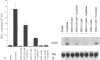Abstract
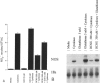
OBJECTIVE: The authors determine the relationship between glutathione and nitric oxide (NO) synthesis in cultured hepatocytes. SUMMARY BACKGROUND DATA: Glutathione is a cofactor for a number of enzymes, and its presence is essential for maximal enzyme activity by the inducible macrophage nitric oxide synthase (iNOS), which produces the reactive nitric oxide radical. Hepatocytes contain substantial quantities of glutathione, and this important tripeptide is decreased in hepatocytes stressed by ischemia/reperfusion or endotoxemia. Endotoxemia also induces the synthesis of inflammatory cytokines that result in the production of nitric oxide from hepatocytes by iNOS, suggesting that hepatocytes may be attempting to synthesize nitric oxide at times when intracellular glutathione is reduced. METHODS: Hepatocytes were cultured with buthionine sulfoximine and 1,3-bis(chloroethyl)-1-nitrosourea (BCNU) to inhibit glutathione. After exposure to cytokines, NO synthesis was assessed by supernatant nitrite levels, cytosolic iNOS enzyme activity, and iNOS mRNA levels. RESULTS: Inhibition of glutathione synthesis with buthionine sulfoximine or inhibition of glutathione reductase activity with BCNU inhibited nitrite synthesis. Both buthionine sulfoximine and BCNU inhibited the induction of iNOS mRNA, as detected by Northern blot analysis. Exogenous glutathione increased cytokine-stimulated iNOS induction, overcame the inhibitory effects of BCNU, and increased nitrite production by intact hepatocytes, induced hepatocyte cytosol, and partially purified hepatocyte iNOS. CONCLUSIONS: In cultured hepatocytes, adequate glutathione levels are required for optimal nitric oxide synthesis. This finding is predominantly due to an effect on iNOS mRNA levels, although glutathione also participates in the regulation of iNOS enzyme activity.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson G. M., Billings R. E. Cytokine toxicity and induction of NO synthase activity in cultured mouse hepatocytes. Toxicol Appl Pharmacol. 1993 Mar;119(1):100–107. doi: 10.1006/taap.1993.1048. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Harbrecht B. G., Stadler J., Williams D. L., Ochoa J. B., Di Silvio M., Simmons R. L., Murray S. A. Association between synthesis and release of cGMP and nitric oxide biosynthesis by hepatocytes. Am J Physiol. 1992 Apr;262(4 Pt 1):C1077–C1082. doi: 10.1152/ajpcell.1992.262.4.C1077. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Harbrecht B. G., Stuehr D. J., Demetris A. J., Simmons R. L. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990 Dec;48(6):565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., Stadler J., Simmons R. L., Murray S. A. Inducible cytosolic enzyme activity for the production of nitrogen oxides from L-arginine in hepatocytes. Biochem Biophys Res Commun. 1990 May 16;168(3):1034–1040. doi: 10.1016/0006-291x(90)91133-d. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Ferrari F. K., Kispert P. H., Stadler J., Stuehr D. J., Simmons R. L., Billiar T. R. Nitric oxide and nitric oxide-generating compounds inhibit hepatocyte protein synthesis. FASEB J. 1991 Apr;5(7):2085–2092. doi: 10.1096/fasebj.5.7.1707021. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Duval D. L., Sieg D. J., Billings R. E. Regulation of hepatic nitric oxide synthase by reactive oxygen intermediates and glutathione. Arch Biochem Biophys. 1995 Feb 1;316(2):699–706. doi: 10.1006/abbi.1995.1093. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo D., Alessio P., Foco A., Bussolino F., Costamagna C., Heller R., Garbarino G., Pescarmona G. P., Bosia A. Nitric oxide synthesis is impaired in glutathione-depleted human umbilical vein endothelial cells. Am J Physiol. 1993 Sep;265(3 Pt 1):C728–C732. doi: 10.1152/ajpcell.1993.265.3.C728. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Harbrecht B. G., Billiar T. R., Stadler J., Demetris A. J., Ochoa J. B., Curran R. D., Simmons R. L. Nitric oxide synthesis serves to reduce hepatic damage during acute murine endotoxemia. Crit Care Med. 1992 Nov;20(11):1568–1574. doi: 10.1097/00003246-199211000-00015. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. S., Billiar T., Curran R. D., Zdziarski U. E., Simmons R. L., Basford R. E. Inhibition of chemotaxis Ng-monomethyl-L-arginine: a role for cyclic GMP. Blood. 1989 Nov 1;74(6):1885–1887. [PubMed] [Google Scholar]
