Abstract
Purpose
Tumor-derived exosomes (TEX) have been proposed as a new kind of cancer vaccine; however, their in vivo antitumor effects are not satisfactory. In order to further improve the efficacy of vaccination with TEX, we investigated whether interleukin-2 (IL-2) genetic modification of tumor cells can make IL-2 presence in the exosomes, thus increasing antitumor effects of the TEX.
Methods
E.G7-OVA tumor cells expressing Ovalbumin (OVA) as a tumor model antigen were used to prepare TEX by serial centrifugation and sucrose gradients ultracentrifugation. To demonstrate their antitumor effects, IL-2-containing exosomes (Exo/IL-2) were injected subcutaneously into C57BL/C mice: either bearing tumor or followed by tumor inoculation.
Results
We found IL-2 within those exosomes as detected by both ELISA and Western blot. Vaccination with these Exo/IL-2 could induce antigen-specific Th1-polarized immune response and Cytotoxic T lymphocytes (CTL) more efficiently, resulting in more significant inhibition of tumor growth. CD8+ T cells are the main effector cells, however, CD4+ T cells, and NK cells are also involved in the induction of antitumor response of this approach.
Conclusions
Our results demonstrate that IL-2 genetic modification of tumor cells can make the TEX contain IL-2 with the increased antitumor effects, representing a promising way of exosome-based tumor vaccine.
Keywords: Immunotherapy, Exosomes, Interleukin-2, CTL, Cancer vaccine
Introduction
Exosomes are small membrane vesicles secreted by many types of cells. Both dendritic cell (DC)-derived exosomes and tumor-derived exosomes (TEX) have been reported to have potential therapeutic efficacy as cancer vaccine (Thery et al. 2002a, b; Taieb et al. 2005; Zitvogel et al. 1998; Wolfers et al. 2001). DC-derived exosomes, either indirectly loaded with tumor antigen peptide (DC pulsed with tumor antigen peptide followed by the isolation of exosomes) or directly loaded (exosomes mixed with antigen peptide) have been shown to be able to activate T cell immune response, either in patients or in vitro (Morse et al. 2005; Escudier et al. 2005; Hsu et al. 2003). However, DC preparation in vitro is time-consuming and expensive, and difficult to handle for scale up. TEX is another source of exosomes, which contain different pattern of proteins, including Major histocompatibility complex (MHC) class I molecules, shared tumor antigen, HSPs, etc. (Thery et al. 2002a, b; Wolfers et al. 2001; Xiu and Cao 2004; Xiu et al. 2004; Dai et al. 2005). It was reported that TEXs could transfer tumor-shared antigens to DC, leading to crossly prime Cytotoxic T lymphocytes (CTL) response (Wolfers et al. 2001). The problem here is that, the immunogenicity of TEXs is limited, and antitumor effects of TEXs require the presence of mature DC. Therefore, new ways need to be found to enhance the immunogenecity of TEXs.
Interleukin 2 (IL-2) serves as an important growth and activation factor for CTL, macrophages, NK cells, and B lymphocytes. Given alone at high doses, IL-2 produces significant clinical responses in some tumors. It is the paradigm of immunological manipulations to produce definite tumor regression in the cases of advanced renal cell carcinoma and metastatic melanoma (Atkins et al. 2004; Eklund and Kuzel 2005). IL-2 gene-modified tumor cell-based vaccine also has demonstrated significant antitumor activity (Li et al. 2002; Cao et al. 1998). Furthermore, IL-2 also was used as vaccine adjuvant, and now several trials of combination of IL-2 with tumor vaccines are being tested (Overwijk et al. 2000; Shimizu et al. 1999). A very low dose of IL-2 regimen resulted in prolonged persistence of transferred T cells in melanoma patients and augmented the antitumor effects with minimal toxicity. Therefore, IL-2 may be a good adjuvant candidate for exosome-based immunotherapy. Chaput (Chaput et al. 2004) and his colleagues found that there were no enhancing effects when IL-2 simply mixed with exosomes. So, another way to integrate IL-2 with exosomes needs to be found.
Previous studies using liposome-capsulated IL-2 have shown that liposome delivery increased the antitumor activity of IL-2 through controlled releasing when injected into tumor site (Johnston et al. 2005; Cao et al. 1999a, b). Like liposome, exosomes are very small vesicles, with rich lipid in their membrane and therefore, we want to know if IL-2 can be contained in the exosomes derived from IL-2 gene-modified tumor cells. Here, we transfected the E.G7-OVA tumor cells with IL-2 gene, and isolated the exosomes from the culture supernatants of those IL-2 gene transfected E.G7-OVA tumor cells, which express Ovalbumin (OVA) as tumor model antigen. We found that vaccination with these IL-2-containing exosomes (Exo/IL-2) could induce antigen-specific Th1-polarized immune response and CTL more efficiently, resulting in more significant inhibition of tumor growth. Importantly, vaccination with these Exo/IL-2 could induce regression of the pre-established tumor significantly. Therefore, this method may represent a new way of exosome–based immunotherapy of cancer.
Materials and methods
Reagents
Mouse IL-2 and Interferin-γ (IFN-γ) ELISA Kit were purchased from Bender Med Systems, San Bruno, CA, USA. 3H thymidine was purchased from Amersham Pharmacia Biotech, Freiburg, Germany. Recombinant murine IL-2, G418, ConA, Mitomycin C, and OVA were purchased from Sigma–Aldrich, St. Louis, MO, USA. IFN-γ ELISPOT Kit was purchased from R&D system (Minneapolis, MN, USA). OVA257-264 and VSV324–332 were synthesized by GL Biochem Ltd. Shanghai, China. Chemiluminescence reagent was obtained from Pierce Biotechnology, Rockford, USA. LDH-cytotoxicity Assay Kit was purchased from BioVision, Mountain View, CA, USA.
Rabbit anti-heat shock protein 70 (HSP70), rabbit anti-heat shock protein 60 (HSP60) polyclonal antibody, rabbit anti-mouse interleukin-2 (IL-2) polyclonal antibody and rabbit anti-ovalbulin (OVA) polyclonal antibody were purchased from Chemicon International, Temecula, CA, USA. Mouse anti-HSC 70 monoclonal antibody (B6) was purchased from Santa Cruz Biotech, CA, USA. Goat anti-rabbit IgG Horseradish peroxidase conjugated affinity purified antibody and Goat anti-rabbit IgG Horseradish peroxidase conjugated affinity purified antibody were purchased from Chemicon International, Temecula, CA, USA.
