performed.
Several potential confounders affecting the accuracy of CA 19-9 kinetics have been described (Boeck et al. 2006). In order to minimize their influence, the CA 19-9 kinetic analyses were repeated including only patients without signs of cholestasis at baseline (defined as total bilirubin ≤1.5 × upper limit of normal, ULN) and patients with a CA 19-9 level at baseline of above the upper limit of the reference range (>37 U/ml). Furthermore, the landmark method was used at day 56 ± 8 to avoid the effect of a guarantee time bias. A landmark is a time point where all patients still have to be on the treatment protocol and up to which the evaluation of tumor marker response must have taken place. Thus, the allocation to a certain prognostic group depending on definitions of CA 19-9 decline (25 or 50 %) must have taken place before that time point and survival times are considered only after the landmark.
Results
Patient characteristics
Data from 291 PC patients were included into this pooled multicenter analysis: 116 patients from the LMU “Pancreas Center,” 122 patients from the Ro96 trial and 53 patients from the GEMOXCET study. Baseline patient characteristics are summarized within Table 1. At treatment initiation, 243 patients (84 %) had metastatic disease and 48 patients (16 %) had locally advanced tumors. The organ most commonly affected by metastases was the liver (69 %), followed by lung (18 %) and peritoneum (5 %). The majority of patients treated at the LMU received gemcitabine-based chemotherapy (61 %) or a capecitabine-based regimen (23 %); only 16 (of the 24 patients with locally advanced disease) received upfront chemoradiotherapy. Overall, 253 of all patients (87 %) received treatment within a prospective clinical trial. Median TTP from treatment initiation in the study cohort was estimated with 5.1 months; median OS was 9.0 months (Table 1). At the time of final data analysis, 237 of the 291 PC patients (81 %) had died.
Table 1.
Patient characteristics
| Characteristic | LMU patients | Ro96 | GEMOXCET | Overall |
|---|---|---|---|---|
| n | 116 | 122 | 53 | 291 |
| Age (years) | ||||
| Median | 63 | 63 | 63 | 63 |
| Range | 39–78 | 40–75 | 31–75 | 31–78 |
| Gender | ||||
| Male | 73 (63 %) | 82 (67 %) | 37 (70 %) | 192 (66 %) |
| Female | 43 (37 %) | 40 (33 %) | 16 (30 %) | 99 (34 %) |
| Stage of disease (at study entry) | ||||
| Locally advanced | 24 (21 %) | 24 (20 %) | 0 | 48 (16 %) |
| Metastatic | 92 (79 %) | 98 (80 %) | 53 (100 %) | 243 (84 %) |
| Previous surgery | 29 (25 %) | 21 (17 %) | 8 (15 %) | 58 (20 %) |
| Karnofsky status (KPS) | ||||
| 90–100 % | 83 (72 %) | 55 (45 %) | 31 (59 %) | 169 (58 %) |
| 60–80 % | 32 (28 %) | 66 (54 %) | 10 (19 %) | 108 (37 %) |
| Missing | 1 (1 %) | 1 (1 %) | 12 (23 %) | 14 (5 %) |
| Tumor grading | ||||
| G1 + G2 | 46 (40 %) | 49 (40 %) | 22 (42 %) | 117 (40 %) |
| G3 + G4 | 59 (51 %) | 45 (37 %) | 21 (40 %) | 125 (43 %) |
| Missing | 11 (9 %) | 28 (23 %) | 10 (19 %) | 49 (17 %) |
| Localization of primary | ||||
| Pancreatic head | 72 (62 %) | 67 (55 %) | 26 (49 %) | 165 (57 %) |
| Pancreatic body or tail | 44 (38 %) | 28 (23 %) | 19 (36 %) | 91 (31 %) |
| Missing | 0 | 27 (22 %) | 8 (15 %) | 35 (12 %) |
| Histology | ||||
| Adenocarcinoma | 112 (97 %) | 122 (100 %) | 53 (100 %) | 287 (99 %) |
| Acinar cell carcinoma | 4 (3 %) | 0 | 0 | 4 (1 %) |
| Pre-treatment CA 19-9 [U/ml] | ||||
| Median | 749 | 1,114 | 3,461 | 1,137 |
| Range | 8–81,626 | 6–100,000 | 8–100,000 | 6–100,000 |
| Patients treated within a prospective clinical trial | 78 (67 %) | 122 (100 %) | 53 (100 %) | 253 (87 %) |
| Patients deceased | 100 (86 %) | 97 (80 %) | 40 (76 %) | 237 (81 %) |
| Median TTP (months) | 4.1 | 5.5 | 4.9 | 5.1 |
| Median OS* (months) | 10.1 | 9.7 | 7.3 | 9.0 |
LMU Ludwig-Maximilians-University, OS overall survival, TTP time-to-progression (* p = 0.019)
Univariate analysis of pre-treatment prognostic factors
Univariate analyses of 12 different clinical characteristics and laboratory parameters were conducted regarding TTP and OS, respectively (for details see Table 2). Regarding TTP, the following clinical parameters revealed a statistical significance: KPS ≥90 %, tumor grading with good or median differentiated tumors (G1 + G2) and age above 64 years (Table 2). Regarding the endpoint OS, locally advanced tumors, a KPS ≥90 % and good or median differentiated tumors (G1 + G2) had an improved survival.
