Abstract
1. Solitary rod inner segments were obtained by enzymatic dissociation of the tiger salamander (Ambystoma tigrinum) retina. Their membrane currents were studied with the single-pipette voltage-clamp technique. Individual currents were isolated with the aid of pharmacological agents.
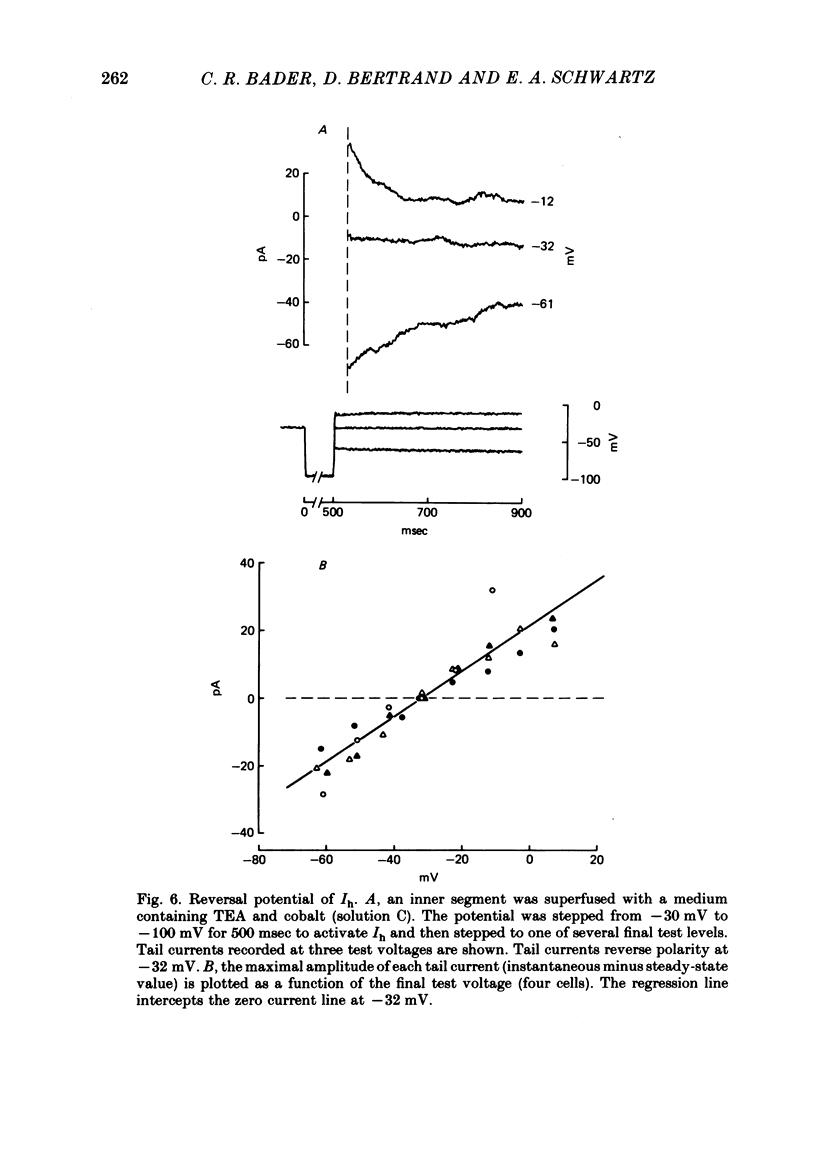
2. Extracellular caesium blocked a current activated by hyperpolarization from -30 mV. Changing external sodium and potassium concentrations altered the value of the reversal potential in a manner consistent with the current being carried equally by both ions.
3. Extracellular tetraethylammonium (TEA) blocked a current activated by depolarization from -70 mV. In normal medium this current had a reversal potential of -72 mV. Changing the external potassium concentration altered the value of the reversal potential in a manner consistent with the current being carried predominantly by potassium.
