Abstract
1. The effects of gamma-aminobutyric acid (GABA) on membrane potential and conductance as well as on the intracellular Cl- activity (aiCl) and intracellular pH (pHi) were studied in crayfish muscle fibres using a three-microelectrode voltage clamp and ion-selective microelectrodes. In the presence of CO2-HCO3-, the intracellular HCO3- activity (aiHCO3) was estimated from pHi. 2. In a nominally HCO3(-)-free solution, a near-saturating concentration of GABA (0.2 mM) produced a marked increase in membrane conductance but little change in potential. In a solution containing 30 mM-HCO3- (equilibrated with 5% CO2 + 95% air; pH 7.4), the GABA-induced increase in conductance was associated with a depolarization of about 15 mV, with an increase in aiCl and with a decrease in aiHCO3. All these effects were blocked by picrotoxin (PTX). The depolarizing action of GABA was augmented following depletion of extracellular and intracellular Cl-. 3. The GABA-induced increase in aiCl which took place in the presence of HCO3- was blocked by clamping the membrane potential at its resting level. This indicates that the increase in aiCl was due to passive redistribution of Cl-. In both the presence and absence of HCO3-, the GABA-activated transmembrane flux of Cl- showed reversal at the level of the resting potential, which indicates that under steady-state conditions the Cl- equilibrium potential (ECl) is identical to the resting potential. 4. In a Cl(-)-free, 30 mM-HCO3(-)-containing solution, 0.5 mM-GABA produced a PTX-sensitive increase in conductance which amounted to 15% of the conductance activated in the presence of Cl-. In the absence of both Cl- and HCO3-, the respective figure was 2.8%. Assuming constant-field conditions, the conductance data yielded a permeability ratio PHCO3/PCl of 0.42 for the GABA-activated channels. 5. In a Cl(-)-containing, HCO3(-)-free solution, the reversal potential of the GABA-activated current (EGABA) was, by about 1 mV, less negative than the resting membrane potential (RP). In a solution containing Cl- and 30 mM-HCO3-, EGABA-RP was 12 mV. Simultaneous measurements of EGABA, aiCl and aiHCO3 (pHi) gave a PHCO3/PCl value of 0.33. 