Abstract
Purpose
Quality of life (QoL) is frequently impaired in patients suffering from malignant disease. Disturbed metabolism of neurotransmitter serotonin might be crucially involved, and serotonin-precursor tryptophan is degraded during pro-inflammatory immune response. In this study, we compared QoL and fatigue self-rating scores of patients with various types of malignancy with tryptophan metabolic changes and immune activation status.
Methods
Venous blood was collected from 146 patients with gastrointestinal tumors (n = 43), hematological malignancy (n = 40), gynecological neoplasms (n = 26), lung cancer (n = 20) and from tumors of other localization (n = 17).
Results
QoL was significantly reduced in patients suffering from progressive tumor disease in comparison to stable or remitting disease, also feeling of fatigue was increased (both P < 0.001). Serum tryptophan concentrations were lower in patients with progressive disease (P < 0.01), and decreased tryptophan concentrations were related to decreased QoL (r s = 0.256, P < 0.01) and increased fatigue (r s = −0.179; P < 0.05). Concentrations of tryptophan and kynurenine and the kynurenine to tryptophan ratio were predictive for impaired QoL and increased fatigue in univariate regression analysis, in multivariate analysis higher ESR and neopterin concentration in combination with stage of disease predicted QoL deterioration.
Conclusions
Results suggest that immune-mediated tryptophan degradation may contribute to cancer-induced QoL deterioration.
Keywords: Tryptophan degradation, Cancer, Quality of life, Neopterin, Immune activation, Fatigue
Introduction
Quality of life (QoL) is often deteriorated in patients suffering from malignant disease in comparison to the normal population. In addition to other consequences of tumor progression like metastasis or tumor-cachexia, immune response was also suggested earlier to be involved in the impairment of QoL (Earlam et al. 1996). In patients suffering from colorectal liver metastasis, QoL was even shown to be related more closely with the extent of immune activation than with liver metastasis volume or the serum level of the tumor marker carcinoembryonic antigen (Allen-Mersh et al. 1998). However, the underlying mechanisms are only poorly understood. Fatigue is another frequent symptom in cancer patients, and it was also proposed to represent a result of chronic immune system activation (Kurzrock 2001; Bower et al. 2002). By contrast, a more recent study on patients with hematological malignancy failed to find such a relationship (Dimeo et al. 2004).
Signs of inflammation and immune activation are often found in patients with malignant disease, and higher concentrations of immune activation markers like neopterin have been shown to predict disease progression in patients with, e.g., gynecological cancer, hepatocellular and colon cancer (Lewenhaupt et al. 1986; Reibnegger et al. 1986, 1987; Weiss et al. 1993; Kronberger et al. 1995; Murr et al. 1999). Well in line with this data, increased concentrations of C-reactive protein (CRP) are also associated with worse outcome in cancer patients (Mahmoud and Rivera 2002). Increased amounts of neopterin are released by human macrophages upon stimulation with Th1-type cytokine interferon-γ, which induces GTP-cyclohydrolase I, the key enzyme for the formation of neopterin. In parallel, interferon-γ triggers expression of the enzyme indoleamine (2,3)-dioxygenase (IDO) (Carlin et al. 1989; Werner et al. 1989; Taylor and Feng 1991; Brown et al. 1991), which converts the essential amino acid tryptophan to kynurenine. Tryptophan is not only important for protein biosynthesis but also serves as precursor for neurotransmitter 5-hydroxytryptamine, serotonin (Wirleitner et al. 2003, see also Fig. 1). Decreased availability of tryptophan and serotonin is discussed to be crucial in the pathogenesis of mood disturbances and depression (Widner et al. 2002).