Animals and cell lines
Six to eight-week-old female C57BL/6 mice were obtained from Joint Ventures Sipper BK Experimental Animal, Shanghai, China, and housed in specific pathogen free conditions. EL-4 is a thymoma cell line derived from C57BL/6 mouse; E.G7-OVA cells (OVA-expressing EL-4 cells) were kindly provided by Dr. E. Gilboa (Duke University, Durham, NC). E.G7-OVA and EL-4 were grown in DMEM supplemented with 10% heat-inactivated FBS (Hyclone, Logan, UT), penicillin (100 u/ml), streptomycin (100 mg/ml), and G 418 (400 μg/ml). P815 cell line and YAC-1 cell line were obtained from ATCC, Manassas, VA, and were maintained according to ATCC instructions.
Preparation of tumor-derived exosomes
E.G7-OVA tumor cells were transfected by recombinant adenovirus encoding mouse IL-2 or LacZ (AdmIL-2 and AdLacZ were kindly provided by Dr. H. Hamada at Sapporo Medical University, Sapporo, Japan). Exosomes were purified from the culture supernatants of E.G7-OVA tumor cells with or without genetic modification by serial centrifugation and sucrose gradients ultracentrifugation as described previously by us (Xiu et al. 2004). Briefly, E.G7-OVA tumor cells were infected with AdmIL-2 or AdLacZ at a multiplicity of infection (MOI) of 100:1. About 2 h later, the cells were washed three times with PBS and then supplemented with complete medium. After 48 h, the culture supernatants were collected and the exosomes were isolated by sequential centrifugation (4°C) at 800g for 10 min, 1,200g for 30 min, 10,000g for 30 min. After concentrated by ultrafiltration through a 500 kDa MWCO hollow fiber membrane (Millipore, Bedford, MA, USA), the post-ultrafiltered supernatant was pelleted at 100,000g for 1 h. To further purify exosomes, the pellet was resuspended in 0.25 M sucrose and floated into a discontinuous density cushion containing of 20 mM Tris/30%sucrose/45%sucrose (PH 7.2) at 100,000g for 2 h in a SW-32 swinging bucket rotor (Beckman, Optima L-80XP). Such exosomes prepared from IL-2 gene-modified tumor cells were regarded as Exo/IL-2. The exosomes from E.G7-OVA cells transfected with AdLacZ or normal E.G7-OVA tumor cells were named as Exo/LacZ and Exo correspondingly. The protein concentrations of exosomes were measured by Bradford assay (Pierce Biotechnology, Rockford, USA). About 500 μg of exosomes was usually obtained from 1 × 109 E.G7-OVA tumor cells cultured for 48 h (5 × 105 cells/ml).
Electron microscopy (EM)
For EM observation, exosome pellets were fixed in 4% paraformaldehyde. The pellets were then loaded onto electro-microscopy grids coated with formvar carbon, followed by contrasted and embedded in a mixture of uranyl acetate and methylcellulose. Sections were observed by a Philips Tecnai-10 transmission electron microscope operating at 80 kV (Phillips Electronic Instruments, Mahway, NJ). Exosome size was measured by the scale bar.
Western blot analysis of exosomes
Western blot was preformed as described previously (Wan et al. 2005). Briefly, exosomal or cellular lysate proteins were first separated by 10% SDS-PAGE gel, and transferred to polyvinylidene difluoride membrane. The membranes were probed with primary antibodies according to recommended dilutions, followed by secondary horse radish peroxidase coupled antibodies and enhanced chemiluminescence revelation (Pierce Biotech, Rockford, France), finally viewed by gel documentation system UVP, c-80 (UVP Inc., Upland, CA, USA). The primary antibodies used here were anti-HSP70, anti-HSC70, anti-HSP60, anti-IL-2, and anti-OVA antibodies.
Immunizations and tumor challenge
To immunize the mice, 10 μg per 100 μl of Exo/IL-2, 10 μg per 100 μl of Exo, 10 μg per 100 μl of Exo/LacZ, and 10 μg exosomes mixed with 75 pg IL-2 in 100 μl volume (named Exo+IL-2), or 100 μl PBS were injected subcutaneously in the left flank and then boosted twice with a 7-days interval. About 7 days after the last immunization, the immunized mice were dedicated to experiments of proliferation test and cytokine secretion assay of T cells, ELISPOT, CTL, and NK assay. Or, the immunized mice were challenged subcutaneously with 3 × 105 E.G7-OVA tumor cells (10 times more than the minimal tumorigenic doses) in the opposite flank. Each group contained eight mice. Tumor growth was evaluated by measuring the diameters of tumor with a caliper every 2 days and recorded as the average of two perpendicular diameter measurements. Mice were killed when their tumor size reached 30 mm in diameter. Survival following tumor challenge also was recorded.
Proliferation and cytokine secretion assay
About 7 days after the last immunization, the splenocytes from immunized mice were isolated and depleted of red blood cells with lysis buffer (0.15 M NH4Cl, 1 M KHCO3, 0.1 mM Na2EDTA), and then transferred to nylon hair column. After 1 h incubation at 37°C, the T cells were enriched and the concentration of the T cells was adjusted to 1 × 106/ml. Purified T cells (1 × 105) were co-cultured with inactivated E.G7-OVA tumor cells (50 μg/ml of mitomycin C for 45 min) (2 × 104) for 7 days in a 5% CO2 incubator at 37°C. For T-cell proliferation test, 1 μCi of 3HTdR was added to each well 16 h prior to the end of the culture; then cells were harvested and 3HTdR incorporation was assayed by liquid scintillation counting. For cytokine secretion measurement by ELISA, supernatants were collected at 24 h culture (for IL-2 assay) and 48 h culture (for IFN-γ assay).
ELISPOT assay
Splenocytes were isolated from the immunized mice 7 days after the last immunization, stimulated at 1 × 106 per 100 μl with 10 μg/ml of OVA, OVA257-264, VSV324-332, and 5 μg/ml of ConA for 72 h, then transferred to IFN-γ ELISPOT plates and assayed as described previously (Wu et al. 2005). The results were evaluated by calculating the number of spot-forming cells (SFCs) per number of cells added into the well.