6. In a Cl(-)-free, HCO3(-)-containing solution EGABA was close to the HCO3- equilibrium potential (EHCO3) and an experimental acidosis which produced a negative shift in EHCO3 was associated with a similar shift in EGABA.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


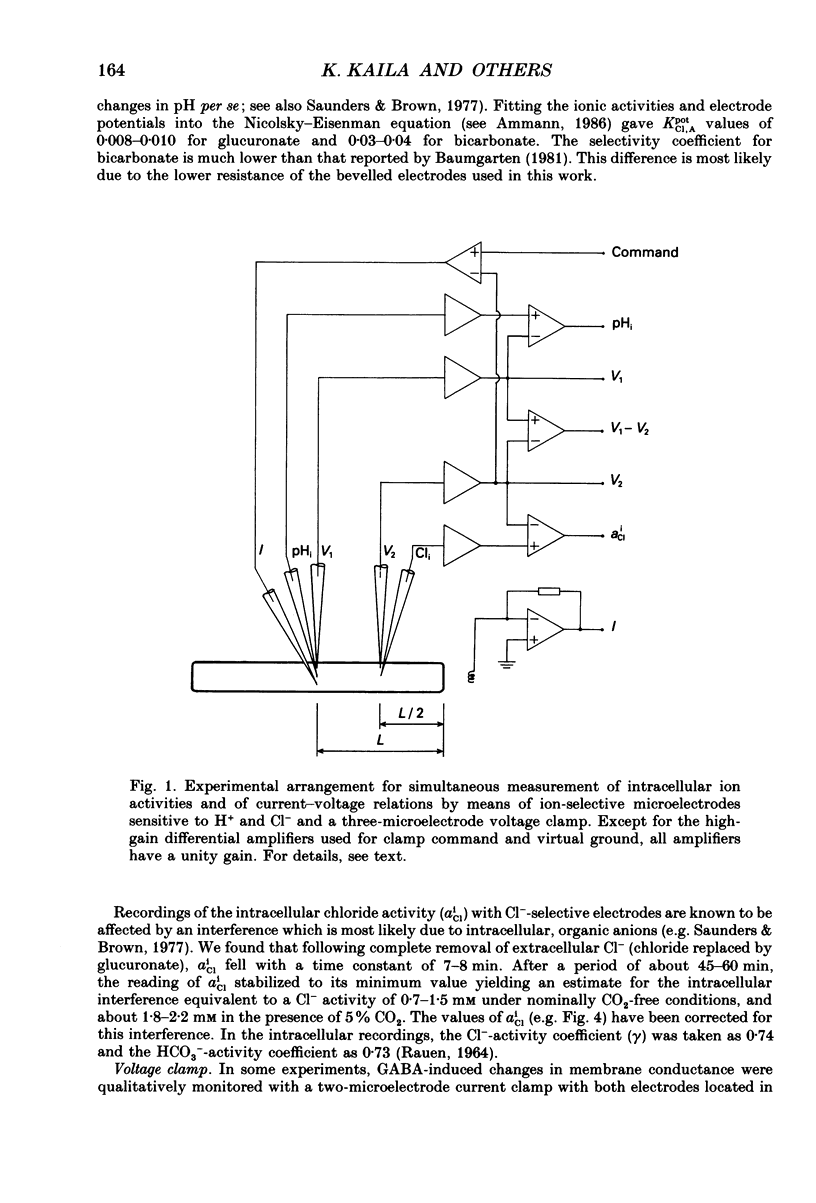





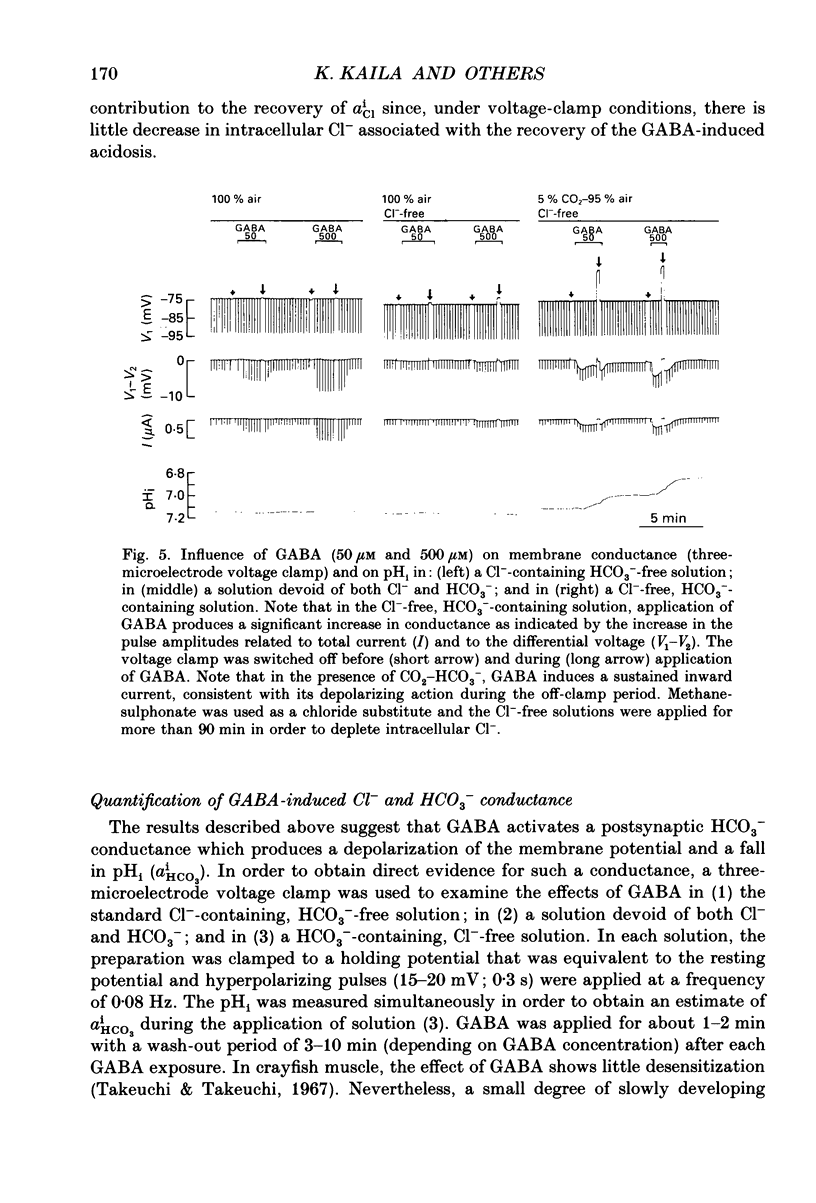




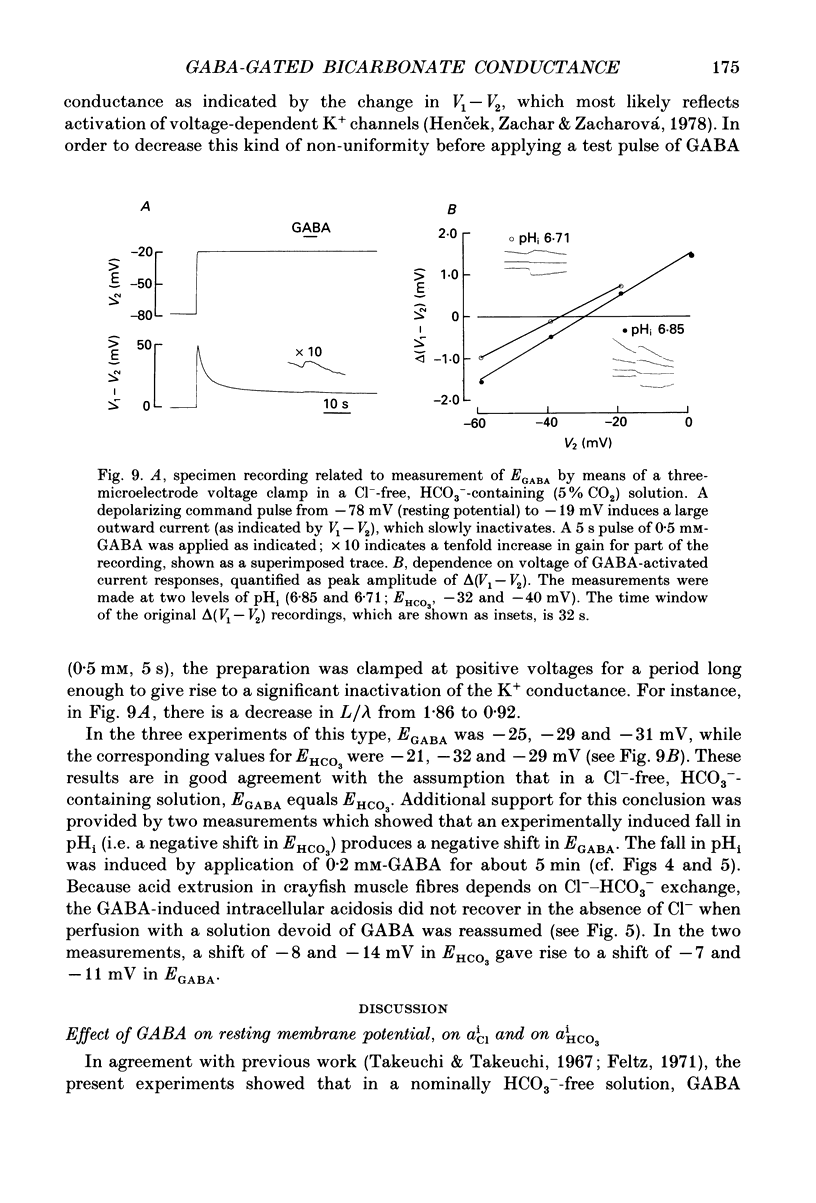






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., ITO M., OSCARSSON O. Anion permeability of the synaptic and non-synaptic motoneurone membrane. J Physiol. 1961 Dec;159:410–435. doi: 10.1113/jphysiol.1961.sp006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979 Sep 27;281(5729):315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans F. J., Gamiño S. M., Giraldez F., Noguerón I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988 Dec;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P. An intracellular analysis of gamma-aminobutyric-acid-associated ion movements in rat sympathetic neurones. J Physiol. 1985 Aug;365:41–58. doi: 10.1113/jphysiol.1985.sp015758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten C. M. An improved liquid ion exchanger for chloride ion-selective microelectrodes. Am J Physiol. 1981 Nov;241(5):C258–C263. doi: 10.1152/ajpcell.1981.241.5.C258. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Depolarization of neurones in the isolated olfactory cortex of the guinea-pig by gamma-aminobutyric acid. Br J Pharmacol. 1979 Feb;65(2):339–345. doi: 10.1111/j.1476-5381.1979.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Smart T. G. Measurement of GABA-evoked conductance changes of lobster muscle fibres by a three-microelectrode voltage clamp technique. Proc R Soc Lond B Biol Sci. 1982 Jun 22;215(1200):343–364. doi: 10.1098/rspb.1982.0046. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N., Simpson H. K. Concerning the ionic basis of presynaptic inhibition. Experientia. 