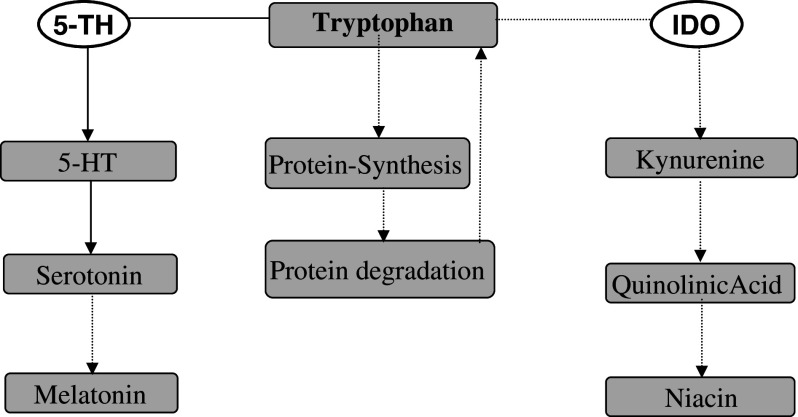
Fig. 1.
(1) Tryptophan is incorporated in proteins. (2) Metabolic conversion of tryptophan via tryptophan-5-hydroxylase (5-TH) to form 5-hydroxytryptophan (5-TH) in first step which is further converted to 5-hydroxytryptamine (serotonin) and melatonin. (3) During Th1-type immune response, indoleamine-2,3-dioxygenase (IDO) converts tryptophan to N-formyl-kynurenine and kynurenine which are further converted to the quinolinic acid and niacin
Enhanced degradation of tryptophan, in parallel with increased production of neopterin is observed in several clinical conditions like virus infections, autoimmune disorders and also malignant diseases (Fuchs et al. 1991; Widner et al. 2000; Denz et al. 1990; Huang et al. 2002; Giusti et al. 1996; Schroecksnadel et al. 2005). QoL is often impaired in patients suffering from such chronic disorders, and susceptibility for mood disturbances or depressive episodes is increased (Cruess et al. 2003). Decreased availability of tryptophan could thus play an important role. Indeed, tryptophan degradation was found recently to be associated with impaired QoL in patients suffering from colorectal cancer (Huang et al. 2002) and in patients with malignant melanoma treated with interferon-α (Capuron et al. 2003). In this study, we compared QoL and fatigue with tryptophan metabolism and cellular immune activation in patients suffering from malignant disease.
Methods
Subjects
One hundred-forty six consecutive patients (mean age ± S.D.: 67 ± 15 years, 53 males and 93 females) suffering from malignant disease were recruited from the district hospital of Natters near Innsbruck/Tyrol. Patients suffering from different types of cancer (Table 1), on the whole 35 different tumors were diagnosed (see Table 1). Staging classification was done according to UICC 1997 guidelines (UICC 1997) for all tumors except hematological malignancies.
Table 1.
Diagnoses of patients included in the study split into five different groups [gastrointestinal (GI)-tumors, hematologic/lymphatic malignancy, gynecological cancer, lung cancer, tumors of other localization]
| GI-tumors | 43 |
| Oesophageal cancer | 7 |
| Carcinoma of the stomach | 5 |
| Colon carcinoma | 12 |
| Rectal carcinoma | 4 |
| Hepatocellular carcinoma | 3 |
| Pancreatic carcinoma | 5 |
| Carcinoid | 3 |
| Klatskin-tumor | 1 |
| Sigmoid carcinoma | 3 |
| Hematologic/lymphatic malignancy | 40 |
| AML | 3 |
| CLL | 5 |
| Osteomyelosclerosis | 1 |
| Non Hodgkin lymphoma | 11 |
| Malignant lymphnode | 1 |
| Hodgkin´s disease | 3 |
| Multiple myeloma | 7 |
| Myelodysplastic syndrome | 5 |
| Histiocytoma | 1 |
| Histiocytosis | 1 |
| Thrombocytemia | 1 |
| Thymoma | 1 |
| Gynecological cancer | 26 |
| Breast carcinoma | 20 |
| Ovarian carcinoma | 3 |
| Endometrium carcinoma | 3 |
| Colli uteri carcinoma | 1 |
| Lung cancer | 20 |
| Bronchus carcinoma | 19 |
| Small-cell lung cancer | 1 |
| Tumors of other localization | 17 |
| Pharyngeal tumors | 5 |
| Melanoma | 4 |
| Leiomyosarcoma | 1 |
| Liposarcoma | 2 |
| Neoplasm of the prostate | 1 |
| Carcinoma of the tonsills | 2 |
| Carcinoma of the tongue bottom | 2 |
According to tumor progression, patients were grouped into patients with remitting (n = 32), with stable (n = 28) and with progressive disease (n = 86) according to their disease status at study entry. Nineteen patients died within the following 3 months after samples were taken. To account for differences caused by insufficient information given by the classification into stable/remitting and progressive diseases (the prognosis of patients with stable CLL cannot compare with the prognosis of aggressive tumors), an experienced oncologist estimated the survival time of patients by categorizing patients’ survival-probability into three categories: estimated time of survival (ETS) (1) lower than 6 months, (2) between 6 and 12 months and (3) more than 1 year.
