Abstract
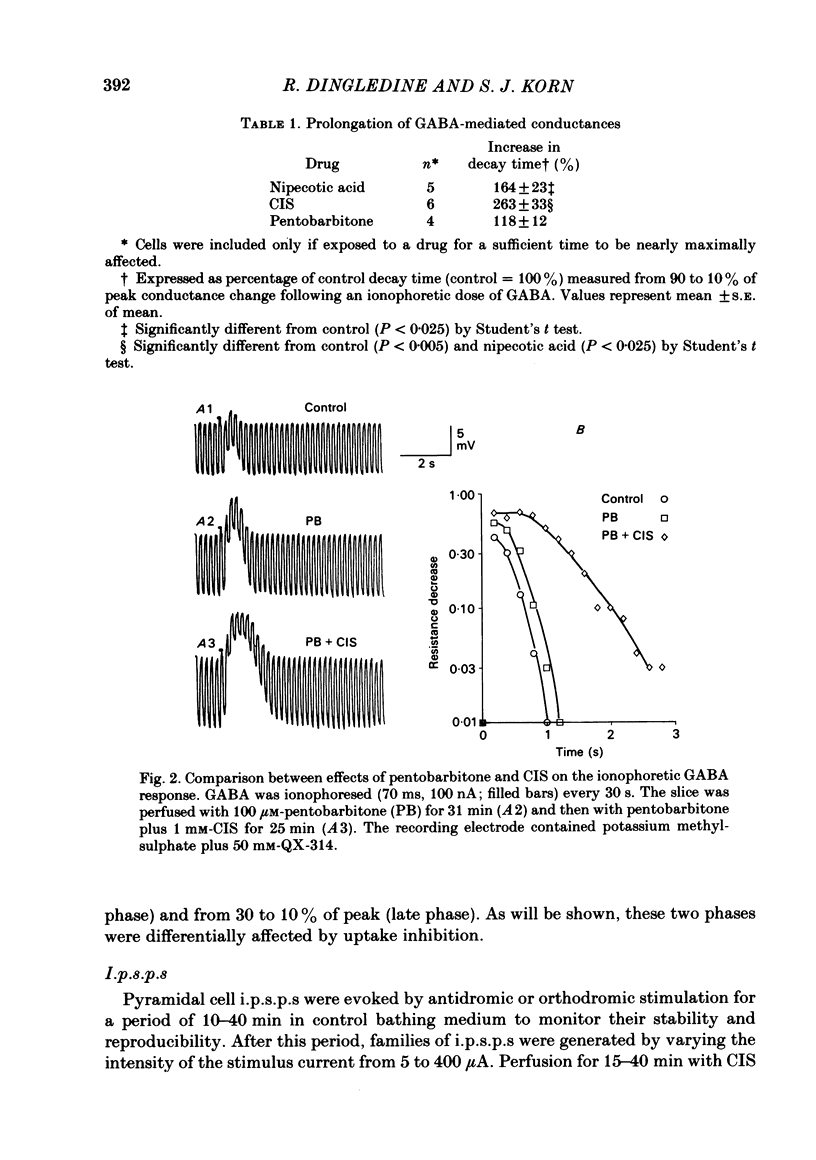
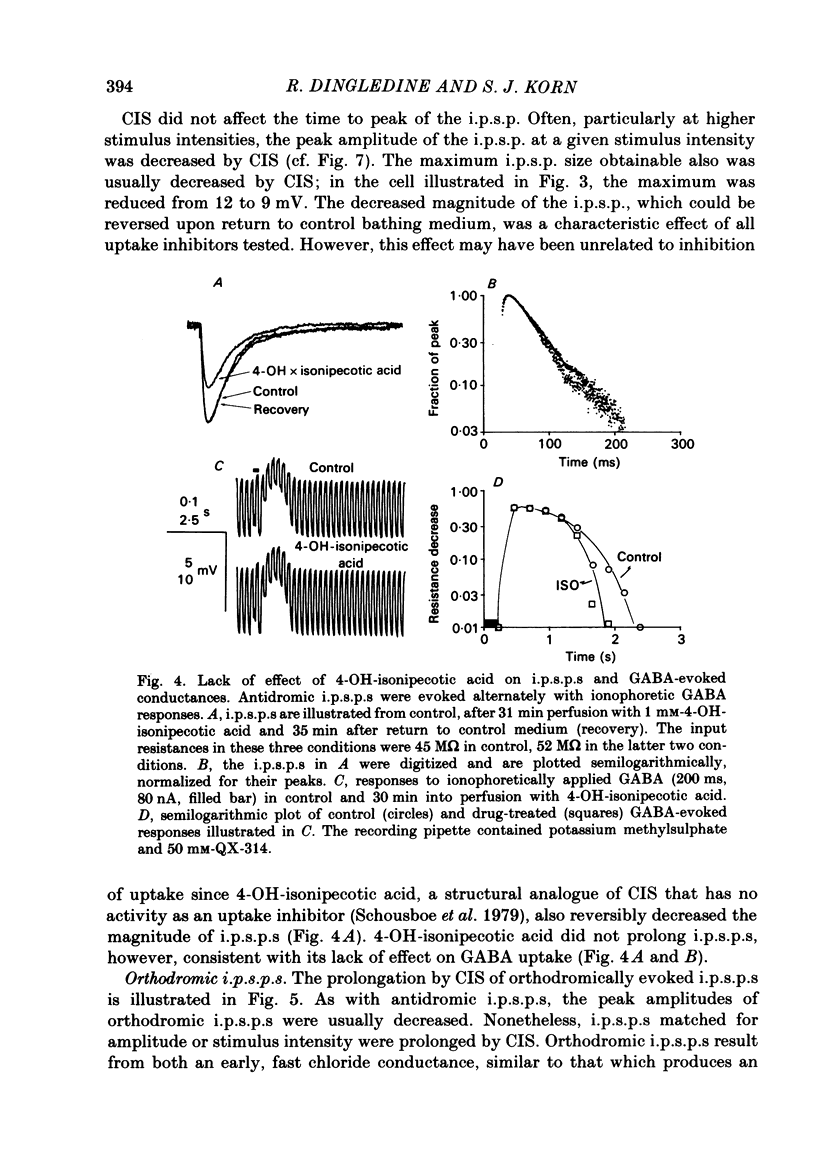
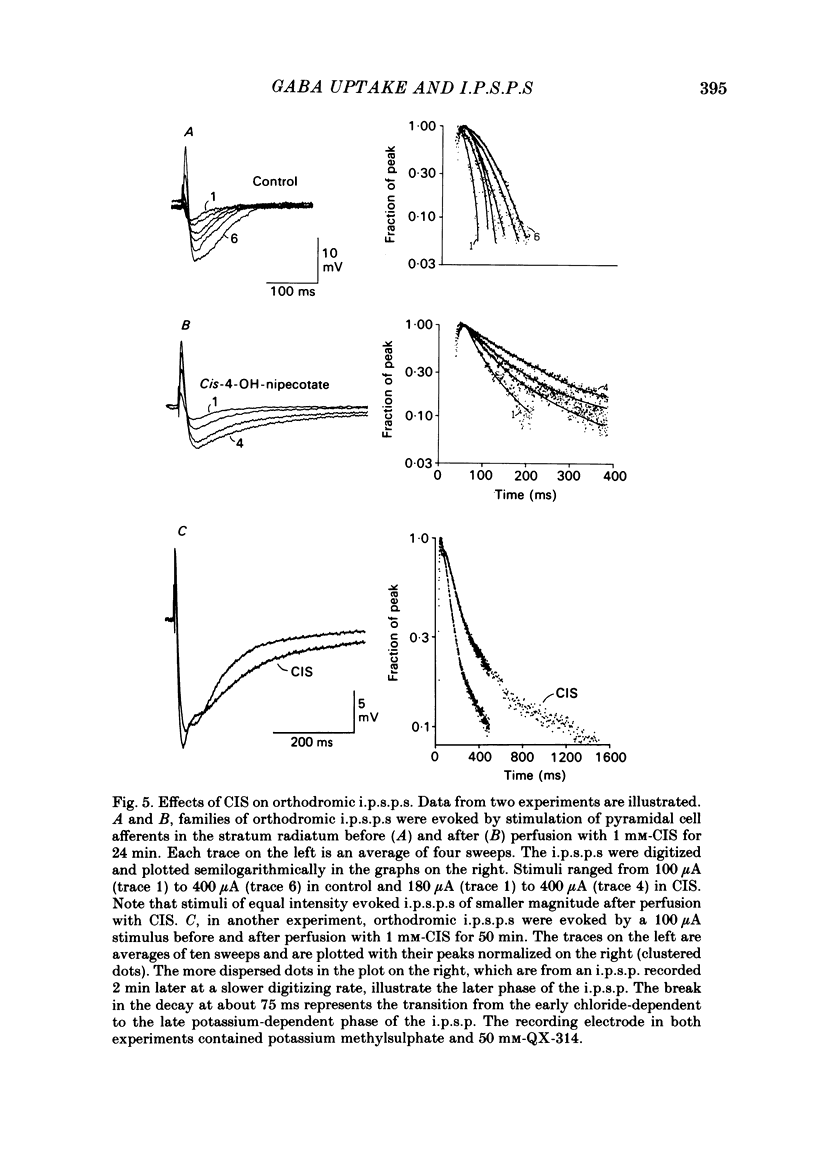
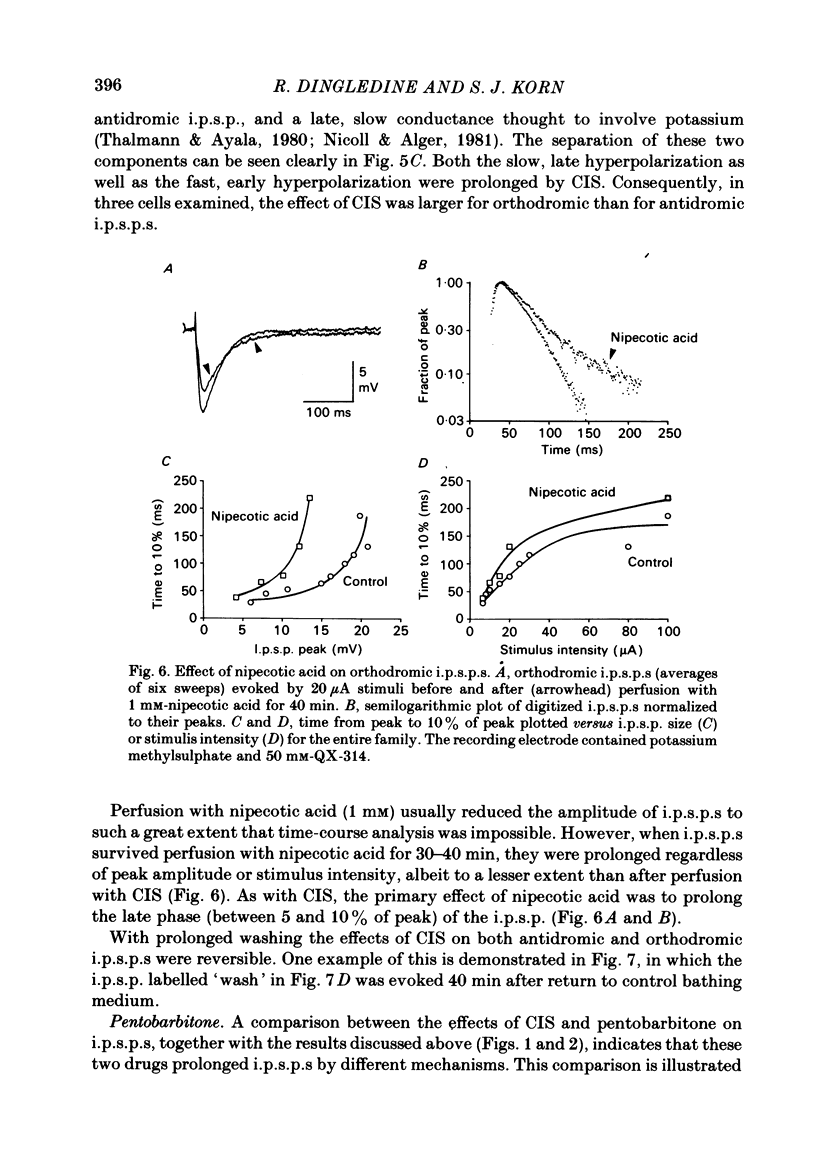
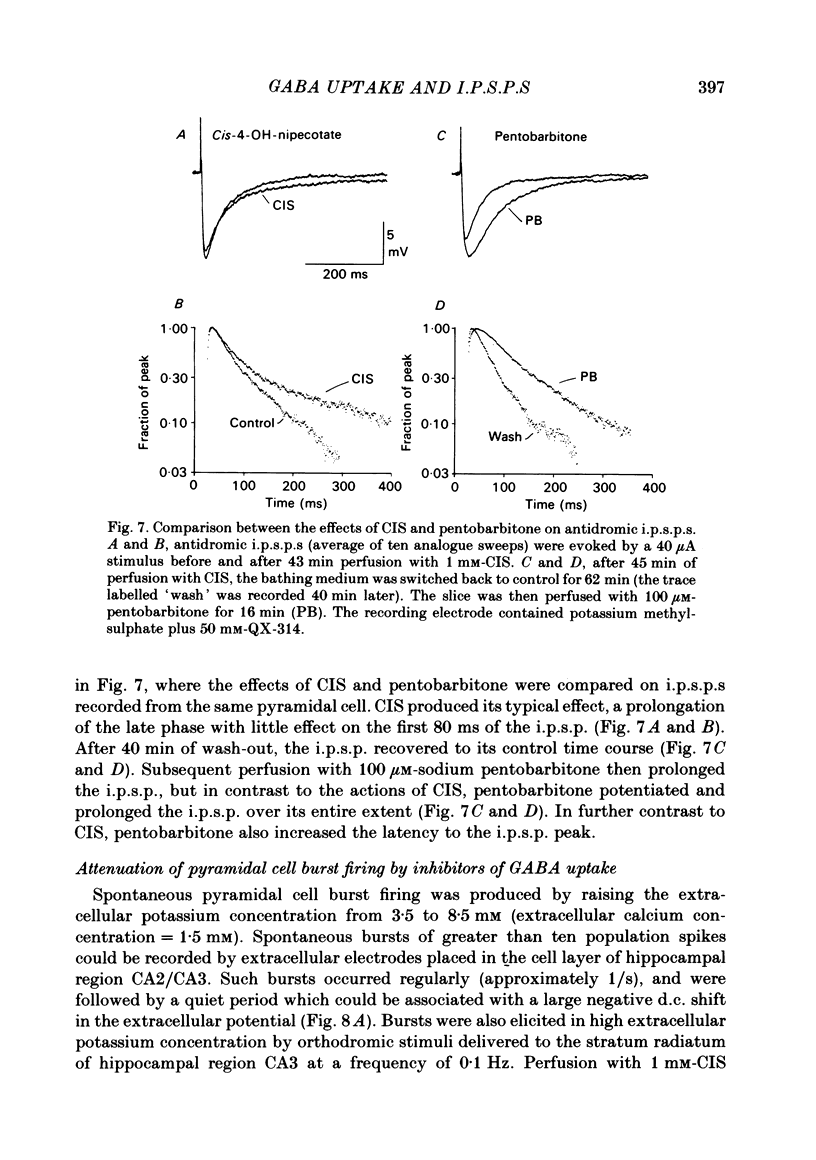
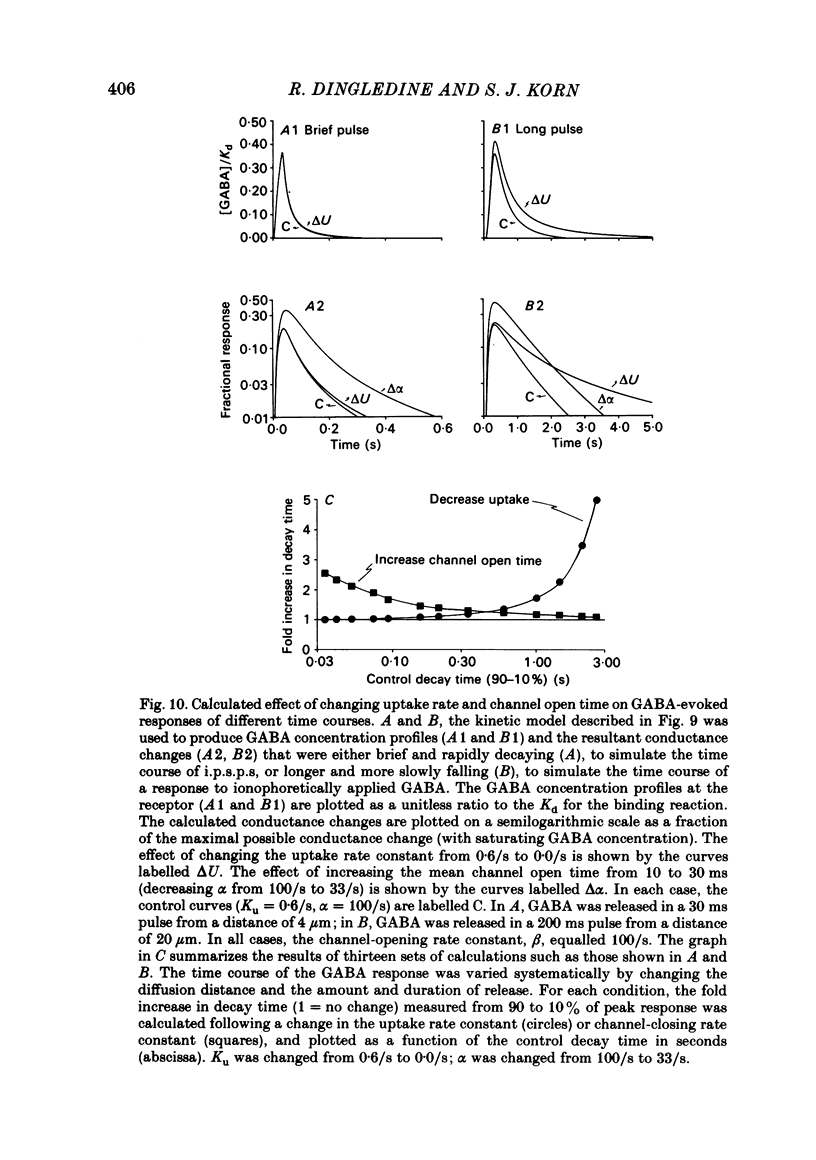
Intracellular recordings were made from CA1 pyramidal cells in the rat hippocampal slice to study the processes that influence the time course of inhibitory post-synaptic potentials (i.p.s.p.s) mediated by gamma-aminobutyric acid (GABA), and conductance changes evoked by ionophoretically applied GABA. The GABA-uptake inhibitors, nipecotic acid and cis-4-OH-nipecotic acid (1 mM), greatly prolonged conductance increases associated with both hyperpolarizing and depolarizing responses to ionophoretically applied GABA. In contrast to their effects on GABA-evoked conductances, uptake inhibitors only slightly prolonged antidromically evoked i.p.s.p.s. Their primary effect occurred after the i.p.s.p. had decayed to 5-30% of its peak. 4-OH-isonipecotic acid, a nipecotic acid analogue that does not inhibit GABA uptake, did not prolong i.p.s.p.s or ionophoretically evoked conductance changes. Sodium pentobarbitone (100 microM), a drug that prolongs the open time of GABA-activated chloride