1976 Mar 15;32(3):348–349. doi: 10.1007/BF01940831. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. The role of intracellular chloride in hyperpolarizing post-synaptic inhibition of crayfish stretch receptor neurones. J Physiol. 1982 May;326:123–138. doi: 10.1113/jphysiol.1982.sp014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Finger W., Stettmeier H. Inhibitory synaptic channels activated by gamma-aminobutyric acid (GABA) in crayfish muscle. Pflugers Arch. 1980 Sep;387(2):143–151. doi: 10.1007/BF00584265. [DOI] [PubMed] [Google Scholar]
- Dudel J., Rüdel R. Voltage controlled contractions and current voltage relations of crayfish muscle fibers in chloride-free solutions. Pflugers Arch. 1969;308(4):291–314. doi: 10.1007/BF00587182. [DOI] [PubMed] [Google Scholar]
- Dudel J. Voltage dependence of amplitude and time course of inhibitory synaptic current in crayfish muscle. Pflugers Arch. 1977 Oct 19;371(1-2):167–174. doi: 10.1007/BF00580786. [DOI] [PubMed] [Google Scholar]
- Feltz A. Competitive interaction of beta-guanidino propionic acid and gamma-aminobutyric acid on the muscle fibre of the crayfish. J Physiol. 1971 Jul;216(2):391–401. doi: 10.1113/jphysiol.1971.sp009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz P., Rasminsky M. A model for the mode of action of GABA on primary afferent terminals: depolarizing effects of GABA applied iontophoretically to neurones of mammalian dorsal root ganglia. Neuropharmacology. 1974 Jun;13(6):553–563. doi: 10.1016/0028-3908(74)90145-2. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Nakamura J., Shinnick-Gallagher P. The effects of temperature, pH and Cl-pump inhibitors on GABA responses recorded from cat dorsal root ganglia. Brain Res. 1983 May 16;267(2):249–259. doi: 10.1016/0006-8993(83)90877-6. [DOI] [PubMed] [Google Scholar]
- Galler S., Moser H. The ionic mechanism of intracellular pH regulation in crayfish muscle fibres. J Physiol. 1986 May;374:137–151. doi: 10.1113/jphysiol.1986.sp016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hencek M., Zachar J., Zacharová D. Membrane currents in a calcium type muscle membrane under voltage clamp. Physiol Bohemoslov. 1978;27(5):457–466. [PubMed] [Google Scholar]