Table 2.
Univariate analysis of pre-treatment prognostic factors
| Variable | n | Median TTP (months) | p | Hazard ratio (95 % CI) |
|---|---|---|---|---|
| A: Time-to-progression (TTP) | ||||
| Stage of disease | ||||
| Locally advanced | 48 | 4.4 | 1 | |
| Metastatic | 243 | 5.1 | 0.868 | 0.97 (0.68–1.39) |
| Karnofsky performance status (KPS) | ||||
| 90–100 % | 169 | 5.5 | 1 | |
| 60–80 % | 108 | 3.6 | 0.004 | 1.51 (1.14–2.01) |
| Tumor grading | ||||
| G1 + G2 | 117 | 6.1 | 1 | |
| G3 + G4 | 125 | 4.4 | 0.008 | 1.50 (1.10–2.03) |
| T stage | ||||
| T1 + T2 | 48 | 5.2 | 1 | |
| T3 + T4 | 209 | 4.7 | 0.412 | 0.85 (0.58–1.25) |
| T (continuous) | 256 | 0.832 | 1.02 (0.84–1.25) | |
| Age (at study entry) | ||||
| ≤63 years | 151 | 4.2 | 1 | |
| ≥64 years | 140 | 5.8 | 0.015 | 0.72 (0.54–0.94) |
| Gender | ||||
| Male | 192 | 4.5 | 1 | |
| Female | 99 | 5.3 | 0.915 | 1.02 (0.77–1.34) |
| Pre-treatment CA 19-9 | ||||
| Not detectable* | 20 | 3.8 | 1 | |
| ≤1,000 U/ml | 128 | 6.1 | 0.76 (0.45–1.27) | |
| >1,000 U/ml | 140 | 4.0 | 0.003 | 1.11 (0.66–1.84) |
| Pre-treatment CA 19-9 | ||||
| ≤1,000 U/ml | 128 | 6.1 | 1 | |
| >1,000 U/ml | 140 | 4.0 | 0.002 | 1.55 (1.16–2.06) |
| log [CA 19-9] | 268 | <0.001 | 1.15 (1.08–1.22) | |
| Pre-treatment CA 19-9 (Elecsys®) | ||||
| ≤1,000 U/ml | 62 | 6.6 | 1 | |
| >1,000 U/ml | 61 | 3.5 | 0.002 | 2.00 (1.29–3.11) |
| log [CA 19-9] | 123 | <0.001 | 1.19 (1.08–1.31) | |
| LDH | ||||
| ≤250 U/l | 168 | 5.4 | 1 | |
| >250 U/l | 79 | 3.4 | <0.001 | 2.04 (1.47–2.84) |
| log [LDH] | 247 | 0.004 | 1.65 (1.18–2.31) | |
| CRP | ||||
| ≤1.0 mg/dl | 59 | 6.1 | ||
| >1.0 mg/dl | 56 | 3.8 | 0.062 | 1.52 (0.97–2.36) |
| log [CRP] | 110 | 0.026 | 1.24 (1.03–1.49) | |
| Bilirubin | ||||
| ≤1.0 mg/dl | 224 | 4.9 | 1 | |
| >1.0 mg/dl | 58 | 4.4 | 0.202 | 1.26 (0.88–1.79) |
| log [Bilirubin] | 282 | 0.228 | 1.14 (0.92–1.41) | |
| CEA | ||||
| ≤4.5 ng/ml | 102 | 6.1 | ||
| >4.5 ng/ml | 101 | 3.5 | <0.001 | 1.89 (1.35–2.63) |
| log [CEA] | 203 | 0.004 | 1.11 (1.03–1.19) | |
| Hemoglobin | ||||
| ≥12 g/dl | 115 | 4.1 | ||
| <12 g/dl | 47 | 4.4 | 0.964 | 0.99 (0.66–1.50) |
| log [Hemoglobin] | 148 | 0.351 | 2.17 (0.43–11.12) | |
| B: Overall survival (OS) | ||||
| Stage of disease | ||||
| Locally advanced | 48 | 12.9 | 1 | |
| Metastatic | 243 | 8.3 | 0.005 | 1.63 (1.16–2.32) |
| Karnofsky performance status (KPS) | ||||
| 90–100 % | 169 | 9.9 | 1 | |
| 60–80 % | 108 | 8.0 | 0.037 | 1.33 (1.02–1.74) |
| Tumor grading | ||||
| G1 + G2 | 117 | 10.8 | 1 | |
| G3 + G4 | 125 | 8.3 | 0.013 | 1.42 (1.08–1.88) |
| T stage | ||||
| T1 + T2 | 48 | 8.3 | 1 | |
| T3 + T4 | 209 | 9.2 | 0.271 | 0.82 (0.58–1.17) |