channels, potentiated both i.p.s.p.s and responses to ionophoretically applied GABA. Whereas pentobarbitone also prolonged i.p.s.p.s, it did not prolong responses to ionophoretically applied GABA. The prolongation of i.p.s.p.s by pentobarbitone occurred equally in both the early and late phases of the i.p.s.p., in contrast to the effects of GABA-uptake inhibitors. I.p.s.p.s did not usually decay exponentially. The observation that uptake inhibitors prolonged the late but not the early decay phase of the i.p.s.p., together with the previous finding that the conductance change persists for the duration of the i.p.s.p., indicate that GABA is present in the synapse throughout much of the i.p.s.p. These data suggest that diffusion of GABA out of the synapse, a non-exponential process, is an important determinant of the i.p.s.p. decay time course. Increasing the extracellular potassium concentration from 3.5 to 8.5 mM resulted in spontaneously occurring, synchronous burst firing of pyramidal cells. Cis-4-OH-nipecotic acid significantly reduced the number and amplitude of extracellularly recorded population spikes within each burst. We conclude that diffusion, channel open time and GABA uptake all influence the time course of GABA-mediated i.p.s.p.s. The time course of a single, brief i.p.s.p. is determined predominantly by post-synaptic channel kinetics and diffusion of GABA out of the synapse, whereas the inhibition produced by prolonged synaptic bursts or relatively long application of exogenous GABA can be markedly influenced by GABA uptake.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. High affinity uptake of transmitters: studies on the uptake of L-aspartate, GABA, L-glutamate and glycine in cat spinal cord. J Neurochem. 1973 Feb;20(2):529–539. doi: 10.1111/j.1471-4159.1973.tb12152.x. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C., Grabsch B. Involvement of uptake2 in the termination of activity of neurogenic noradrenaline in the rat isolated atrium. J Physiol. 1976 Jan;254(1):203–212. doi: 10.1113/jphysiol.1976.sp011229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Galvan M. Influence of neuroglial transport on the action of gamma-aminobutyric acid on mammalian ganglion cells. Br J Pharmacol. 1977 Feb;59(2):373–378. doi: 10.1111/j.1476-5381.1977.tb07502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Inhibition of GABA uptake potentiates the conductance increase produced by GABA-mimetic compounds on single neurones in isolated olfactory cortex slices of the guinea-pig. Br J Pharmacol. 