Cytotoxic assay of CTL and NK cells
Splenic lymphocytes were isolated from the tumor-bearing mice 1 week after the last immunization. For detection of NK cell cytotoxicity against YAC-1 cells, lymphocytes were directly used as effector cells. For measuring of CTL activity, lymphocytes were cultured at 2 × 106/ml and re-stimulated with 4 × 105/ml inactivated E.G7-OVA tumor cells (by 50 μg/ml of mitomycin for 45 min) for 7 days in the presence of recombinant mouse IL-2 (100 μg/ml) and then collected as CTL effector cells. E.G7-OVA tumor cells were used as OVA-specific target cells, EL-4 cells, and P815 as control target cells. Cytotoxic activity was tested by LDH assay as described previously (Xia et al. 2002) at the ratios of 1:20, 1:40, and 1:80 (Effector/Target) in triplicate. The corresponding target cells (1 × 104) were mixed with different ratios of effector cells for 6 h, in a humidified incubator at 37°C. The cytotoxicity was calculated as follows: percentage of specific lysis = 100 × (experimental−effector spontaneous-target spontaneous)/(target maximum−target spontaneous).
In vivo depletion of specific cell subsets
C57BL/6 mice were immunized three times with Exo/IL-2. About 1 week after the last immunization, mice were challenged subcutaneously with 3 × 105 E.G7-OVA tumor cells. For selective depletion, mice were injected intraperitoneally with 100 μg of GK1.5 (anti-CD4), 2.43 (anti-CD8), or PK136 (anti-NK1.1), respectively. Antibodies or normal rat IgG were injected at 4 days before immunization (induction phase) or inoculation of tumor cells (effector phase). Another three injections of antibodies were performed with a 2-day interval. Depletion of T cell subsets and NK cells was confirmed by flow cytometry, showing >90% specific depletion in splenocytes. The tumor size and survival were monitored (Cao et al. 1998).
Immunotherapy of the established E.G7-OVA tumor
For immunotherapy of established tumor, models of tumor-bearing mice were established by subcutaneous inoculation of 3 × 105 E.G7-OVA tumor cells. About 3 days after tumor inoculation, 10 μg of Exo/IL-2, 10 μg of Exo, 10 μg of Exo/LacZ, 10 μg of Exo+IL-2, or 100 μl PBS were injected subcutaneously into the flank region of the tumor-bearing mice. The identical treatment was repeated three times every other day. Each group contained eight mice. Tumor growth was monitored as described in Immunizations and Tumor Challenge.
Statistical Analysis
Statistical analysis was performed using Student’s t-test, except of long-rank test for survival analysis. The statistical significance was determined at P < 0.05.
Results
Identification of exosomes derived IL-2 gene-modified tumor cells
Up to now, exosomes can be identified by electron microscope, protein composition pattern and preparation method. Through serial centrifugation and sucrose gradients ultracentrifugation, we isolated and purified the exosomes from the E.G7-OVA tumor cells transfected with AdmIL-2; then viewed them by EM. As shown in Fig. 1, the exosomes have characteristic of ‘saucer’ or round morphology, limited by a bi-layer membrane and their diameters are between 40 and 100 nm. We also prepared exosomes from E.G7-OVA tumor cells without IL-2 gene transfection and E.G7-OVA tumor cells transfected with AdLacZ, respectively, and visualized them under EM (only the later shown here). We found no significant differences in morphology and size among those exosomes.
Fig. 1.
Identification of tumor-derived exosomes. The exosomes were isolated and purified from E.G7-OVA tumor cells transfected with IL-2 gene (Exo/IL-2) or from E.G7-OVA tumor cells without IL-2 gene transfection, and then visualized under EM. They are saucer-like vesicles limited by bi-layer membrane with sizes between 40 and 100 nm Magnification: ×30,000. Bars = 100 nm (a), Exo/IL-2 (b), conventional exosomes (c), Western blot analysis of protein compositions of exosomes. Equal amount of exosomal protein derived from E.G7-OVA tumor cell with or without IL-2 gene transfection or E.G7-OVA tumor cell lysate were separated on 10% SDS-PAGE gel and the desired proteins were probed with corresponding antibodies. The primary antibodies used were anti-HSP70, anti-HSC70, anti-HSP60, anti-IL-2, and anti-OVA antibodies
To testify whether the IL-2 gene transfection of tumor cells can influence the protein components of the TEX, we compared the protein pattern of Exo/IL-2, Exo/LacZ, and Exo by Western blot. As shown in Fig. 2 Exo/IL-2 contained IL-2, whereas there was no IL-2 detectable in the exosomes derived from E.G7-OVA tumor cells (Exo) or E.G7-OVA tumor cells transfected with AdLacZ (Exo/LacZ). The amount of IL-2 in EXo/IL-2 was further quantified by ELISA assay and the amount of IL-2 in 100 μg of Exo/IL-2 was 750 pg. Those results showed that IL-2 could be sorted into exosomes, through unknown mechanisms, when the tumor cells were transfected with IL-2 gene. Interestingly, we also found the Exo/IL-2 richly contained several important proteins, including OVA, HSC70, HSP70, and HSP60, which are important for the induction of antitumor response by cancer vaccine, but there was no obvious difference in quantity of these proteins among Exo/IL-2, Exo/LacZ, and Exo.
Fig. 2.
