Abstract
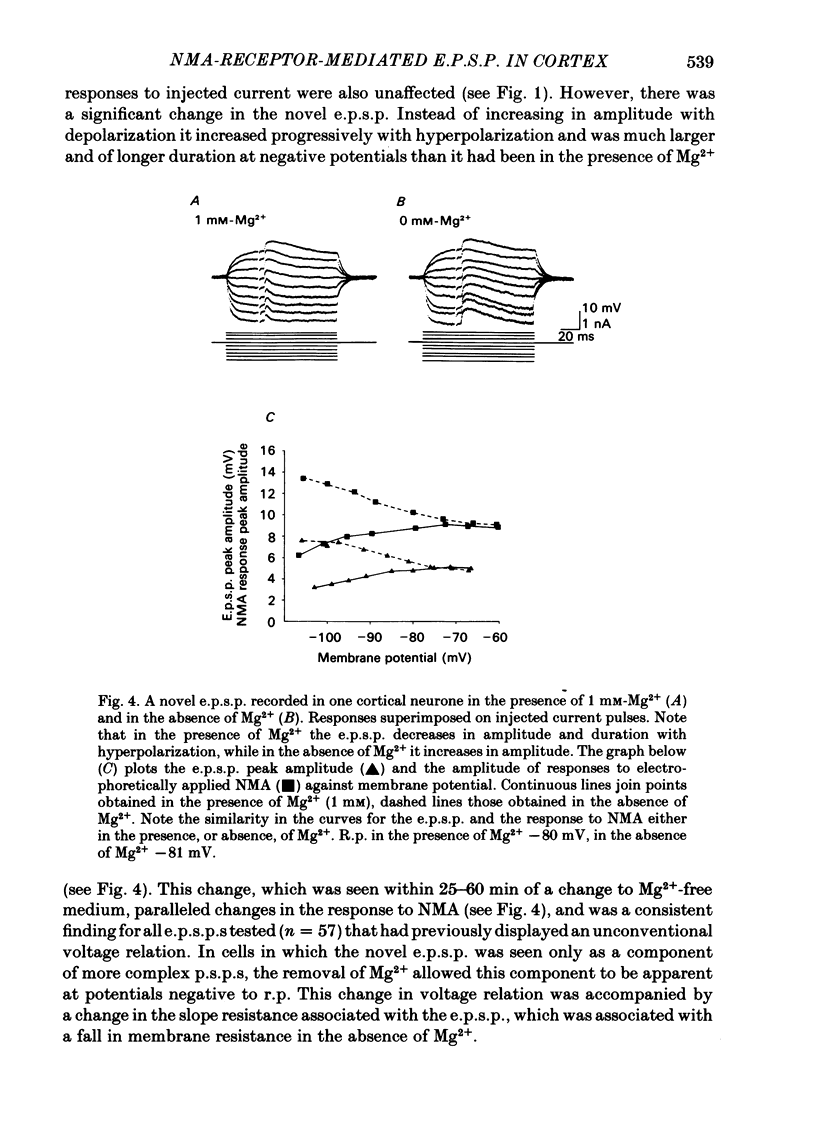
In isolated slices of rat cerebral cortex, intracellular recordings were obtained from pyramidal cells that were predominantly in layers II/III. These cells could be antidromically activated from the underlying white matter and had resting potentials of greater than -75 mV, action potentials with amplitudes of greater than 70 mV (measured from threshold), overshoots of 20-30 mV, and thresholds 20-30 mV positive to the resting potential. The responses of these cells to short (1-2 s) pulses of electrophoretically applied N-methylaspartate (NMA) decreased in amplitude with membrane hyperpolarization between -40 and -120 mV, and were associated with an apparent increase in membrane resistance when recorded in the presence of 1 mM-Mg2+. However, in the absence of Mg2+, responses to NMA increased progressively in amplitude with hyperpolarization and were associated with a decrease in membrane resistance. In addition to conventional excitatory post-synaptic potentials (e.p.s.p.s) and inhibitory post-synaptic potentials (i.p.s.p.s), electrical stimulation of the underlying white matter evoked a novel e.p.s.p. This e.p.s.p. displayed a similar voltage relation to the response evoked by NMA and was associated with an apparent increase in membrane resistance. When Mg2+ was removed from the bathing medium, the properties of the novel e.p.s.p. changed, and it displayed a conventional voltage relation and was associated with a decrease in membrane resistance. In the absence of Mg2+, novel e.p.s.p.s showed potentiation on low frequency repetitive stimulation (0.5-2 Hz). A fully potentiated response could evoke bursts of slow potentials each of which could evoke a burst of fast spikes. In contrast, the more conventional e.p.s.p.s and i.p.s.p.s evoked in pyramidal neurones were unaffected by reducing the Mg2+ concentration from 1.0 to near 0 mM and conventional e.p.s.p.s showed no potentiation on repetitive, low frequency repetition, even after several hours exposure to Mg2+-free medium. The NMA antagonists: 2-amino-5-phosphonovaleric acid, ketamine and cyclazocine, applied electrophoretically at doses that blocked responses to NMA, but which had little effect on responses to glutamate, blocked the novel e.p.s.p. and its potentiation.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES A., 3rd, SAKANOUE M., ENDO S. NA, K, CA, MG, AND C1 CONCENTRATIONS IN CHOROID PLEXUS FLUID AND CISTERNAL FLUID COMPARED WITH PLASMA ULTRAFILTRATE. J Neurophysiol. 1964 Jul;27:672–681. doi: 10.1152/jn.1964.27.4.672. [DOI] [PubMed] [Google Scholar]