| T (continuous) | 256 | 0.290 | 0.91 (0.77–1.08) | |
| Age (at study entry) | ||||
| ≤63 years | 151 | 9.1 | 1 | |
| ≥64 years | 140 | 8.8 | 0.554 | 0.93 (0.72–1.20) |
| log [age] | 291 | 0.204 | 0.58 (0.26–1.34) | |
| Gender | ||||
| Male | 192 | 8.6 | 1 | |
| Female | 99 | 9.7 | 0.196 | 0.84 (0.64–1.01) |
| Pre-treatment CA 19-9 | ||||
| Not detectable* | 20 | 8.9 | 1 | |
| ≤1,000 U/ml | 128 | 10.5 | 0.76 (0.45–1.27) | |
| >1,000 U/ml | 140 | 8.0 | 0.020 | 1.11 (0.66–1.84) |
| Pre-treatment CA 19-9 | ||||
| ≤1,000 U/ml | 128 | 10.5 | 1 | |
| >1,000 U/ml | 140 | 8.0 | 0.006 | 1.46 (1.11–1.90) |
| log [CA 19-9] | 268 | <0.001 | 1.13 (1.06–1.19) | |
| Pre-treatment CA 19-9 (Elecsys®) | ||||
| ≤1,000 | 62 | 10.3 | 1 | |
| >1,000 | 61 | 8.1 | 0.048 | 1.49 (1.00–2.22) |
| log [CA 19-9] | 123 | 0.002 | 1.15 (1.06–1.26) | |
| LDH | ||||
| ≤250 U/l | 168 | 9.9 | 1 | |
| >250 U/l | 79 | 5.9 | <0.001 | 2.07 (1.55–2.78) |
| log [LDH] | <0.001 | 1.93 (1.40–2.65) | ||
| CRP | ||||
| ≤1.0 mg/dl | 59 | 11.4 | 1 | |
| >1.0 mg/dl | 56 | 6.8 | 0.009 | 1.69 (1.14–2.52) |
| log [CRP] | 115 | 0.001 | 1.40 (1.16–1.69) | |
| Bilirubin | ||||
| ≤1.0 mg/dl | 224 | 9.7 | 1 | |
| >1.0 mg/dl | 58 | 7.2 | 0.003 | 1.62 (1.18–2.24) |
| log [Bilirubin] | <0.001 | 1.43 (1.20–1.70) | ||
| CEA | ||||
| ≤4.5 ng/ml | 102 | 10.3 | 1 | |
| >4.5 ng/ml | 101 | 9.0 | 0.054 | 1.35 (0.99–1.83) |
| log [CEA] | 0.053 | 1.07 (1.00–1.15) | ||
| Hemoglobin | ||||
| ≥12 g/dl | 115 | 9.0 | 1 | |
| <12 g/dl | 47 | 7.1 | 0.463 | 1.15 (0.79–1.67) |
| log [Hemoglobin] | 0.547 | 0.64 (0.15–2.73) | ||
Bold values indicate statistical significance (p < 0.05)
* Defined as CA 19-9 < 2.6 U/ml
Among laboratory parameters, normal LDH values (defined as ≤250 U/l) had a prognostic significance for both TTP (5.4 vs. 3.4 months, p < 0.001) and OS (9.9 vs. 5.9 months, p < 0.001). When LDH was analyzed as continuous variable—after transformation into the natural logarithm (log [LDH])—and included into a Cox proportional hazard regression model, the level of significance remained with a HR of 1.65 (95 % CI 1.18–2.31, p = 0.004) for TTP and a HR of 1.93 (95 % CI 1.40–2.65, p < 0.001) for OS (Table 2). Measurements of serum CRP levels at baseline also had a prognostic impact for TTP and OS: while our pre-defined cutoff of 1.0 mg/dl did not reach a level of statistical significance for TTP, this could be observed after transformation into the natural logarithm log [CRP] (HR 1.24; 95 % CI 1.03–1.49, p = 0.026). Regarding OS, both the cutoff of 1.0 mg/dl (11.4 vs. 6.8 months, p = 0.009) and the transformed values log [CRP] (HR 1.40, 95 % CI 