Abstract
1. Transmitter release at the squid giant synapse was stimulated by photolytic release of Ca2+ from the 'caged' Ca2+ compound DM-nitrophen (Kaplan & Ellis-Davies, 1988) inserted into presynaptic terminals. 2. Competing binding reactions cause the amount of Ca2+ released by DM-nitrophen photolysis to depend on the concentrations of DM-nitrophen, total Ca2+, Mg+, ATP and native cytoplasmic Ca2+ buffer. Measurements of presynaptic [Ca2+] changes by co-injection of the fluorescent indicator dye Fura-2 show that DM-nitrophen photolysis causes a transient rise in Ca2+ followed by decay within about 150 ms to an increased steady-state level. 3. Rapid photolysis of Ca2(+)-loaded nitrophen within the presynaptic terminal was followed in less than a millisecond by depolarization of the postsynaptic membrane. As with action potential-evoked excitatory postsynaptic potentials (EPSPs), the light-evoked response was partially and reversibly blocked by 1-3 mM-kainic acid which desensitizes postsynaptic glutamate receptors. 4. Release was similar in magnitude and rate to normal action potential-mediated EPSPs. 5. The release of transmitter by photolysis of Ca2(+)-loaded DM-nitrophen was not affected by removal of Ca2+ from the saline or addition of tetrodotoxin. Photolysis of DM-nitrophen injected into presynaptic terminals without added Ca2+ did not stimulate release of transmitter nor did it interfere with normal action potential-mediated release. 6. Stimulation of presynaptic action potentials in Ca2(+)-free saline during the light-evoked response did not elicit increased release of transmitter if the ganglion was bathed in Ca2(+)-free saline, i.e. in the absence of Ca2+ influx. Increasing the intensity of the light or stimulating presynaptic action potentials in Ca2(+)-containing saline increased the release of transmitter. Therefore the failure of presynaptic voltage change to increase transmitter release resulting from release of caged Ca2+ was not due to saturation or inhibition of the release mechanism by light-released Ca2+. 7. Decreasing the temperature of the preparation increased the delay to onset of the light-evoked response and reduced its amplitude and rate of rise to an extent similar to that observed for action potential-evoked EPSPs.
Full text
PDF









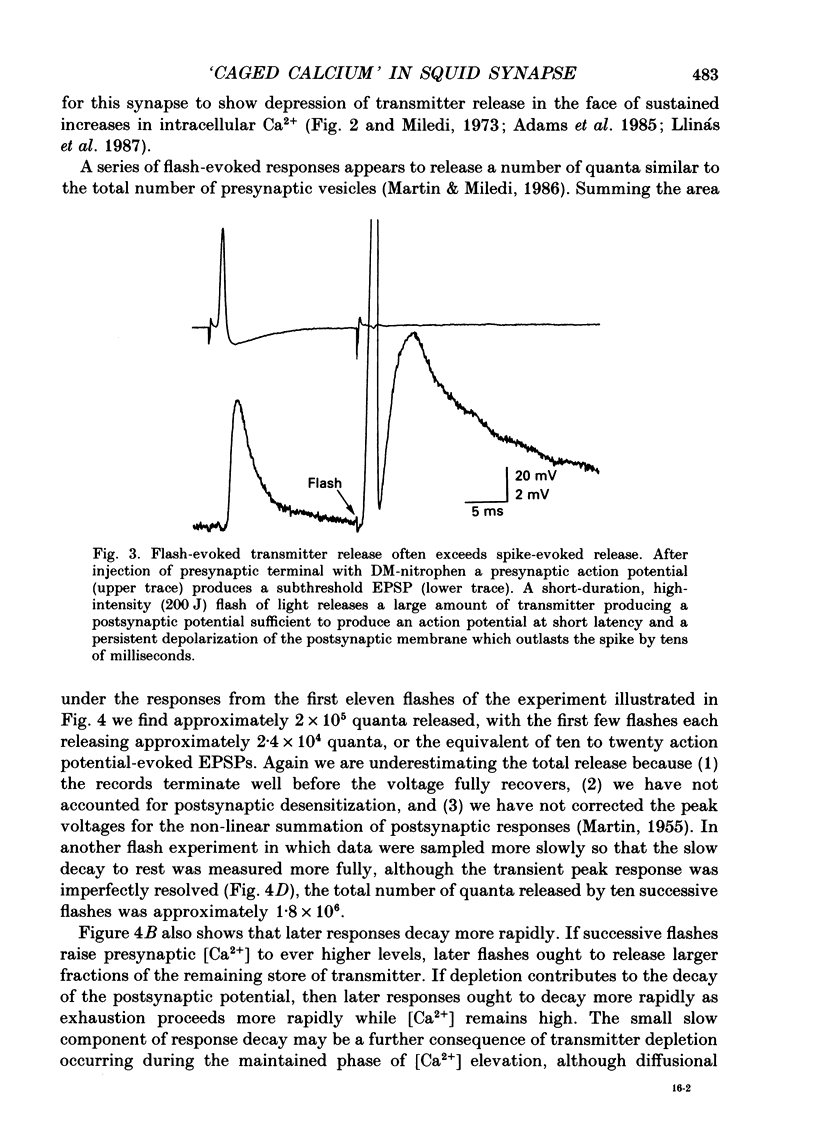
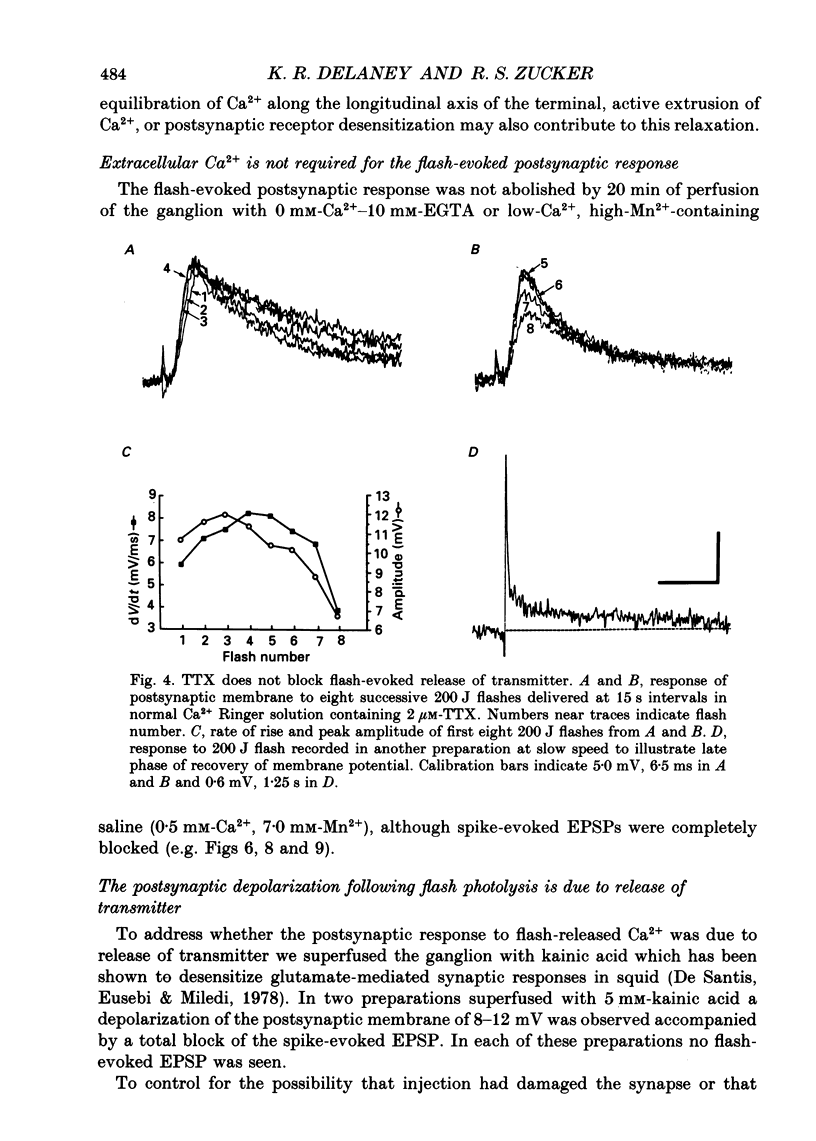














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Takeda K., Umbach J. A. Inhibitors of calcium buffering depress evoked transmitter release at the squid giant synapse. J Physiol. 1985 Dec;369:145–159. doi: 10.1113/jphysiol.1985.sp015893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabás K., Keszthelyi L. Temperature dependence of ATP release from "caged" ATP. Acta Biochim Biophys Acad Sci Hung. 1984;19(3-4):305–309. [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. L., Fay F. S. Photobleaching of fura-2 and its effect on determination of calcium concentrations. Am J Physiol. 