Forty-four patients received chemotherapy at the time of blood sampling: on the whole, the majority of patients (71.9%) had not had any chemotherapy (CHT) before. Of those patients, who had already received chemotherapy, 13 had had one prior CHT cycle, 10 two cycles, 9 three cycles and 3 patients each had received 4, 5 or 6 cycles of CHT.
Within the scope of routine blood examinations in the morning, fractions of serum samples of patients were collected and frozen at −20°C until analysis. The study was approved by the ethics committee of the Innsbruck Medical University and patients gave informed consent to participate in the study.
Measurements
Tryptophan metabolic changes
Tryptophan and kynurenine concentrations were determined by high-performance liquid chromatography as described (Widner et al. 1997). After precipitation of protein with trichloroacetic acid, tryptophan was measured by fluorescence detection at 285 nm excitation and 365 nm emission wavelengths. Kynurenine was monitored by UV-absorption at 360-nm-wavelength. To estimate IDO activity, the ratio of the concentrations of the enzyme product kynurenine to the substrate tryptophan (kynurenine to tryptophan ratio = kyn/trp) was calculated (Fuchs et al. 1991).
Inflammatory and immune activation markers
Neopterin concentrations were measured by ELISA (BRAHMS Diagnostica, Berlin, Germany), and CRP concentrations were determined with Ektachem clinical chemistry slides according to instructions of the manufacturers. Blood sedimentation rate was measured according to Westergren. Leukocyte counts as well as hemoglobin concentrations were determined by a fully automatized counter (MAX-M-Counter, Beckmann Coulter, Krefeld, Germany).
Quality of life and fatigue scores
To assess the QoL of patients, patients were asked to assess their QoL on a scale from 1 to 5 (patients’ self-report: 1 = best score, very good QoL; 5 = worst score, very bad QoL), independently of a questionnaire. Patients furthermore scored their fatigue on the same scale from 1 to 5 (1 = no fatigue; 5 = high grade of fatigue).
Statistical analysis
As data did not show normal distribution, Kruskal–Wallis test was employed to test for differences between subgroups. If differences were found, subgroups of patients were compared with non-parametric Mann–Whitney U-test. Spearman rank correlation analysis was applied to assess correlation; partial correlation analysis was employed to adjust for confounders like tumor progression. Binary logistic regression analysis was used to identify parameters indicative of impaired QoL (univariate and multivariate, stepwise and inclusion method). P-values < 0.05 were considered to indicate statistical significance.
Results
Median tryptophan concentration in patients was 42.9 μmol/l (interquartile range: 31.1–51.5 μmol/l), median kynurenine concentration was 2.21 μmol/l (1.57–3.24) and median kyn/trp was 55.7 μmol/mmol [41.4–78.9, reference ranges, as published by Widner et al. (1997) are shown in the left column of Table 2]. When compared to all other groups, patients with gastrointestinal tumors presented with lowest tryptophan concentrations (median: 36.6 μmol/l) and highest kyn/trp (median: 68.9 μmol/mmol), levels being significantly different from patients with hematologic malignancy and gynecological cancer (both P < 0.05).
Table 2.