1.16–1.69, p = 0.001) had a highly significant impact on OS. Bilirubin values below the ULN (1.0 mg/dl) did not influence TTP (4.9 vs. 4.4 months, p = 0.202) nor did log [bilirubin]. However, patients with normal bilirubin levels at chemotherapy initiation lived significantly longer (9.7 vs. 7.2 months, p = 0.003), and also, the continuous variable log [bilirubin] significantly influenced OS (HR 1.43; 95 % CI 1.20–1.70, p < 0.001).
Among the assessed serum tumor markers, CEA values below or above a cutoff of 4.5 ng/ml showed a significant correlation with TTP (6.1 vs. 3.5 months, p < 0.001), and comparable results were seen when log [CEA] was analyzed as continuous variable (HR 1.11, 95 % CI 1.03–1.19, p = 0.004). However, the correlation of pre-therapeutic CEA values with OS did not reach a level of statistical significance. The distribution of median pre-treatment CA 19-9 levels in the different study populations is shown in Table 1. The median value of CA 19-9 among all patients was 1,137 U/ml. LMU patients had the lowest median value of 749 U/ml, whereas the participants of the GEMOXCET trial had the highest median count of 3,641 U/ml. Using the pre-defined CA 19-9 cut-off of 1,000 U/ml, a significant correlation with TTP was observed (6.1 vs. 4.0 months, p = 0.002). These results were also seen when only values measured with the Elecsys® assay (n = 123) were taken into account (median TTP: 6.6 vs. 3.5 months, p = 0.002). Concerning OS, CA 19-9 values below 1,000 U/ml were linked with an improved OS, regardless whether all CA 19-9 values (10.5 vs. 8.0 months, p = 0.006) or only those CA 19-9 levels determined with the Elecsys® assay (10.3 vs. 8.1 months, p = 0.048) were taken into account. After transformation of the CA 19-9 values into the natural logarithm, a highly significant correlation of baseline CA 19-9 as continuous variable with both TTP and OS was observed through all subgroups (see Table 2).
Multivariate analysis of pre-treatment prognostic factors
In a multivariate Cox model for TTP where the variables age, tumor grading, KPS, log [LDH], log [CEA] and log [CA 19-9] were included, only age and log [CA 19-9] showed independent statistical significance (Table 3). A multivariate model for the endpoint OS contained the parameters tumor stage, tumor grading, KPS, log [CA 19-9], log [LDH] and log [bilirubin]. Log [bilirubin] was the only laboratory parameter that kept statistical significance besides tumor stage and KPS. A subgroup analysis including a smaller number of patients furthermore revealed an independent prognostic value for log [CRP] (HR 1.32; 1.06–1.63, p = 0.011) (see Table 3).