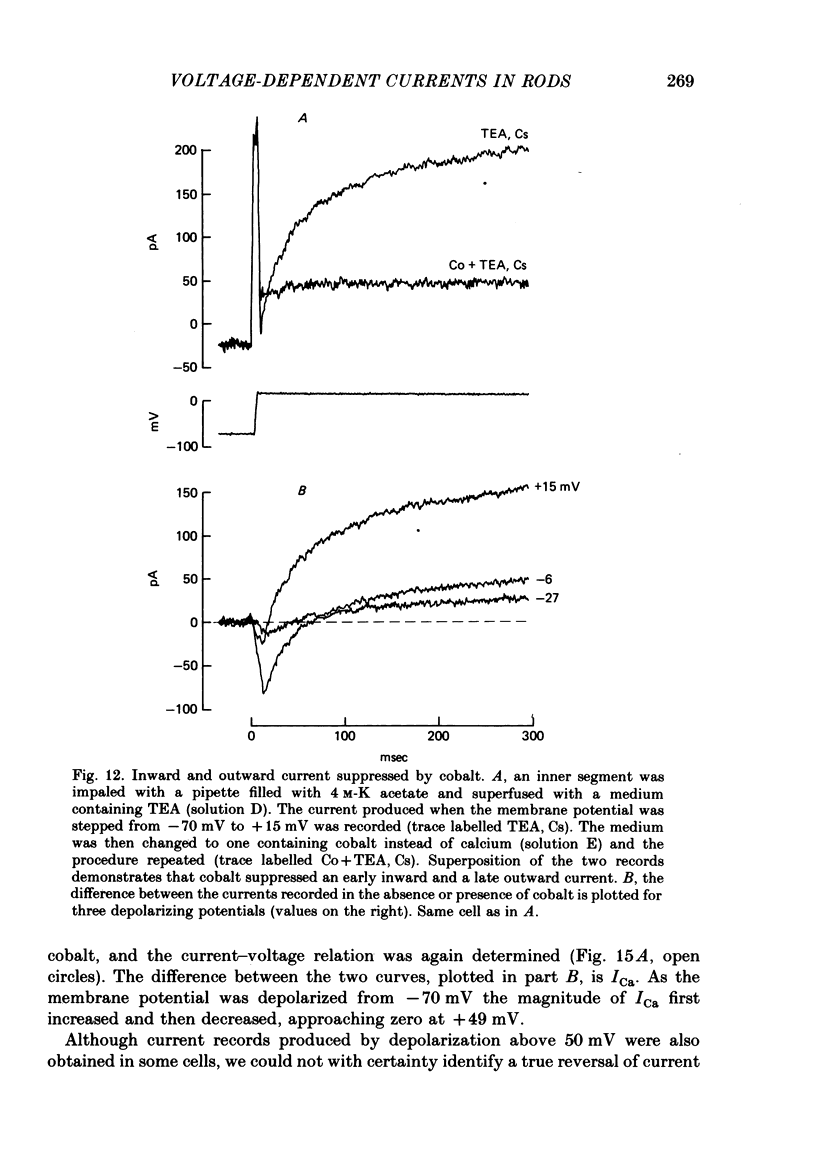
4. Extracellular cobalt blocked a current activated by depolarization that had an initial inward and a later outward component.