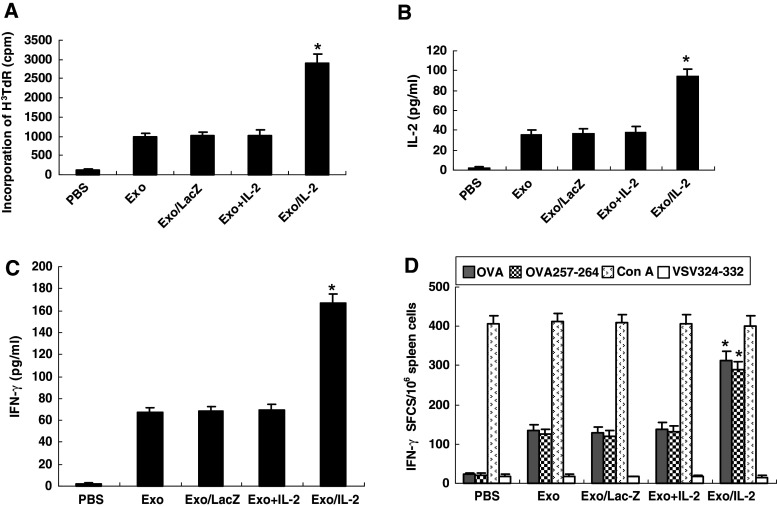
Augmentation of T-cell proliferation and Th1 type cytokine secretion by immunization with IL-2-containing tumor-derived exosomes (Exo/IL-2). Splenic T cells were purified from the mice immunized with Exo/IL-2 7 days after last immunization. Then T cells were co-cultured with inactivated E.G7-OVA tumor cells for 5 days. T cell proliferation was evaluated by the method of 3H thymidine incorporation. Supernatants were collected after 24 h co-culture (for IL-2 assay) or 48 h co-culture (for IFN-γ assay), cytokine levels were measured by ELISA. a Lymphocyte proliferation. Columns, mean [3H]TdR incorporation; bars, SE; *P < 0.01, for [3H]TdR incorporation of T cells from Exo/IL-2 immunized mice compared with other groups. b IL-2 production, *P < 0.01, for IL-2 concentration secreted by splenic T cells from Exo/IL-2 immunized mice compared with other groups. c IFN-γ production, *P < 0.01, for IFN-γ concentration secreted by splenic T cells from Exo/IL-2 immunized mice compared with other groups. d IFN-γ ELISPOT assay. The splenocytes from the immunized mice were restimulated with OVA, OVA257-264 peptide, Con A, or unrelated control peptide VSV324–332, respectively. ELISPOT results were expressed as the number of IFN-γ–positive spot-forming cells (SFCs)/106 splenocytes. Columns, mean SFCs per 106 splenocytes; bars, SE; *P < 0.01, for the number of IFN-γ–positive SFCs per 106 splenocytes from Exo/IL-2 immunized mice compared with other groups, indicating that increased induction of OVA-specific Th1 response by immunization with Exo/IL-2
Augmentation of antigen-specific Th1 response by immunization with IL-2-containing tumor-derived exosomes
To determine whether Exo/IL-2 immunization could induce tumor-specific cellular immune responses more efficiently, we measured the proliferation of T cells from the mice immunized with Exo/IL-2, Exo, Exo/LacZ, Exo+IL-2, or injected with PBS, respectively, in the mixed lymphocyte-tumor reaction (MLTR) as shown in Fig. 2a. Immunization with Exo+IL-2, Exo or Exo/Lac-Z alone could mount proliferating reaction of T cells more significantly compared with PBS group, respectively. However, the most significant T cell proliferation was observed from T cells of the mice immunized with Exo/IL-2 as compared with other four groups (P < 0.01). Then, we measured the cytokine secretion of the lymphocytes stimulated with inactivated E.G7-OVA tumor cells. As shown in Fig. 2b, c, the splenocytes from mice immunized with Exo/IL-2, secreted the highest levels of IL-2 and IFN-γ as compared to those from other group mice (P < 0.01).
To examine the IFN-γ secretion in single cell level and antigen-specific model, we detected the IFN-γ production of the lymphocytes stimulated at 1 × 106 per 100 μl with 10 μg/ml of OVA, OVA257-264, 5 μg/ml of ConA and VSV324-332, by ELISPOT assay. The results in Fig. 2d showed that the splenocytes from mice immunized with Exo/IL-2 had more IFN-γ–secreting cells as compared to that from other groups (P < 0.01). The splenocytes from mice immunized with Exo+IL-2, Exo, and Exo/LacZ also generated more IFN-γ–secreting cells than PBS control; however, there was no significant difference among these groups. Taken together, these results indicated that immunization with Exo/IL-2 could induce antigen-specific Th1 immune response more significantly than immunization with conventional exosomes, suggesting their application in vivo might have potent antitumor effects.