Table 3.
Multivariate analysis of pre-treatment prognostic factors
| Variable | HR (95 % CI) | p |
|---|---|---|
| A: Time-to-progression (TTP), n = 133 | ||
| Age (≤63 years vs. older) | 0.66 (0.44–0.98) | 0.040 |
| Tumor grading (G1 + G2 vs. G3 + G4) | 1.07 (0.69–1.65) | 0.766 |
| KPS (90–100 % vs. 60–80 %) | 1.30 (0.87–1.95) | 0.208 |
| log [CA 19-9] | 1.18 (1.09–1.28) | <0.001 |
| log [LDH] | 1.13 (0.64–2.00) | 0.678 |
| log [CEA] | 1.07 (0.98–1.18) | 0.115 |
| log [CA 19-9] Elecsys® (n = 84) | 1.08 (0.94–1.24) | 0.257 |
| log [CRP] (n = 84) | 1.03 (0.82–1.30) | 0.794 |
| B: Overall survival (OS), n = 183 | ||
| Stage of disease (locally advanced vs. metastatic) | 1.56 (1.03–2.35) | 0.036 |
| Tumor grading (G1 + G2 vs. G3 + G4) | 1.32 (0.94–1.85) | 0.107 |
| KPS (90–100 % vs. 60–80 %) | 1.91 (1.37–2.67) | <0.001 |
| log [CA 19-9] | 1.02 (0.95–1.10) | 0.581 |
| log [LDH] | 1.21 (0.83–1.75) | 0.321 |
| log [Bilirubin] | 1.83 (1.43–2.36) | <0.001 |
| log [CA 19-9] Elecsys® (n = 102) | 1.04 (0.94–1.50) | 0.429 |
| log [CRP] (n = 93) | 1.32 (1.06–1.63) | 0.011 |
Bold values indicate statistical significance (p < 0.05)
Prognostic role of serial CA 19-9 measurements during treatment
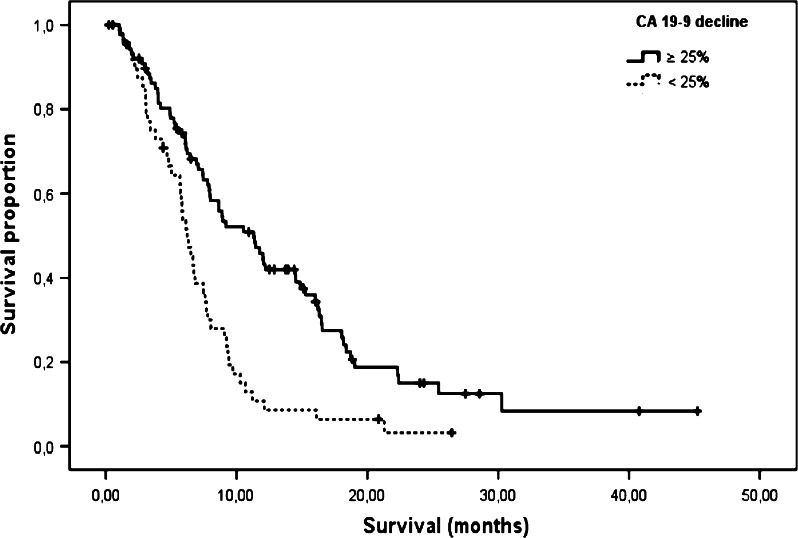
Overall, at least one follow-up measurement of CA 19-9 during the first two cycles of chemotherapy was available for 186 patients (64 %), among them 83 patients whose values were measured by the Elecsys® method. Two cutoffs of a CA 19-9 response during treatment were applied (25 and 50 %, respectively) in order to define a biochemical treatment response. As summarized within Table 4, a strong correlation was observed between a CA 19-9 decline of at least 25 % with OS (11.9 vs. 8.2 months, p = 0.003) for all values and for the subgroup determined by the Elecsys® assay (13.4 vs. 8.6 months, p = 0.004). Adopting the 50 % cutoff in the univariate analysis, no significant correlation with TTP or OS could be observed—neither for all 186 patients nor for the subgroup of 83 patients measured with the Elecsys® assay (Table 4). In a second step, more stringent inclusion criteria (serum bilirubin ≤1.5 × ULN and baseline CA 19-9 > 37 U/ml) were applied for the landmark analysis of the serial CA 19-9 kinetics (see “validation analysis” in Table 4): this resulted in 138 eligible patients, 64 of them were assessed only by the Elecsys® assay. Again, a robust correlation with OS was seen for the 25 % cutoff (11.3 vs. 6.2 months, p < 0.001; Fig. 1) for all patients and also for the Elecsys® subgroup (11.8 vs. 7.5 months, p = 0.001).