- ITO M., KOSTYUK P. G., OSHIMA T. Further study on anion permeability of inhibitory post-synaptic membrane of cat motoneurones. J Physiol. 1962 Oct;164:150–156. doi: 10.1113/jphysiol.1962.sp007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. THE EFFECT OF ANION INJECTION AND CHANGES IN THE EXTERNAL POTASSIUM AND CHLORIDE CONCENTRATION ON THE REVERSAL POTENTIALS OF THE IPSP AND ACETYLCHOLINE. Comp Biochem Physiol. 1964 Feb;11:199–213. doi: 10.1016/0010-406x(64)90163-x. [DOI] [PubMed] [Google Scholar]
- Kaila K., Mattsson K., Voipio J. Fall in intracellular pH and increase in resting tension induced by a mitochondrial uncoupling agent in crayfish muscle. J Physiol. 1989 Jan;408:271–293. doi: 10.1113/jphysiol.1989.sp017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Morris M. E., Yim G. K. Anionic permeability of cortical neurones. Exp Brain Res. 1969;7(1):11–31. doi: 10.1007/BF00236105. [DOI] [PubMed] [Google Scholar]
- Llinas R., Baker R., Precht W. Blockage of inhibition by ammonium acetate action on chloride pump in cat trochlear motoneurons. J Neurophysiol. 1974 May;37(3):522–532. doi: 10.1152/jn.1974.37.3.522. [DOI] [PubMed] [Google Scholar]
- Lux H. D. Ammonium and chloride extrusion: hyperpolarizing synaptic inhibition in spinal motoneurons. Science. 1971 Aug 6;173(3996):555–557. doi: 10.1126/science.173.3996.555. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Loracher C., Neher E. The action of ammonium on postsynaptic inhibition of cat spinal motoneurons. Exp Brain Res. 1970;11(5):431–447. doi: 10.1007/BF00233967. [DOI] [PubMed] [Google Scholar]
- Motokizawa F., Reuben J. P., Grundfest H. Ionic permeability of the inhibitory postsynaptic membrane of lobster muscle fibers. J Gen Physiol. 1969 Oct;54(4):437–461. doi: 10.1085/jgp.54.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild T. O., Thomas R. C. Intracellular chloride activity and the effects of acetylcholine in snail neurones. J Physiol. 