- Keller G. A., Barke R., Harty J. T., Humphrey E., Simmons R. L. Decreased hepatic glutathione levels in septic shock. Predisposition of hepatocytes to oxidative stress: an experimental approach. Arch Surg. 1985 Aug;120(8):941–945. doi: 10.1001/archsurg.1985.01390320065013. [DOI] [PubMed] [Google Scholar]
- Kuo P. C., Slivka A. Nitric oxide decreases oxidant-mediated hepatocyte injury. J Surg Res. 1994 Jun;56(6):594–600. doi: 10.1006/jsre.1994.1094. [DOI] [PubMed] [Google Scholar]
- Marczin N., Ryan U. S., Catravas J. D. Sulfhydryl-depleting agents, but not deferoxamine, modulate EDRF action in cultured pulmonary arterial cells. Am J Physiol. 1993 Sep;265(3 Pt 1):L220–L227. doi: 10.1152/ajplung.1993.265.3.L220. [DOI] [PubMed] [Google Scholar]
- McKelvey T. G., Höllwarth M. E., Granger D. N., Engerson T. D., Landler U., Jones H. P. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol. 1988 May;254(5 Pt 1):G753–G760. doi: 10.1152/ajpgi.1988.254.5.G753. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Murphy M. E., Piper H. M., Watanabe H., Sies H. Nitric oxide production by cultured aortic endothelial cells in response to thiol depletion and replenishment. J Biol Chem. 1991 Oct 15;266(29):19378–19383. [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Harrison D. G. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am J Physiol. 1991 Feb;260(2 Pt 1):C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R., Liu Z. Z., Morris S. M., Jr Coinduction of nitric oxide synthase and argininosuccinate synthetase in a murine macrophage cell line. Implications for regulation of nitric oxide production. J Biol Chem. 1994 Jan 14;269(2):1257–1261. [PubMed] [Google Scholar]
- Nüssler A. K., Geller D. A., Sweetland M. A., Di Silvio M., Billiar T. R., Madariaga J. B., Simmons R. L., Lancaster J. R., Jr Induction of nitric oxide synthesis and its reactions in cultured human and rat hepatocytes stimulated with cytokines plus LPS. Biochem Biophys Res Commun. 1993 Jul 30;194(2):826–835. doi: 10.1006/bbrc.1993.1896. [DOI] [PubMed] [Google Scholar]
- Nüssler A., Drapier J. C., Rénia L., Pied S., Miltgen F., Gentilini M., Mazier D. L-arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991 Jan;21(1):227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- Okabe H., Irita K., Taniguchi S., Kurosawa K., Tagawa K., Yoshitake J., Takahashi S. Endotoxin causes early changes in glutathione concentrations in rabbit plasma and liver. J Surg Res. 1994 Sep;57(3):416–419. doi: 10.1006/jsre.1994.1163. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shan X. Q., Aw T. Y., Jones D. P. Glutathione-dependent protection against oxidative injury. Pharmacol Ther. 1990;47(1):61–71. doi: 10.1016/0163-7258(90)90045-4. [DOI] [PubMed] [Google Scholar]
- Stadler J., Curran R. D., Ochoa J. B., Harbrecht B. G., Hoffman R. A., Simmons R. L., Billiar T. R. Effect of endogenous nitric oxide on mitochondrial respiration of rat hepatocytes in vitro and in vivo. Arch Surg. 1991 Feb;126(2):186–191. doi: 10.1001/archsurg.1991.01410260074010. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985 Jan 10;260(1):86–92. [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F. FAD and GSH participate in macrophage synthesis of nitric oxide. Biochem Biophys Res Commun. 1990 Apr 30;168(2):558–565. doi: 10.1016/0006-291x(90)92357-6. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]