Table 4.
Prognostic value of CA 19-9 kinetics during the first two treatment cycles (day 20–64)
| Overall survival (OS) | Time-to-progression (TTP) | ||||||
|---|---|---|---|---|---|---|---|
| CA 19-9 decline | n | OS (months) | p | HR (95 % CI) | TTP (months) | p | HR (95 % CI) |
| All patients (n = 186) | |||||||
| ≥25 % | 110 | 11.9 | 0.003 | 1.62 (1.17–2.25) | 5.8 | 0.018 | 1.49 (1.01–2.09) |
| <25 % | 76 | 8.2 | 4.4 | ||||
| ≥50 % | 77 | 11.9 | 0.076 | 1.34 (0.97–1.87) | 5.5 | 0.372 | 1.16 (0.84–1.61) |
| <50 % | 109 | 8.8 | 5.4 | ||||
| Elecsys® patients only (n = 83) | |||||||
| ≥25 % | 46 | 13.4 | 0.004 | 2.03 (1.24–3.33) | 7.0 | 0.003 | 2.18 (1.28–3.72) |
| <25 % | 37 | 8.6 | 2.6 | ||||
| ≥50 % | 27 | 12.4 | 0.259 | 1.36 (0.79–2.34) | 5.8 | 0.785 | 1.08 (0.63–1.83) |
| <50 % | 56 | 9.5 | 6.1 | ||||
| Validation* all patients (n = 138) | |||||||
| ≥25 % | 89 | 11.3 | <0.001 | 2.12 (1.44–3.14) | 3.8 | 0.011 | 1.69 (1.12–2.55) |
| <25 % | 49 | 6.2 | 2.8 | ||||
| ≥50 % | 59 | 11.3 | 0.032 | 1.51 (1.03–2.22) | 3.6 | 0.508 | 1.14 (0.78–1.66) |
| <50 % | 79 | 6.8 | 3.5 | ||||
| Validation* Elecsys® patients only (n = 64) | |||||||
| ≥25 % | 39 | 11.8 | 0.001 | 2.52 (1.40–4.52) | 5.9 | 0.016 | 2.11 (1.13–3.95) |
| <25 % | 25 | 7.5 | 2.8 | ||||
| ≥50 % | 22 | 11.8 | 0.146 | 1.57 (0.85–2.88) | 4.7 | 0.748 | 1.11 (0.60–2.04) |
| <50 % | 42 | 7.6 | 4.7 | ||||
Bold values indicate statistical significance (p < 0.05)
* Patients selected for validation: landmark at day 56 ± 8; serum bilirubin ≤1.5 mg/dl; CA 19-9 ≥ 37 U/ml
Fig. 1.
Kaplan–Meier curve for OS based on a CA 19-9 decline of ≥25 % during the first two chemotherapy cycles: subgroup “CA 19-9 validation—all patients” (n = 138; for details see text and Table 4); median OS: 11.3 versus 6.2 months (HR 2.12, p < 0.001)
The baseline parameters log [CA 19-9], tumor stage, tumor grading and KPS together with the variable “CA 19-9 decline of at least 25 %” were subsequently included into a multivariate Cox model: besides tumor grading and KPS, also the 25 % cutoff for the CA 19-9 decline during treatment showed an independent prognostic significance for the endpoint OS (HR 2.40, 95 % CI 1.54–3.74, p < 0.001).