1984 Sep;83(1):195–202. doi: 10.1111/j.1476-5381.1984.tb10135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Fricke R. A., Perkel D. H. Passive electrical constants in three classes of hippocampal neurons. J Neurophysiol. 1981 Oct;46(4):812–827. doi: 10.1152/jn.1981.46.4.812. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Gration K. A., Usherwood P. N. Influence of glutamate and aspartate on time course of decay of excitatory synaptic currents at locust neuromuscular junctions. Brain Res. 1980 Jun 16;192(1):205–216. doi: 10.1016/0006-8993(80)91020-3. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Prince D. A. Effects of local anesthetic QX-314 on the membrane properties of hippocampal pyramidal neurons. J Pharmacol Exp Ther. 1982 Mar;220(3):476–481. [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The termination of transmitter action at the crustacean excitatory neuromuscular junction. J Physiol. 1977 Jul;268(3):711–729. doi: 10.1113/jphysiol.1977.sp011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher M. J., Meldrum B. S., Krogsgaard-Larsen P. Anticonvulsant activity of GABA uptake inhibitors and their prodrugs following central or systemic administration. Eur J Pharmacol. 1983 May 6;89(3-4):217–228. doi: 10.1016/0014-2999(83)90497-1. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Lodge D. The in vivo inactivation of GABA and other inhibitory amino acids in the cat nervous system. Exp Brain Res. 1976 Jun 30;25(4):413–428. doi: 10.1007/BF00241731. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Langmoen I. A. Conductance changes and inhibitory actions of hippocampal recurrent IPSPs. Brain Res. 1980 Mar 10;185(2):277–287. doi: 10.1016/0006-8993(80)91068-9. [DOI] [PubMed] [Google Scholar]
- Dingledine R. Possible mechanisms of enkephalin action on hippocampal CA1 pyramidal neurons. J Neurosci. 1981 Sep;1(9):1022–1035. doi: 10.1523/JNEUROSCI.01-09-01022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., Henderson G., North R. A., Williams J. T. Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. J Physiol. 1983 Dec;345:477–488. doi: 10.1113/jphysiol.1983.sp014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi H., Nishi S. Effect of barbiturates on the GABA receptor of cat primary afferent neurones. J Physiol. 1982 Nov;332:299–314. doi: 10.1113/jphysiol.1982.sp014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Lodge D., Johnston G. A., Curtis D. R., Brand S. J. Effects of the Areca nut constituents arecaidine and guvacine on the action of GABA in the cat central nervous system. Brain Res. 1977 Nov 18;136(3):513–522. doi: 10.1016/0006-8993(77)90075-0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews W. D., McCafferty G. P., Setler P. E. An electrophysiological model of GABA-mediated neurotransmission. Neuropharmacology. 1981 Jun;20(6):561–565. doi: 10.1016/0028-3908(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984 Mar;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981 Dec;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981 May 22;212(4497):957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Rovira C., Ben-Ari Y., Cherubini E. Somatic and dendritic actions of gamma-aminobutyric acid agonists and uptake blockers in the hippocampus in vivo. Neuroscience. 1984 Jun;12(2):543–555. doi: 10.1016/0306-4522(84)90072-1. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Thorbek P., Hertz L., Krogsgaard-Larsen P. Effects of GABA analogues of restricted conformation on GABA transport in astrocytes and brain cortex slices and on GABA receptor binding. J Neurochem. 1979 Jul;33(1):181–189. doi: 10.1111/j.1471-4159.1979.tb11720.x. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]