More potent protective and therapeutic antitumor effects by immunization with IL-2-containing tumor-derived exosomes
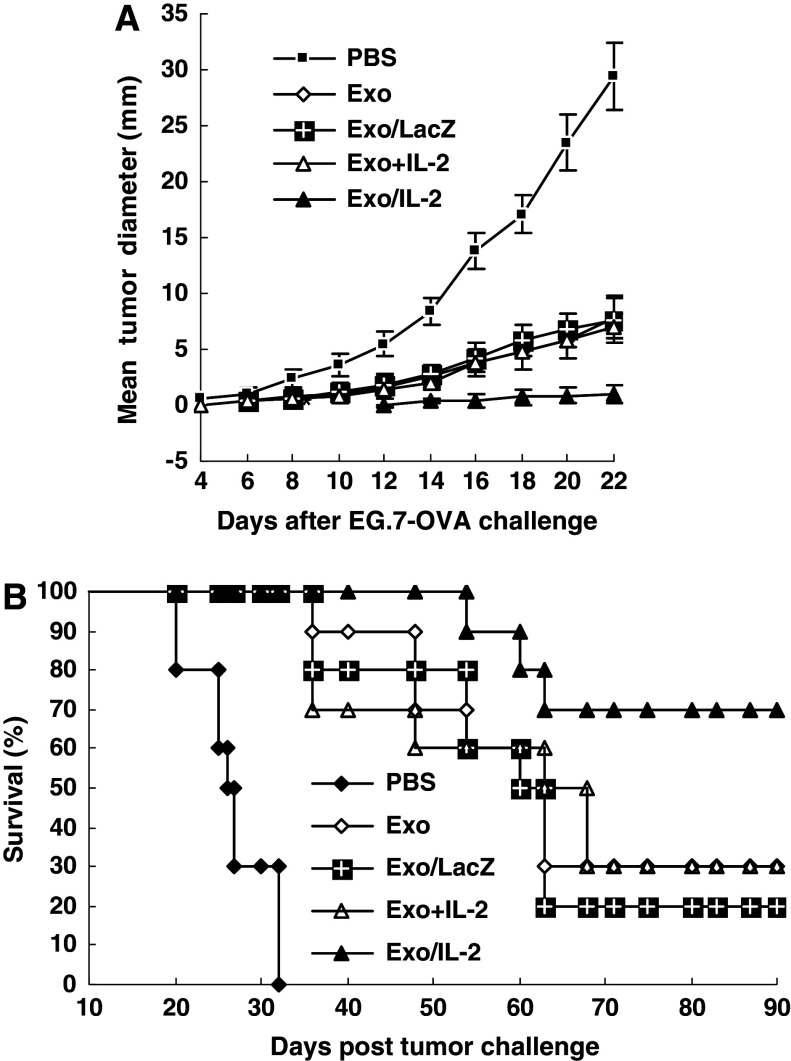
To explore whether Exo/IL-2 immunization could elicit more potent protective antitumor effects, mice were immunized with Exo/IL-2, Exo, Exo/LacZ, Exo+IL-2, or injected with PBS for three times, respectively, with a 7-day interval. About 7 days after the last immunization, the mice were challenged subcutaneously with 3 × 105 E.G7-OVA tumor cells in the opposite flank. The results illustrated in Fig. 3a showed that immunization with Exo, Exo/LacZ or Exo+IL-2 could inhibit tumor growth markedly as compared with PBS group (P < 0.01); however, immunization with Exo/IL-2 inhibited tumor growth most significantly (P < 0.01). No tumor was observed in Exo/IL-2 immunized mice until the 14th day, whereas the PBS control mice developed palpable tumors even at the sixth day. Accordingly, the survival of mice challenged with E.G7-OVA cells was prolonged most significantly by immunization with Exo/IL-2, while 70% of the mice remained tumor-free for 90 days (Fig. 3b). Thirty-two days after tumor challenge with E.G7-OVA cells, all the mice died in PBS group. About 30% of mice vaccinated with Exo+IL-2 or Exo, 20% of mice immunized with Exo/LacZ were tumor-free for 90 days, when compared with PBS (P < 0.01). Therefore, immunization with Exo+IL-2, Exo, or Exo/LacZ all can inhibit tumor growth significantly, and the most potent antitumor effect was observed after immunization with Exo/IL-2.
Fig. 3.
More potent protective antitumor immunity induced by immunization with Exo/IL-2. a Tumor growth of the immunized mice challenged with parental tumor cells. C57BL/6 mice were immunized subcutaneously with Exo/IL-2, Exo, Exo/LacZ, Exo+IL-2, or injected with PBS respectively for three times with 1 week interval. About 1 week after the last immunization, the mice were challenged subcutaneously with 3 × 105 EG7-OVA tumor cells. Tumor size was measured with calibers every 2 days. *P < 0.01, for tumor diameter from Exo/IL-2 immunized mice compared with that from other groups. b Survival of immunized mice after E.G7-OVA tumor challenge. Each group contained eight mice
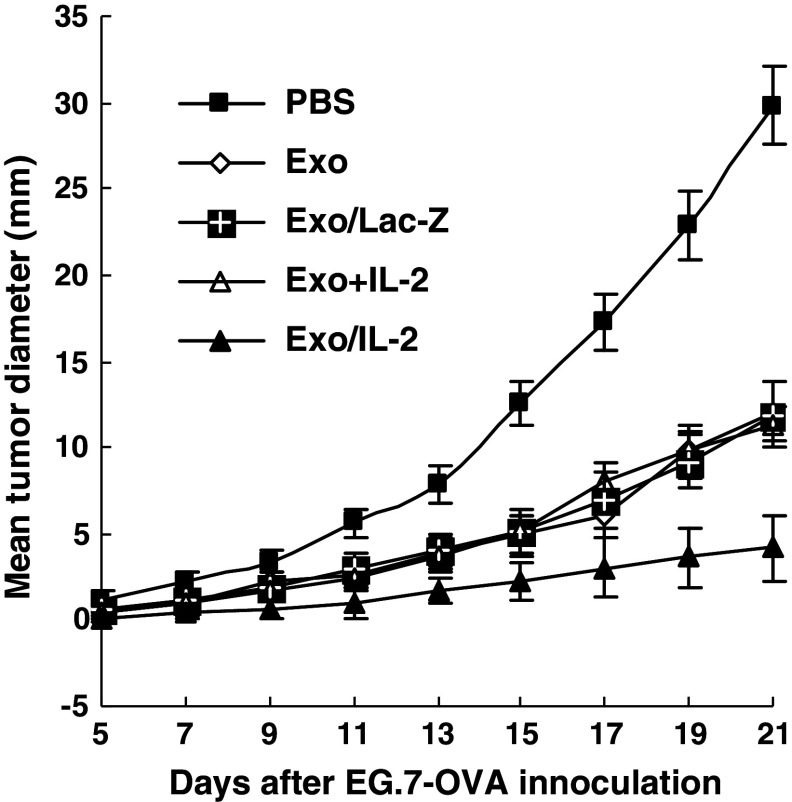
We went further to examine the therapeutic effect of Exo/IL-2 immunization on the established tumor. Immunization with Exo, Exo/LacZ or Exo+IL-2, could significantly inhibit tumor growth of the pre-established tumor-bearing mice as compared with PBS group (P < 0.01). However, the tumor growth of the pre-established tumor-bearing mice was most significantly inhibited by immunization with Exo/IL-2 (Fig. 4), suggesting the increased therapeutic efficacy of IL-2-containing TEX in treatment of cancer.
Fig. 4.