Discussion
Within a pooled analysis based on data from two German randomized phase II trials in conjunction with data from one high-volume German Cancer Center, we were able to investigate the prognostic role of 12 clinical and biochemical variables in patients with advanced PC. As expected, stage of disease and KPS were significant prognostic factors for TTP and OS also in our study population (Heinemann et al. 2012). Interestingly, an age of ≥64 years at study entry was associated with a prolonged TTP (5.8 vs. 4.2 months), without having an impact on OS (8.8 vs. 9.1 months), respectively. Thus, one might conclude that age per se should be no contraindication for the use of palliative chemotherapy in PC. We furthermore hypothesize that the prolongation of TTP in older patients might argue for a slower tumor growth, which however was not transferred into a survival benefit (possibly due to age-dependent co-morbidities).
LDH is a known parameter indicating a high turnover of cells with subsequent release of the intracellular enzyme. The upper limit of normal (250 U/l) was chosen as a cutoff for this study, and significant differences for TTP and OS were observed in univariate (but not in multivariate) analyses. The transformation into the natural logarithm, potentially a more adequate method for the evaluation of a continuous variable with a broad range, also revealed a correlation with clinical outcome. Up to now, there are only few reports describing a potential prognostic role of LDH in patients with PC (Bramhall et al. 2001; Stocken et al. 2008). Remarkably, baseline CRP levels also indicated a highly significant correlation with OS when using a cutoff of 1.0 mg/dl in the univariate analysis (Falconer et al. 1995). The transformation of CRP into the natural logarithm was similarly associated with TTP and OS. On multivariate analysis, log [CRP] retained independent significance for OS. These results are consistent with other reports on CRP in the perioperative or palliative setting for PC (Moses et al. 2009; Pine et al. 2009). Data from resectable colorectal cancer demonstrated that a low CD4+ T-lymphocyte infiltration in the tumor was linked with elevated CRP serum levels, both reflecting a poor cancer-specific survival. Thus, tumor progression might not merely be determined by tumor biology only, but also by the immunologic response of the host (Canna et al. 2005). Both parameters, LDH and CRP, were also shown to have a significant impact on OS in an univariate subgroup analysis (published online only) from the large phase III AViTA study (n = 607): LDH levels at baseline of >ULN resulted in a HR for death of 0.59 (95 % CI 0.43–0.82) as did pre-treatment CRP levels of >1.4 mg/dl (HR 0.65, 95 % CI 0.51–0.84) (van Cutsem et al. 2009).
Baseline CA 19-9 levels were significant predictors of TTP in uni- and multivariate analyses in our patient cohort (Tables 2 and 3). This observation was detected when we applied a cutoff of 1,000 U/ml for dichotomizing the CA 19-9 variable as well as for the analysis of log [CA 19-9] as continuous variable. Of note, this association seems not to be assay dependent, as results were the same when analyzing the Elecsys® only subgroup in univariate analysis. Regarding OS, pre-treatment CA 19-9 also had a highly significant impact on survival, irrespective of the applied assay (Table 2). One important scientific (and clinically relevant) finding from this study is summarized in Table 4: a CA 19-9 decline of ≥25 % during treatment seems appropriate to define subgroups with a different outcome for TTP and OS. In contrast, the 50 % cutoff seems inappropriate as a marker for a biochemical treatment response. Interestingly, this observation again was independent of the applied CA 19-9 assay. These data are consistent with the result reported from Hess and colleagues who found that an early decrease in CA 19-9 concentration of at least 50 % after two cycles of chemotherapy was not associated with a prolonged OS in their biomarker analysis from the large randomized SAKK 44/00 trial (Hess et al. 2008). Thus, one might conclude that an early CA 19-9 decline of 25 % during treatment is adequate in order to define a prognostically favorable subgroup of PC patients and that is important to use the same assay in the follow-up of a single patient (intraindividual approach); however, the use of a unique assay for all patients (e.g., within the setting of a multicenter study) does not necessarily seem to be required for generating valid tumor marker data (Boeck et al. 2006).