Differences between patients suffering from stable or remitting malignant disease (n = 62) and progressive disease (n = 86) regarding age, erythrocyte sedimentation rate (ESR-1 and ESR-2), leukocytes and concentrations of C-reactive protein (CRP), neopterin, tryptophan, kynurenine, hemoglobin and the ratio of kynurenine to tryptophan (kyn/trp)
| All patients | Stable/remitting disease | Progressive disease | P-value | Reference range | |
|---|---|---|---|---|---|
| Age (year) | 66.6 ± 1.2 | 68.9 ± 1.4 | 64.9 ± 1.9 | NS | |
| Tryptophan (μmol/l) | 41.4 ± 1.3 | 45.5 ± 1.86 | 38.7 ± 1.7 | P < 0.001 | 73.0 ± 14.9 |
| Kynurenine (μmol/l) | 2.58 ± 0.12 | 2.44 ± 0.13 | 2.70 ± 0.19 | NS | 1.9 ± 0.6 |
| Kyn/trp (μmol/mmol) | 73.9 ± 5.1 | 58.9 ± 4.5 | 85.0 ± 7.8 | P < 0.05 | 26.9 ±8.10 |
| Neopterin (nmol/l) | 30.2 ± 2.8 | 23. 7 ± 2.8 | 35.1 ± 4.2 | P < 0.07 | <8.7 |
| ESR-1 (mm/1 h) | 37.8 ± 2.4 | 30.8 ± 3.2 | 43.2 ± 3.4 | P < 0.05 | <10 |
| ESR-2 (mm/2 h) | 61.4 ± 2.7 | 53.9 ± 3.7 | 67.8 ± 3.6 | P < 0.001 | <20 |
| CRP (mg/dl) | 4.7 ± 0.6 | 3.0 ± 0.7 | 5.9 ± 0.9 | P < 0.001 | <0.7 |
| Hemoglobin (g/dl) | 11.2 ± 0.2 | 11.6 ± 0.2 | 10.9 ± 0.2 | P < 0.05 | >12 |
| Leukocytes (×103/μl) | 8.4 ± 0.8 | 8.0 ± 1.1 | 8.6 ± 1.2 | NS | 4–10 |
| Quality of life (score) | 3 ± 0 | 2 ± 0 | 3 ± 0 | P < 0.001 | |
| Fatigue (score) | 3 ± 0 | 2 ± 0 | 3 ± 0 | P < 0.001 |
Values represent mean ± standard error of the mean and P-values
NS no significant difference between the two groups
Concentrations of neopterin were highly elevated in comparison to reference ranges (see Table 2). Patients with hematologic/lymphatic malignancy had highest neopterin concentrations (median: 25.0 nmol/l), and they were significantly higher compared to patients with gynecological cancer (median: 12.7 nmol/l) and tumors of other localization (median: 15.7 nmol/l; both P < 0.05). Similarly, concentrations of CRP (median: 2.1; interquartile range: 0.6–5.3 mg/dl) and erythrocyte sedimentation rate after one (ESR-1) and 2 h (ESR-2) were elevated in patients: ESR-1: 33 mm/h (16–52), ESR-2: 65 mm/2 h (35–88).
Patients suffering from progressive malignancy had significantly higher concentrations of CRP, ESR-1 and ESR-2 and kyn/trp than patients who had stable or remitting disease (all P < 0.05, Table 2). Tryptophan concentrations and kyn/trp depended on the stage of disease: lower tryptophan concentrations and higher kyn/trp were observed in patients with progressive disease (both P < 0.01). Kynurenine concentrations did not differ in patients with stable or progressive disease, but were significantly higher in patients who died within 3 months (both P < 0.01).
Median QoL score of patients was 3—17.8% of patients rated their QoL as bad (score 4; n = 18) or very bad (score 5; n = 8). Median fatigue score was 3—17.1% of patients rated it high (score 4; n = 18) or very high (score 5; n = 7). Fifty-four patients suffered from depression and had to take antidepressant medication. Rather unexpectedly, the diagnosis of depression and also of anemia was not associated significantly with QoL and fatigue scores (see Table 3), however, a correlation existed between fatigue feeling and hemoglobin counts (r s = −0.204, P < 0.05). On the other hand, a significant correlation was observed between QoL and fatigue scores (r s = 0.555; P < 0.001), which were both strongly associated with the performance status of patients (r s = −0.510 for QoL and Karnofsky-Index, r s = 0.519 for QoL and WHO/ECOG score; r s = −0.651 for fatigue and Karnofsky-Index, r s = 0.666 for fatigue and WHO/ECOG score, all P < 0.001). Patients with different types of cancer did not differ regarding QoL and fatigue scores; however, significantly lower QoL and fatigue scores were observed in patients suffering from more advanced disease in comparison with patients suffering from remitting or stable disease (both P < 0.001).