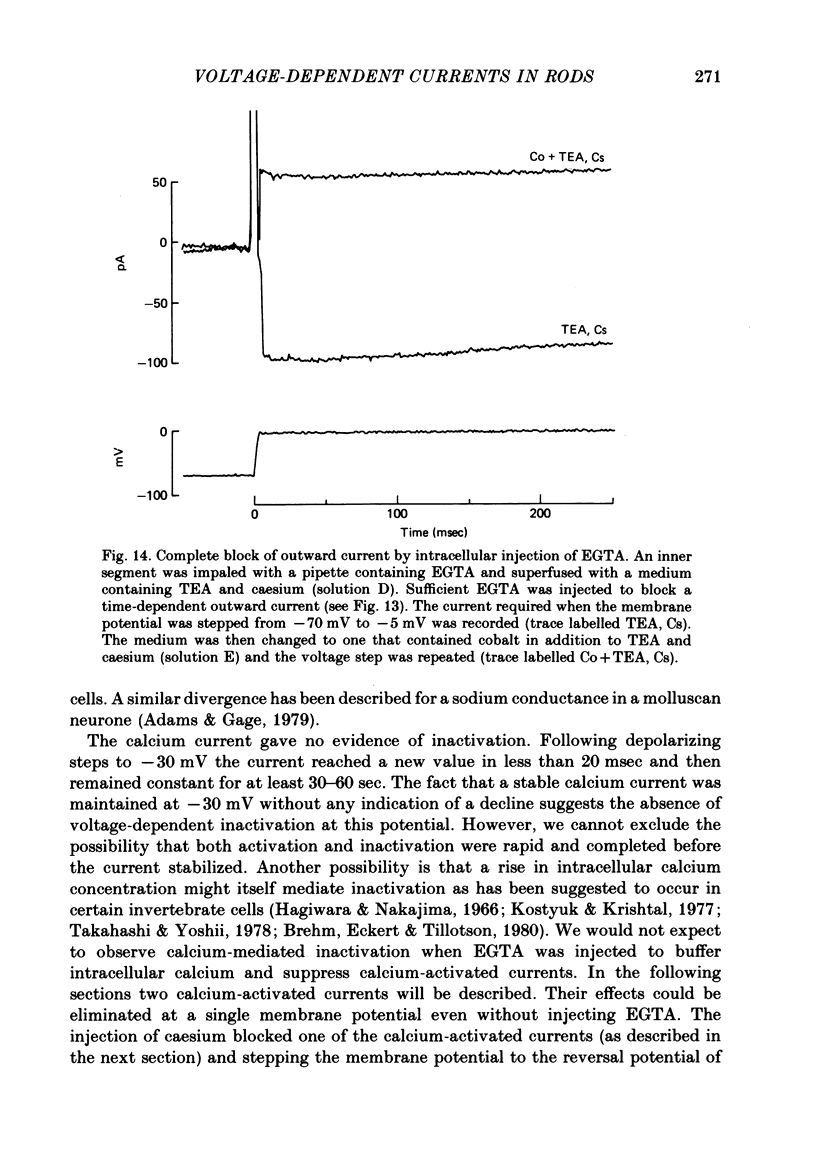
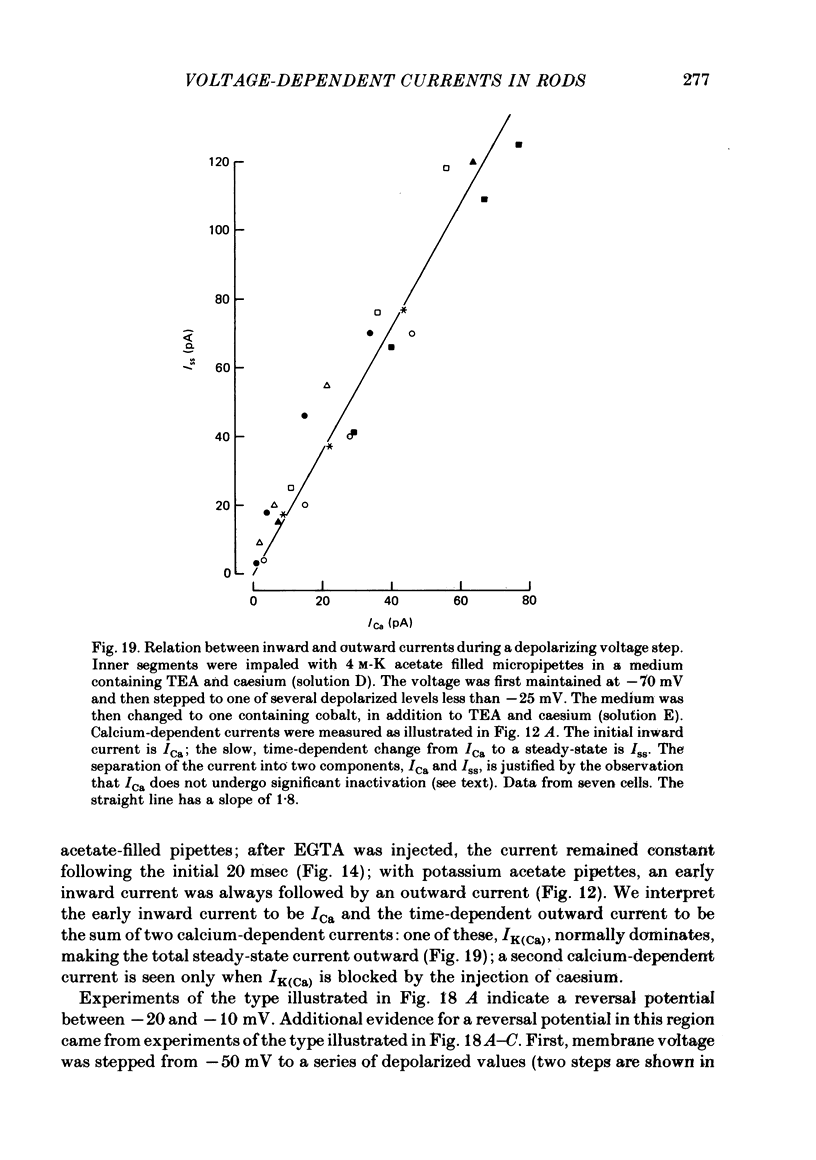
5. After EGTA was injected into an inner segment the outward component was suppressed. Cobalt then blocked an inward current. This current is believed to be carried predominantly by calcium. The conductance increased with depolarization from -45 mV and reached a maximum at approximately 0 mV. Following a step of depolarization the current activated rapidly (< 20 msec) and then remained constant for at least several seconds without evidence of inactivation.
6. Injecting caesium into an inner segment eliminated a calcium-activated outward current believed to be carried by potassium ions.
7. After the injection of caesium there remained another calcium-activated current with a reversal potential of -17 mV. Changing extracellular chloride concentration altered the value of the reversal potential in a manner consistent with chloride carrying at least 70% of the current. Another anion may carry the balance.
8. When the five currents mentioned in items 2, 3, 5, 6 and 7 were blocked, the membrane resistance between -90 and -25 mV was linear, time-independent, and had a high value (2·1 GΩ).
9. The five identified currents can all be activated in the physiological range of voltage in which salamander rods normally operate.