The strength of this pooled retrospective, multicenter study arises from the fact that we were able to analyze a large patient number [according to the REMARK guidelines (McShane et al. 2005)] and that most of the patients included (87 %) were treated within prospective clinical trials. All marker data included in the profound statistical analyses (e. g., including a landmark method) were collected prospectively. Furthermore, we were able to generate differentiated data on the value of the applied CA 19-9 assay; an approach that is—at least to our knowledge—unique up to now. Limitations are based on the different numbers that were available for each single variable (e. g., CRP data were available from LMU patients only) and on a significant proportion of missing data for the analysis of the CA 19-9 kinetics (n = 186/291)—a problem that often is observed when subgroup data from multicenter PC trials are reported.
In conclusion, this study confirms the important role of CA 19-9 as tumor marker in advanced PC and also highlights the potential role of other serum markers like CRP, LDH and bilirubin to serve as prognostically relevant factors in this disease. These findings may play an important role for future stratification procedures in clinical trials and also for the selection of “good risk” and “poor risk” PC patients for different treatment strategies (e. g., singe-agent gemcitabine vs. intensive FOLFIRINOX chemotherapy; Conroy et al. 2011; Heinemann et al. 2012). However, a prospective validation of these novel hypothesis-generating data is recommended.
Acknowledgments
The authors would like to thank all patients and their families, nurses, study coordinators and investigators that actively participated in the Ro96 and the GEMOXCET study, thereby enabling this pooled analysis. This work is part of the doctoral thesis of Michael Haas.
Conflict of interest
All authors declare that they have no conflict of interest.
References
- Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V (2006) Prognostic and therapeutic significance of carbohydrate antigen 19–9 as tumor marker in patients with pancreatic cancer. Oncology 70(4):255–264 [DOI] [PubMed] [Google Scholar]
- Boeck S, Hoehler T, Seipelt G, Mahlberg R, Wein A, Hochhaus A et al (2008) Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann Oncol 19(2):340–347 [DOI] [PubMed] [Google Scholar]
- Boeck S, Haas M, Laubender RP, Kullmann F, Klose C, Bruns CJ et al (2010) Application of a time-varying covariate model to the analysis of CA 19–9 as serum biomarker in patients with advanced pancreatic cancer. Clin Cancer Res 16(3):986–994 [DOI] [PubMed] [Google Scholar]
- Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA (2001) Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol 19(15):3447–3455 [DOI] [PubMed] [Google Scholar]
- Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF et al (2005) The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer 92(4):651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825 [DOI] [PubMed] [Google Scholar]
- Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R et al (2010) Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol 21(3):441–447 [DOI] [PubMed] [Google Scholar]
- Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ et al (1995) Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer 75(8):2077–2082 [DOI] [PubMed] [Google Scholar]
- Heinemann V, Haas M, Boeck S (2012) Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev 38(7):843–853 [DOI] [PubMed] [Google Scholar]
- Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T et al (2008) CA 19–9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 9(2):132–138 [DOI] [PubMed] [Google Scholar]
- Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD et al (2012) The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol 23(7):1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann F, Hollerbach S, Dollinger MM, Harder J, Fuchs M, Messmann H et al (2009) Cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line metastatic pancreatic cancer: a multicentre phase II study. Br J Cancer 100(7):1032–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93(4):387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical trials group. J Clin Oncol 25(15):1960–1966 [DOI] [PubMed] [Google Scholar]
- Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA (2009) Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep 21(4):1091–1095 [DOI] [PubMed] [Google Scholar]
- Pine JK, Fusai KG, Young R, Sharma D, Davidson BR, Menon KV et al (2009) Serum C-reactive protein concentration and the prognosis of ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol 35(6):605–610 [DOI] [PubMed] [Google Scholar]
- Reni M, Cereda S, Balzano G, Passoni P, Rognone A, Fugazza C et al (2009) Carbohydrate antigen 19–9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer 115(12):2630–2639 [DOI] [PubMed] [Google Scholar]
- Stocken DD, Hassan AB, Altman DG, Billingham LJ, Bramhall SR, Johnson PJ et al (2008) Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer 99(6):883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL et al (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27(13):2231–2237 [DOI] [PubMed] [Google Scholar]
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M (2011) Pancreatic cancer. Lancet 378(9791):607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]