Table 3.
Power of diverse parameters to predict reduced QoL and fatigue, respectively in cancer patients in univariate regression analysis: concentrations of tryptophan, kynurenine, neopterin, hemoglobin and CRP, the kynurenine to tryptophan ratio (kyn/trp), leukocyte counts and erythrocyte sedimentation rate after 1 h (ESR-1) and 2 h (ESR-2) as well as stage of disease, anemia, fatigue, depression and performance status of patients (measured by WHO/ECOG and Karnofsky-index)
| OR QoL (95% CI) | P-value | OR Fatigue (95% CI) | P-value | |||
|---|---|---|---|---|---|---|
| Tryptophan | 0.966 | (0.937–0.966) | 0.025 | 0.980 | (0.952–1.010) | 0.185 |
| Kynurenine | 1.379 | (1.054–1.803) | 0.019 | 1.197 | (0.930–1.541) | 0.163 |
| Kyn/trp | 1.008 | (1.002–1.015) | <0.01 | 1.006 | (1.000–1.012) | 0.057 |
| Neopterin | 1.018 | (1.006–1.031) | <0.01 | 1.016 | (1.004–1.028) | <0.01 |
| ESR-1 | 1.026 | (1.009–1.044) | <0.01 | 1.010 | (0.993–1.028) | 0.252 |
| ESR-2 | 1.027 | (1.008–1.047) | <0.01 | 1.010 | (0.993–1.028) | 0.243 |
| CRP | 1.098 | (1.039–1.160) | 0.001 | 1.080 | (1.026–1.138) | <0.01 |
| Hemoglobin | 0.767 | (0.598–0.982) | 0.036 | 0.767 | (0.598–0.982) | 0.036 |
| Leukocytes | 1.027 | (0.992–1.064) | 0.136 | 1.022 | (0.987–1.059) | 0.216 |
| Anemia | 1.750 | (0.611–5.011) | 0.297 | 1.244 | (0.457–3.389) | 0.669 |
| Depression | 0.622 | (0.259–1.499) | 0.290 | 0.669 | (0.277–1.619) | 0.373 |
| Stage | 3.890 | (1.555–9.731) | <0.01 | 3.756 | (1.508–9.355) | <0.01 |
| Karnofsky index | 0.885 | (0.841–0.932) | <0.001 | 0.882 | (0.836–0.930) | <0.001 |
| WHO/ECOG | 7.957 | (3.349–18.901) | <0.001 | 9.869 | (3.805–25.599) | <0.001 |
P-values indicating significant differences are written in bold
Both, QoL and fatigue scores, were associated with the clinical stage and also the prognosis of tumor disease in our study: patients with more advanced stages were more likely to suffer from impaired QoL (r s = 0.362; P < 0.001) and increased fatigue feeling (r s = 0.456; P < 0.001, see Table 3). While patients suffering from remitting disease had QoL scores of 2.25 ± 0.67 (mean ± SD) and fatigue of 1.97 ± 0.70, patients with stable disease had a mean QoL score of 2.54 ± 0.74 and fatigue of 2.39 ± 0.88. Patients with progressive tumor disease had the worst QoL (QoL score 3.04 ± 0.92) and fatigue feeling (2.98 ± 0.96; both P < 0.001). Furthermore also patients who died within 3 months of the study had decreased QoL (mean ± SD: 3.47 ± 1.22) and suffered more often from fatigue (3.58 ± 1.02) compared to patients still alive after 3 months (QoL score: 2.66 ± 0.80; P < 0.01; fatigue: 2.49 ± 0.89; P < 0.001).