1987 Oct;253(4 Pt 1):C613–C618. doi: 10.1152/ajpcell.1987.253.4.C613. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A. Ionized magnesium concentration in axoplasm of dialyzed squid axons. FEBS Lett. 1975 Jan 15;50(1):82–85. doi: 10.1016/0014-5793(75)81046-5. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A., Mullins L. J. Intracellular calcium buffering capacity in isolated squid axons. J Gen Physiol. 1977 Sep;70(3):355–384. doi: 10.1085/jgp.70.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Atwood H. L. Synaptic transmission: temperature-sensitivity of calcium entry in presynaptic terminals. Brain Res. 1979 Jul 20;170(3):543–546. doi: 10.1016/0006-8993(79)90972-7. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Bittner G. D. Facilitation of transmitter release at squid synapses. J Gen Physiol. 1978 Oct;72(4):471–486. doi: 10.1085/jgp.72.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Control of quantal transmitter release at frog's motor nerve terminals. I. Dependence on amplitude and duration of depolarization. Pflugers Arch. 1984 Nov;402(3):225–234. doi: 10.1007/BF00585504. [DOI] [PubMed] [Google Scholar]
- Dudel J. Control of quantal transmitter release at frog's motor nerve terminals. II. Modulation by de- or hyperpolarizing pulses. Pflugers Arch. 1984 Nov;402(3):235–243. doi: 10.1007/BF00585505. [DOI] [PubMed] [Google Scholar]
- Dudel J., Parnas I., Parnas H. Neurotransmitter release and its facilitation in crayfish muscle. VI. Release determined by both, intracellular calcium concentration and depolarization of the nerve terminal. Pflugers Arch. 1983 Sep;399(1):1–10. doi: 10.1007/BF00652515. [DOI] [PubMed] [Google Scholar]
- EIGEN M., HAMMES G. G. ELEMENTARY STEPS IN ENZYME REACTIONS (AS STUDIED BY RELAXATION SPECTROMETRY). Adv Enzymol Relat Areas Mol Biol. 1963;25:1–38. doi: 10.1002/9780470122709.ch1. [DOI] [PubMed] [Google Scholar]
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987 Aug 13;925(2):133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Tsien R. Y. Ca2+ binding kinetics of fura-2 and azo-1 from temperature-jump relaxation measurements. Biophys J. 1988 Apr;53(4):635–639. doi: 10.1016/S0006-3495(88)83142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinks M. H., Klee C. B., Pant H. C., Gainer H. Identification and quantification of calcium-binding proteins in squid axoplasm. J Neurosci. 1988 Jun;8(6):2172–2182. doi: 10.1523/JNEUROSCI.08-06-02172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Landau E. M. Depression and recovery of transmission at the squid giant synapse. J Physiol. 1975 Feb;245(1):13–32. doi: 10.1113/jphysiol.1975.sp010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landò L., Zucker R. S. "Caged calcium" in Aplysia pacemaker neurons. Characterization of calcium-activated potassium and nonspecific cation currents. J Gen Physiol. 