1974 Oct;242(2):453–470. doi: 10.1113/jphysiol.1974.sp010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The blockade of GABA mediated responses in the frog spinal cord by ammonium ions and furosemide. J Physiol. 1978 Oct;283:121–132. doi: 10.1113/jphysiol.1978.sp012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Tsuda K. Membrane permeability change during inhibitory transmitter action in crayfish stretch receptor cell. J Neurophysiol. 1973 Sep;36(5):805–816. doi: 10.1152/jn.1973.36.5.805. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Saunders J. H., Brown H. M. Liquid and solid-state Cl- -sensitive microelectrodes. Characteristics and application to intracellular Cl- activity in Balanus photoreceptor. J Gen Physiol. 1977 Oct;70(4):507–530. doi: 10.1085/jgp.70.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickholm A. Reduction of response time for potential in salt bridge reference electrodes for electrophysiology. Nature. 1968 Jan 6;217(5123):80–81. doi: 10.1038/217080a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. LOCALIZED ACTION OF GAMMA-AMINOBUTYRIC ACID ON THE CRAYFISH MUSCLE. J Physiol. 1965 Mar;177:225–238. doi: 10.1113/jphysiol.1965.sp007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion permeability of the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1967 Aug;191(3):575–590. doi: 10.1113/jphysiol.1967.sp008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Experimental displacement of intracellular pH and the mechanism of its subsequent recovery. J Physiol. 1984 Sep;354:3P–22P. doi: 10.1113/jphysiol.1984.sp015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Analysis of Cl- -HCO3(-) exchange during recovery from intracellular acidosis in cardiac Purkinje strands. Am J Physiol. 1984 May;246(5 Pt 1):C391–C400. doi: 10.1152/ajpcell.1984.246.5.C391. [DOI] [PubMed] [Google Scholar]
- Wojtowicz J. M., Nicoll R. A. Selective action of piretanide on primary afferent GABA responses in the frog spinal cord. Brain Res. 1982 Mar 18;236(1):173–181. doi: 10.1016/0006-8993(82)90043-9. [DOI] [PubMed] [Google Scholar]