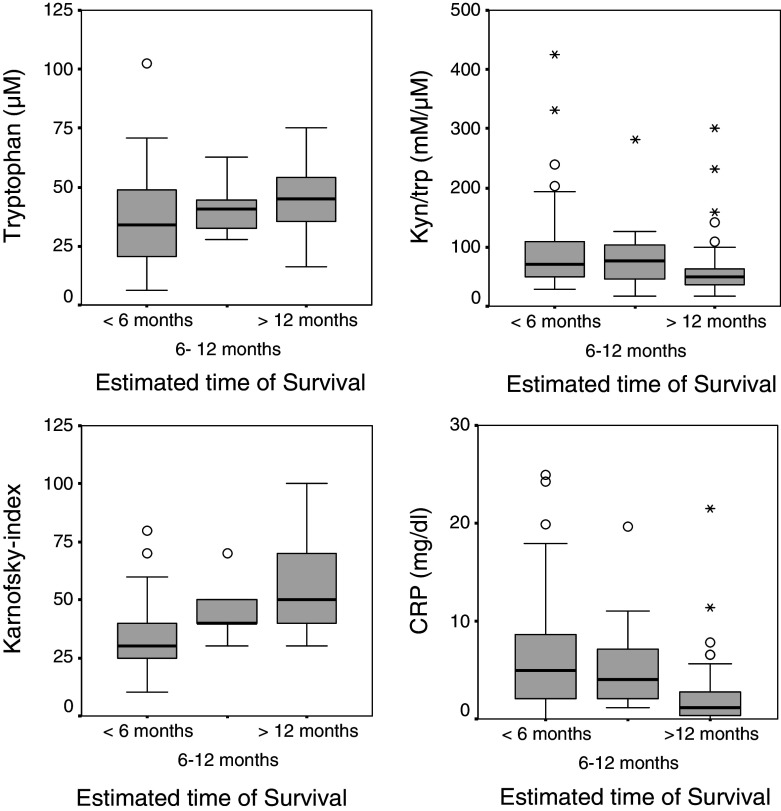
QoL and fatigue as well as Karnofsky index were better in patients with an ETS above 1 year, WHO/ECOG score was worse (all P < 0.001) in comparison to patients with an ETS below 6 months. Patients with an ETS between 6 and 12 months had a lower ESR compared to an ETS of below 6 months (P < 0.01). Patients with an ETS below 6 months had significantly lower tryptophan and hemoglobin concentrations (both P < 0.001); in parallel they presented with higher CRP (P < 0.001), and higher neopterin (P < 0.001) concentrations than patients with an ETS above 1 year and kyn/trp was significantly higher in patients with an ETS lower than 6 months (see Fig. 2).
Fig. 2.
Relationship between estimated time of survival, tryptophan metabolism and immune activation: decreasing tryptophan concentrations (upper left) and increasing kynurenine to tryptophan ratio (kyn/trp)—(upper right) are found in patients with worse prognosis. A better performance status of patients (bottom right) and lower CRP concentrations (bottom left) are observed in patients with a better estimated time of survival; boxplots with medians (black line), interquartile range (grey boxes), range (whiskers), outliers (circles) and stars (extrema)
QoL was better in patients under CHT (median 2 vs. 3, P < 0.01), who also had higher tryptophan (median: 45.5 vs. 41.1, P < 0.01) and lower neopterin (9.6 vs. 20.0 nM, P < 0.001) and CRP (1.3 vs. 2.9 mg/dl, P < 0.001) concentrations. Interestingly, neither age nor disease stage or ETS were different in patients under CHT, whereas Karnofski index was higher in patients under CHT (P < 0.05), indicating that patients under CHT were in a better physical condition than the other patients.
Table 3 shows all parameters that were predictors of impaired QoL and fatigue, respectively, in binary logistic regression analysis (univariate). In multivariate regression analysis, when all laboratory parameters that were predictive for impaired QoL in univariate analysis were included, neopterin concentrations, in combination with tumor stage and ESR were the best indicators of impaired QoL: (Z = −7.459 + 1.309 × stage + 0.023 × ESR-2 + 0.024 × neopterin; P < 0.01 for neopterin, P < 0.05 for ESR-1 and stage).