Regression of the pre-established tumor induced by immunization with Exo/IL-2. Mice were subcutaneously inoculated with 3 × 105 E.G7-OVA tumor cells. About 3 days later, the tumor-bearing mice were immunized subcutaneously with Exo/IL-2, Exo+IL-2, Exo, Exo/LacZ or injected with PBS, respectively, for three times with 1 week intervals. Sizes of tumor were measured with calibers at 3 days interval. Each group contained eight mice. Bars, SE; *P < 0.01, for the tumor volume of the mice immunized with Exo/IL-2 compared with that of other groups
More efficient induction of specific CTL by immunization with IL-2-containing tumor-derived exosomes
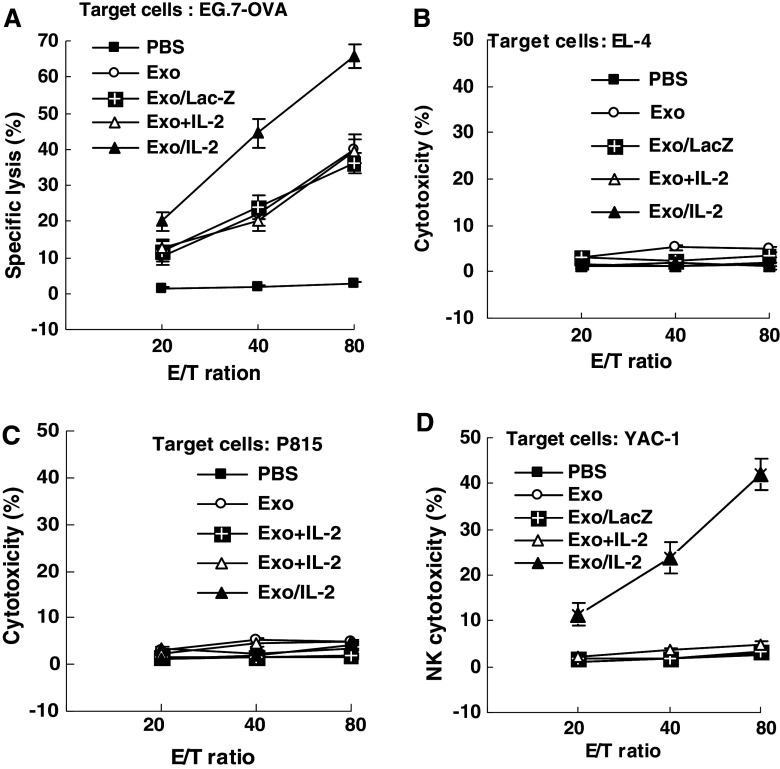
To illuminate mechanisms for the increased antitumor effect of Exo/IL-2 immunization, we observed the CTL response in the immunized mice. The results showed that highest CTL activity was induced in the Exo/IL-2 immunized mice as compared to those from other groups (P < 0.01). Immunization with Exo+IL-2, Exo or Exo/LacZ also induced certain level of E.G7-OVA specific CTL responses as compared with no CTL induction in mice injected with PBS (P < 0.01), but no significant difference among them. The CTLs induced by Exo/IL-2, and even by Exo+IL-2, Exo, and Exo/LacZ at different levels in the cytotoxicity against E.G7-OVA cells, were specific because they were not able to kill control target cells including EL-4 cells or P815 cells (Fig. 5a, b, c).
Fig. 5.
Increased induction of specific CTL and NK activity by immunization with Exo/IL-2. The splenocytes were purified from the mice immunized by Exo/IL-2 7 days after last immunization. Splenic T lymphocytes were re-stimulated with inactivated E.G7-OVA tumor cells in vitro for 7 days, then assayed for CTL cytotoxicity against the targets of E.G7-OVA (b) and EL-4 (c), respectively. The splenocytes without re-stimulation in vitro were used directly as NK effector cells against YAC-1 (d). Data are representative of three independent experiments
Enhanced NK activity in the mice immunized with IL-2-containing tumor-derived exosomes
The splenocytes from the mice immunized with various vaccines were directly used as NK effector cells for detection of cytotoxicity by LDH assay. As shown in Fig. 5d, Exo/IL-2 immunization could enhance the NK cell cytotoxicity more markedly than other groups (P < 0.01), whereas immunization with Exo+IL-2, Exo or Exo/LacZ was not able to enhance NK cytotoxicity as compared with PBS injection (P > 0.05), suggesting that the nonspecific immunity may also be involved in the antitumor responses of Exo/IL-2 vaccine.
Roles of T cells and NK cells in the antitumor immunity induced by Exo/IL-2 immunization
As observed above, immunization with Exo/IL-2 could activate Th1, CTL and NK cells. To further investigate the roles of CD4+T cells, CD8+T cells, and NK cells in the protective antitumor immunity induced by Exo/IL-2 immunization, the mice were depleted of CD4+T cells, CD8+T cells, or NK cells during immunization or during tumor challenge. Tumor development was observed after tumor challenge. As shown in Fig. 6, depletion of CD8+T cells during both immunization phase and effector phase all blocked induction of protective antitumor immunity by Exo/IL-2. Mice, depleted of CD4+T cells during immunization, failed to reject tumor challenge but could resist tumor challenge during challenge phase. The data suggested that CD8+T cells were the main effector cells of this approach, and CD4+T cells were required for helping induction of CD8+ dependent T-cell immunity in protective immunity but were not necessary in the effector phase. Mice, depleted of NK cells could resist tumor challenge to some degree, but tumor size was smaller than the mice depleted of CD8+T cells (P < 0.01), indicating that NK cells also participated in the antitumor immunity, but were less important than CD8+T cells. Our results demonstrated that CD8+T cells play critical role in the antitumor effect of Exo/IL-2 immunization, and CD4+, NK cells are also involved in the induction of antitumor response by Exo/IL-2 immunization.
Fig. 6.
Roles of lymphocyte subsets in the antitumor immunity induced by immunization with Exo/IL-2 C57BL/6 mice were immunized with Exo/IL-2 for three times with 1 week intervals, followed by challenge with 3 × 105 E.G7-OVA tumor cells 1 week after last immunization. About 4 days before first Exo/IL-2 immunization (a) or tumor challenge (b), the mice (eight per group) started to receive a total of four intraperitoneal injections (0.5 ml per injection) of ascites from hybridoma-bearing mice at intervals of 3 days. The antibodies used were GK1.5 (anti-CD4) and 2.43 (anti-CD8) McAbs, respectively. Normal rat IgG was used as control antibody. The tumor volume was monitored every other day
Discussion
Ideal cancer vaccine should be able to elicit CTL responses against a broad repertoire of tumor rejection antigens. However, this remains hampered by the lack of molecularly defined tumor antigen delivery or targeting systems. The source of tumor antigens currently used includes tumor cell lysates, irradiated tumor cells, apoptotic cells or recombinant proteins, etc. But all those vaccination cannot cross-prime CTL and surpass the MHC limitation. As a cell-free vaccine, TEX should be a new source of tumor antigens and may avoid those shortcomings associated with traditional cancer vaccines. But recent experimental results have shown that TEX alone had low efficacy of T-cell stimulation. Raposo demonstrated that the exosomes derived from EBV-transformed B cells could stimulate T cell, however, with low efficacy (Raposo et al. 1996). DEX do not directly activate CD4+ T cells except with the help of mature DC (Thery et al. 2002a, b). And, the results from clinical trials for treatment of tumor patients with DEX so far are not satisfactory (Morse et al. 2005; Escudier et al. 2005). So, the exosome-based immunotherapy needs to be modified to increase therapeutic efficacy. One way is to simply mix adjuvant with the exosomes, however, with low stability. We also tried to anchor target protein onto the surface of exosomes by protein transfer of fusion protein, leading to more potent antitumor effect of exosomes. In this study, we demonstrated that preparation of exosomes from cytokine gene-modified tumor cells is another way to promote anti-tumor effects of TEX.