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S. C., Dawkins S. L., Lodge D. Comparison of sigma- and kappa-opiate receptor ligands as excitatory amino acid antagonists. Br J Pharmacol. 1984 Sep;83(1):179–185. doi: 10.1111/j.1476-5381.1984.tb10133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Courtney K. R., Prince D. A. Epileptogenesis in neocortical slices. Brain Res. 1977 May 20;127(1):191–196. doi: 10.1016/0006-8993(77)90393-6. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Kelly J. S. The reversal potential of excitatory amino acid action on granule cells of the rat dentate gyrus. J Physiol. 1984 Jun;351:327–342. doi: 10.1113/jphysiol.1984.sp015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Selective antagonism of amino acid-induced and synaptic excitation in the cat spinal cord. J Physiol. 1979 Dec;297(0):621–635. doi: 10.1113/jphysiol.1979.sp013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. N-methyl aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells. J Physiol. 1983 Oct;343:385–405. doi: 10.1113/jphysiol.1983.sp014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick M. J., Connors B. W., Prince D. A. Mechanisms of neocortical epileptogenesis in vitro. J Neurophysiol. 1982 Dec;48(6):1321–1335. doi: 10.1152/jn.1982.48.6.1321. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Schaerer B., Vosmansky M. A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods. 1979 Dec;1(4):323–325. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Porietis A. V., Wojtowicz J. M. L-Aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons. Brain Res. 1982 Apr 8;237(1):248–253. doi: 10.1016/0006-8993(82)90575-3. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McLennan H., Lodge D. The antagonism of amino acid-induced excitation of spinal neurones in the cat. Brain Res. 1979 Jun 15;169(1):83–90. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. A depolarizing inhibitory potential in neurones of the olfactory cortex in vitro. J Physiol. 1978 Feb;275:547–557. doi: 10.1113/jphysiol.1978.sp012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. Electrical properties of neurones in the olfactory cortex slice in vitro. J Physiol. 1978 Feb;275:535–546. doi: 10.1113/jphysiol.1978.sp012206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C., Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985 Feb 7;313(6002):479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Gorman A. L. Responses of cortical neurons to stimulation of corpus callosum in vitro. J Neurophysiol. 1982 Dec;48(6):1257–1273. doi: 10.1152/jn.1982.48.6.1257. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]