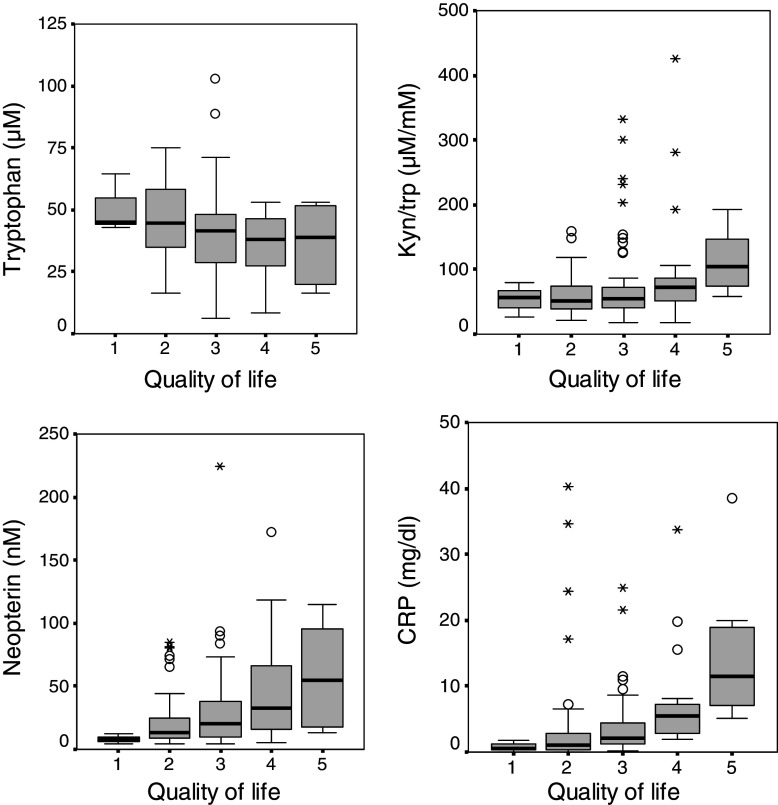
Lower tryptophan concentrations coincided with decreased QoL (r s = −0.256; P < 0.01) and increased fatigue feeling (r s = −0.179; P < 0.05). Similarly, significant correlations were observed between fatigue scores and kyn/trp (r s = 0.276; P < 0.01). Furthermore, fatigue scores correlated with concentrations of neopterin (r s = 0.274; P < 0.01), CRP (r s = 0.375; P < 0.001), ESR-1 (r s = 0.234; P < 0.01) and ESR-2 (r s = 0.241; P < 0.01), and QoL correlated with concentrations of neopterin (r s = 0.307; P < 0.001) and CRP (r s = 0.508; P < 0.001) (Fig. 3). After adjustment for tumor stage, (partial) correlations between QoL scores and concentrations of tryptophan, neopterin, CRP and kyn/trp remained significant (all P < 0.05).
Fig. 3.
Relationship between quality of life (QoL), tryptophan metabolism and immune activation: decreasing tryptophan concentrations (upper, left figure) and increasing tryptophan degradation—which is expressed as the kynurenine to tryptophan ratio (kyn/trp)—(upper, right figure) are associated with impaired QoL (= higher QoL scores); furthermore QoL is also related with the extent of immune activation reflected by neopterin (lower, left figure) and CRP concentrations (lower, right figure)
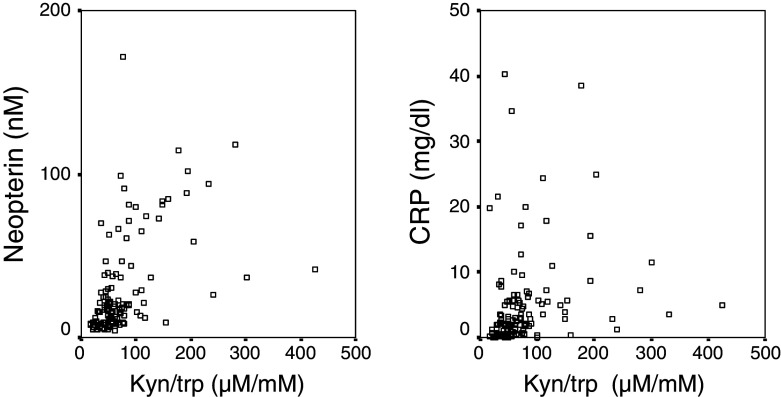
Immune activation status was strongly related to enhanced tryptophan degradation: Elevated concentrations of neopterin correlated with increased kyn/trp (r s = 0.586); furthermore, also CRP (r s = 0.453) and ESR-1 (r s = 0.326) were strongly related to increased kyn/trp (all P < 0.001, Fig. 4). In line with this finding, lower tryptophan concentrations coincided with higher neopterin and CRP concentrations (both P < 0.001) and higher ESR-1 (P < 0.01). CRP correlated with neopterin (r s = 0.410; P < 0.001), ESR-1 (r s = 0.435; P < 0.001) and ESR-2 (r s = 0.406; P < 0.001), and also neopterin and ESR-1 were weakly associated (r s = 0.187; P < 0.05).