Interleukin 2 is a cytokine with definite anti-tumor effects when used systemically. But the major limitations of systemic IL-2 administration are the severe toxicities and the lack of paracrine function. Therefore, local delivery of IL-2 appears to be a safer and more rational approach to IL-2-based cancer therapy. However, the notable drawback to local delivery of the IL-2 is the quick clearance and need for multiple closely spaced injections or many injections successively to maintain an anti-tumor response. One solution to this limitation is the application of gene transfer of IL-2 to provide local sustained release of IL-2 (Li et al. 2002; Cao et al. 1998). Another solution, is the usage of a carrier maintaining IL-2 to ‘secrete’ slowly in the tumor site when administrated peritumorially (Johnston et al. 2005; Cao et al. 1999a, b). Previous study failed to find that IL-2 had adjuvant effect when simply mixed with exosomes (Chaput et al. 2004), suggesting that IL-2 must be stably combined with exosomes in order to assert its effects with the help of exosomes. Herein, we prepared exosomes from IL-2 gene-modified E.G7-OVA tumor cells, and interestingly showed that IL-2 was within the exosomes and those exosomes exhibited much more potent anti-tumor effect than conventional TEX. It is a promising way to make the exosomes ‘express’ the target cytokine by gene transfection of their parental tumor cells.
As to the mechanisms of those exosomes to exhibit much more potent anti-tumor effect than conventional TEX, we proposed the following reasons: First, Exo/IL-2 can activate immune effector cells by directly stimulating DC. As we know, DCs are the most potent antigen-presenting cells, which can directly prime naïve T cells. Mature splenic DCs express IL-2 receptor (IL-2R) (Fukao and Koyasu 2000). IL-2 can act on DC during antigen presentation, thus contributing to the maturation process of DC, which makes DC highly efficient antigen presentation (Bykovskaja et al. 1998; Granucci et al. 2002). How did the IL-2 within exosomes activate DC? It might be that, Exo/IL-2 was captured by immature DC and then IL-2 was released from DC in an unknown manner (Morelli et al. 2004). The released IL-2 then interacted with DC by binding the IL-2 receptors on the surfaces of DC, T cells, and NK cells, triggering stronger anti-tumor effect in vivo. Therefore, IL-2 within exosomes may provide signal for maturation of DC and the activation of naive T cells and NK cell. In this way, IL-2 within exosomes is an adjuvant to enhance the antitumor effects of exosomes. Second, exosomes themselves contain many molecules by which exosomes assert their activities. Similar to DEX in their morphology, density and protein components, TEX are the source of shared tumor rejection antigens for CTL induction. In addition, Exo/IL-2 contains high levels of heat-shock proteins such as HSP70, HSP60, and HSC70. As molecular chaperones and potent adjuvant, HSPs have important functions that help antigenic peptide folding, transporting and are able to activate T cells (Wu et al. 2005; Massa et al. 2005). Those HSPs can bind tumor peptides, be internalized into DCs and then transfer the bound peptides onto MHC class I molecules, by which HSP activate DC and enhance their antigen presentation capability (Tsan and Gao 2004; Gastpar et al. 2005). All those suggested that the presences of HSC70, HSP70, and HSP60 in Exo/IL-2, could significantly enhance the immunogenicity of Exo/IL-2, therefore leading to effective induction of antigen specific T cell immune response. Third, Exo/IL-2 can stimulate the Th1-polarized immune response more significantly. The balance between Th1 and Th2 cells plays an important role in the anti-tumor immune response. Th1 cells, which secrete IL-2 and IFN-γ, are the main cells contributing to the tumor rejection (Shurin et al. 1999). Our results showed that Exo/IL-2 immunization, could significantly promote immune response toward Th1 polarization with increased IL-2 and IFN-γ production. Interestingly, our results also suggested that IL-2 did not synergize with Exo in the anti-tumor activity when simply mixed with Exo, so, prolonged and sustained delivery of IL-2 might contribute to the much more potent antitumor effect of exosomes derived from IL-2 gene-modified tumor cells.
In recent years, IL-2 has been attributed to the regulation of the Treg cells in tumor patients or tumor-bearing mice. However, IL-2 seems to be more important in contributing to the Treg in the later stages of immune response. Studies showed that part of CD4+T cells will switch to the CD4+CD25+T cells by the education of the tumor microenvironment, but that Treg failed to perform their suppressive effects, once the priming of CD8 T cells was initiated (Yu and Fu 2006). We conducted the anti-established tumor effects by using the exosomes for treatment 3 days after tumor inoculation. We think that 3 days still is the early or initial stage of the tumor. Therefore, we suggest that IL-2 should be applied to the tumor patients or experimental tumor model, in the early stage instead of the advanced stage.
In summary, to the best of our knowledge, we, for the first time demonstrate that exosomes derived from IL-2 gene-modified tumor cells contain IL-2, and immunization with these Exo/IL-2 can induce antigen-specific Th1-polarized immune response and CTL more efficiently, resulting in more significant inhibition of tumor growth. Importantly, vaccination with these Exo/IL-2 could induce regression of the pre-established tumor significantly. Therefore, the anti-tumor activity of exosomes can be enhanced by making exosomes display certain target cytokine. However, the mechanisms of how IL-2 is sorted into exosomes are not clear and need to be elucidated in the future.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (30328011, 30490240, 30121002) and the National Key Basic Research Program of China (2001CB510002).