Full text
PDF




























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., TAYLOR R. E. Leakage current rectification in the squid giant axon. Nature. 1961 Jun 3;190:883–885. doi: 10.1038/190883a0. [DOI] [PubMed] [Google Scholar]
- Adams D. J., Gage P. W. Characteristics of sodium and calcium conductance changes produced by membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:143–161. doi: 10.1113/jphysiol.1979.sp012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Kunze D., Neild T. O. Chloride distribution in Aplysia neurones. J Physiol. 1976 Apr;256(2):441–464. doi: 10.1113/jphysiol.1976.sp011332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Bader C. R., MacLeish P. R., Schwartz E. A. Responses to light of solitary rod photoreceptors isolated from tiger salamander retina. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3507–3511. doi: 10.1073/pnas.75.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R., Tillotson D. Calcium-mediated inactivation of calcium current in Paramecium. J Physiol. 1980 Sep;306:193–203. doi: 10.1113/jphysiol.1980.sp013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busis N. A., Weight F. F. Spike after-hyperpolarisation of a sympathetic neurone is calcium sensitive and is potentiated by theophylline. Nature. 1976 Sep 30;263(5576):434–436. doi: 10.1038/263434a0. [DOI] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Effects of changing external potassium and chloride concentrations on the photoresponses of Bufo bufo rods. J Physiol. 1980 Oct;307:529–551. doi: 10.1113/jphysiol.1980.sp013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. O., Alving B. O. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol. 1968 Jul;52(1):1–21. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Bennett M. V. Calcium-activated conductance in skate electroreceptors: voltage clamp experiments. J Gen Physiol. 1977 Feb;69(2):145–182. doi: 10.1085/jgp.69.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Geduldig D. Electrogenic sodium pump in squid giant axon. Science. 1973 Mar 30;179(4080):1326–1328. doi: 10.1126/science.179.4080.1326. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick E., Miller R. F. Light-evoked potassium activity in mudpuppy retina: its relationship to the b-wave of the electroretinogram. Brain Res. 1978 Oct 13;154(2):388–394. doi: 10.1016/0006-8993(78)90711-4. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Gerschenfeld H. M., Quandt F. N. Calcium spikes in toad rods. J Physiol. 1980 Jun;303:495–513. doi: 10.1113/jphysiol.1980.sp013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Gerschenfeld H. M. Calcium-dependent regenerative responses in rods. Nature. 1977 Oct 20;269(5630):707–710. doi: 10.1038/269707a0. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Synaptic transmission from photoreceptors to bipolar and horizontal cells in the carp retina. Cold Spring Harb Symp Quant Biol. 1976;40:537–546. doi: 10.1101/sqb.1976.040.01.050. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium current and the activation of a slow potassium conductance in voltage-clamped mouse neuroblastoma cells. J Physiol. 1979 Jul;292:307–323. doi: 10.1113/jphysiol.1979.sp012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Lague P. H., Potter D. D., Furshpan E. J. Studies on rat sympathetic neurons developing in cell culture. I. Growth characteristics and electrophysiological properties. Dev Biol. 1978 Dec;67(2):384–403. doi: 10.1016/0012-1606(78)90208-7. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Green D. G. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol. 1976 Sep;39(5):1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Russell J. M. ATP-Dependent chloride influx into internally dialyzed squid giant axons. J Membr Biol. 1976 Sep 17;28(4):335–349. doi: 10.1007/BF01869704. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. First events in vision: the generation of responses in vertebrate rods. J Cell Biol. 1981 Aug;90(2):271–278. doi: 10.1083/jcb.90.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. II. On the basis of the ionic mechanism. Vision Res. 1969 Dec;9(12):1443–1451. doi: 10.1016/0042-6989(69)90060-1. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J., Goldin S. M. Reconstitution of active ion transport by the sodium and potassium ion-stimulated adenosine triphosphatase from canine brain. J Biol Chem. 1975 May 25;250(10):4022–4024. [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Effects of internal free calcium upon the sodium and calcium channels in the tunicate egg analysed by the internal perfusion technique. J Physiol. 1978 Jun;279:519–549. doi: 10.1113/jphysiol.1978.sp012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Tillotson D., Horn R. Inactivation without facilitation of calcium conductance in caesium-loaded neurones of Aplysia. Nature. 1978 May 25;273(5660):312–314. doi: 10.1038/273312a0. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov I. U. Izuchenie sinapticheskoi peredachi mezhdu fotoretseptorom i gorizontal'noi kletkoi pri pomoshchi élektricheskikh razdrazhenii setchatki. Biofizika. 1968 Sep-Oct;13(5):809–817. [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Time- and voltage-dependent ionic components of the rod response. J Physiol. 1979 Sep;294:613–626. doi: 10.1113/jphysiol.1979.sp012949. [DOI] [PMC free article] [PubMed] [Google Scholar]