1989 Jun;93(6):1017–1060. doi: 10.1085/jgp.93.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A. Transmitter release by presynaptic impulses in the squid stellate ganglion. Nature. 1970 Aug 1;227(5257):493–496. doi: 10.1038/227493a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Walton K. Further studies on depolarization release coupling in squid giant synapse. Adv Exp Med Biol. 1987;221:1–17. doi: 10.1007/978-1-4684-7618-7_1. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Parnas H., Dudel J., Parnas I. Neurotransmitter release and its facilitation in crayfish. VII. Another voltage dependent process beside Ca entry controls the time course of phasic release. Pflugers Arch. 1986 Feb;406(2):121–130. doi: 10.1007/BF00586672. [DOI] [PubMed] [Google Scholar]
- Parnas H., Segel L. A. Exhaustion of calcium does not terminate evoked neurotransmitter release. J Theor Biol. 1984 Apr 7;107(3):345–365. doi: 10.1016/s0022-5193(84)80096-x. [DOI] [PubMed] [Google Scholar]
- Parnas I., Parnas H. Calcium is essential but insufficient for neurotransmitter release: the calcium-voltage hypothesis. J Physiol (Paris) 1986;81(4):289–305. [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Meiri H., Erulkar S. D., Barenholz Y. Changes in transmitter release induced by ion-containing liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5214–5216. doi: 10.1073/pnas.75.10.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Statham H. E., Duncan C. J. The action of ionophores at the frog neuromuscular junction. Life Sci. 1975 Nov 1;17(9):1401–1406. doi: 10.1016/0024-3205(75)90159-9. [DOI] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Zucker R. S. Control of cytoplasmic calcium with photolabile tetracarboxylate 2-nitrobenzhydrol chelators. Biophys J. 1986 Nov;50(5):843–853. doi: 10.1016/S0006-3495(86)83525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Fogelson A. L. Relationship between transmitter release and presynaptic calcium influx when calcium enters through discrete channels. Proc Natl Acad Sci U S A. 1986 May;83(9):3032–3036. doi: 10.1073/pnas.83.9.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Haydon P. G. Membrane potential has no direct role in evoking neurotransmitter release. Nature. 1988 Sep 22;335(6188):360–362. doi: 10.1038/335360a0. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Landò L., Fogelson A. Can presynaptic depolarization release transmitter without calcium influx? J Physiol (Paris) 1986;81(4):237–245. [PubMed] [Google Scholar]
- Zucker R. S., Landò L. Mechanism of transmitter release: voltage hypothesis and calcium hypothesis. Science. 1986 Feb 7;231(4738):574–579. doi: 10.1126/science.2868525. [DOI] [PubMed] [Google Scholar]
- de Santis A., Eusebi F., Miledi R. Kainic acid and synaptic transmission in the stellate ganglion of the squid. Proc R Soc Lond B Biol Sci. 1978 Sep 29;202(1149):527–532. doi: 10.1098/rspb.1978.0084. [DOI] [PubMed] [Google Scholar]