Abbreviations
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cells
- DEX
DC-derived exosomes
- Exo
Conventional exosomes
- Exo/IL-2
IL-2-containing exosomes
- HSP
Heat shock protein
- IFN-γ
Interferin-γ
- IL-2
Interleukin 2
- MHC
Major histocompatibility complex
- OVA
Ovalbumin
- TEX
Tumor-derived exosomes
Footnotes
The first two authors contributed equally to this work.
References
- Atkins MB, Regan M, McDermott D (2004) Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res 10(18):6342S–6346S [DOI] [PubMed] [Google Scholar]
- Bykovskaja SN, Buffo MJ, Bunker M, Zhang H, Majors A, Herbert M, Lokshin A, Levitt ML, Jaja A, Scalise D, Kosiban D, Evans C, Marks S, Shogan J (1998) Interleukin-2-induces development of denditric cells from cord blood CD34+ cells. J Leukoc Biol 63:620–630 [DOI] [PubMed] [Google Scholar]
- Cao X, Zhang W, He L, Xie Z, Ma S, Tao Q, Yu Y, Hamada H, Wang J (1998) Lymphotactin gene-modified bone marrow dendritic cells act as more potent adjuvants for peptide delivery to induce specific antitumor immunity. J Immunol 161:6238–6244 [PubMed] [Google Scholar]
- Cao X, Zhang W, Wan T, Yu Y, Wang J (1999a) Enhanced antitumor immune responses of IL-2 gene-modified tumor vaccine by combination with IL-1 and low dose cyclophosphamide. J Exp Clin Cancer Res 18(2):173–179 [PubMed] [Google Scholar]
- Cao X, Wang Q, Ju D, Tao Q, Wang J (1999b) Efficient induction of local and systemic antitumor immune response by liposome-mediated intratumoral co-transfer of interleukin-2 gene and interleukin-6 gene. J Exp Clin Cancer Res 18(2):191–199 [PubMed] [Google Scholar]
- Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L (2004) Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG ODN adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol 172:2137–2146 [DOI] [PubMed] [Google Scholar]
- Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X (2005) More efficient induction of HLA-A*0201-restricted and CEA-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumour cells. Clin Cancer Res 11(20):7554–7563 [DOI] [PubMed] [Google Scholar]
- Eklund JW, Kuzel TM (2005) Interleukin-2 in the treatment of renal cell carcinoma and malignant melanoma. Cancer Treat Res 126:263–287 [DOI] [PubMed] [Google Scholar]
- Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med 3(1):10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Koyasu S (2000) Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur J Immunol 30:1453–1457 [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65(12):5238–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granucci F, Andrews DM, Degli-Esposti MA, Ricciardi-Castagnoli P (2002) IL-2 mediates adjuvant effect of dendritic cells. Trends Immunol 23:169–171 [DOI] [PubMed] [Google Scholar]
- Hsu DH, Paz P, Villaflor G, Rivas A, Mehta-Damani A, Angevin E, Zitvogel L, Le Pecq JB (2003) Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother 26:440–450 [DOI] [PubMed] [Google Scholar]
- Johnston D, Reynolds SR, Bystryn JC (2005) Interleukin-2/liposomes potentiate immune responses to a soluble protein cancer vaccine in mice. Cancer Immunol Immunother 54(9):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Ronson B, Guo M, Liu S, Bishop JS, Van Echo DA, O’Malley BW Jr (2002) Interleukin 2 gene transfer prevents NKG2D suppression and enhances antitumor efficacy in combination with cisplatin for head and neck squamous cell cancer. Cancer Res 62:4023–4028 [PubMed] [Google Scholar]
- Massa C, Melani C, Colombo MP (2005) Chaperon and adjuvant activity of hsp70: different natural killer requirement for cross-priming of chaperoned and bystander antigens. Cancer Res 65(17):7942–7949 [DOI] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW (2004) Endocytosis, intracellular sorting and processing of exosomes by dendritic cells. Blood 104:3257–3266 [DOI] [PubMed] [Google Scholar]
- Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3(1):9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Restifo NP (2000) The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am 6(Suppl 1):S76–S80 [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183(3):1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Fields RC, Giedlin M, Mule JJ (1999) Systemic administration of interleukin 2 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines. Proc Natl Acad Sci USA 96:2268–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT (1999) Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol 21(3):339–359 [DOI] [PubMed] [Google Scholar]
- Taieb J, Chaput N, Zitvogel L (2005) Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol 25(3):215–223 [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S (2002a) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579 [DOI] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S (2002b) Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3(12):1156–1162 [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B (2004) Cytokine function of heat shock proteins. Am J Physiol Cell Physiol 286(4):C739–C744 [DOI] [PubMed] [Google Scholar]
- Wan T, Zhou X, Chen G, An H, Chen T, Zhang W, Liu S, Jiang Y, Yang F, Wu Y, Cao X (2005) Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood 103(5):1747–1754 [DOI] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303 [DOI] [PubMed] [Google Scholar]
- Wu Y, Wan T, Zhou X, Wang B, Yang F, Li N, Chen G, Dai S, Liu S, Zhang M, Cao X (2005) Hsp70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine. Cancer Res 65(11):4947–4954 [DOI] [PubMed] [Google Scholar]
- Xia D, Zhang W, Zheng S, Wang J, Pan JP, Wang Q, Zhang LH, Hamada H, Cao X (2002) Lymphotactin cotransfection enhances the therapeutic efficacy of dendritic cells genetically modified with melanoma antigen gp100. Gene Ther 9(9):592–601 [DOI] [PubMed] [Google Scholar]
- Xiu F, Cao X (2004) Exosomes in the immune response and tolerance. J Microbiol Immunol 2(4):231–236 [Google Scholar]
- Xiu F, Yang Y, Cai Z, Wang J, Cao X (2004) Isolation and characterization of exosomes derived from tumor cells genetically expressing model antigen. J Microbiol Immunol 2(4):278–285 [Google Scholar]
- Yu P, Fu Y (2006) Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest 86(3):231–245 [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600 [DOI] [PubMed] [Google Scholar]