Fig. 4.
Correlations between neopterin concentrations and tryptophan degradation—which is expressed as the kynurenine to tryptophan ratio (Kyn/trp)—(left figure; r s = 0.586; P < 0.001) and between C-reactive protein (CRP) and Kyn/trp (right figure; r s = 0.453; P < 0.001)
Discussion
Our patients, suffering from malignant disease, experienced deteriorating QoL and fatigue feeling dependent on disease progression, data confirming and extending earlier observations. The investigation of tryptophan catabolism indicates that depletion of this essential amino acid may, among other factors, contribute to impairment of both, QoL and feeling of fatigue. Tryptophan degradation was found to be accelerated in our patients and correlated with immune activation. Immune-mediated tryptophan degradation has already been shown earlier to be associated with impaired QoL when psychological questionnaires were applied (Huang et al. 2002). In our study, a less objective but more practicable method was performed, namely the subjective view of patients was asked for to judge their QoL and fatigue feeling. Nevertheless, results appear to confirm existing data, the query used was obviously suited rather well to assess psychological status of patients in a more general sense. Both scores were associated with clinical outcome and correlated significantly with tumor stage as well as survival/death within 3 months. Certainly, more sophisticated psychological questionnaires can define better which specific problems may impair the patient’s QoL, e.g., whether the patient suffers rather from physical or mental health disturbances.
QoL and fatigue scores were also strongly associated with markers of inflammation and immune activation and with the degradation of tryptophan. Enhanced conversion of tryptophan to kynurenine in patients coincided with enhanced concentrations of neopterin. Thus, immune activation appears to underlie degradation of tryptophan, and thus most likely IDO is involved. Data are consistent with and extend earlier studies in patients with hematologic and gastrointestinal malignancy (Denz et al. 1993; Giusti et al. 1996; Iwagaki et al. 1995).
Tumor-mediated IDO-expression was recently proposed as effective evasion strategy of tumors to escape immune response (Uyttenhove et al. 2003). However, the coincidence of tryptophan degradation with neopterin formation suggests that mostly interferon-γ-stimulated monocytes are responsible for enhanced tryptophan conversion. Well in accordance with this observation, elevated interferon-γ concentrations were found to correlate with increased neopterin concentrations in patients with hematologic disorders earlier (Denz et al. 1993). However, in a few patients, very high kyn/trp, together with only moderately elevated neopterin levels were observed. Data may indicate that, in addition to macrophages and dendritic cells, also tumor-associated IDO activity may be of some relevance in these patients. Such data agree well with observations made earlier in a pilot study of patients with gynecological tumors (Schroecksnadel et al. 2005). Interestingly, increased tryptophan degradation was found in this study in nearly all cancer patients. Thus, this phenomenon appears not to be confined to special types of tumors. Anti-tumor immune response was earlier suggested to lead to impaired QoL in cancer patients (Earlam et al. 1996), one study in patients with colorectal cancer even observed that QoL was associated stronger with immune activation than with liver metastasis or tumor marker elevation (Allen-Mersh et al. 1998).
Reduced QoL was recently shown to be associated with low tryptophan concentrations in patients suffering from colorectal cancer (Huang et al. 2002). Our data confirm that decreased tryptophan availability in parallel with impaired QoL is also found in patients suffering from other types of cancer. Immune activation cascades with on-going production of pro-inflammatory cytokines like interferon-γ and TNF-α (= cachectin) may in fact account for “cancer symptoms” of patients: most cancer patients show symptoms, which resemble cytokine-induced sickness behavior in animal models and impair QoL severely (Cleeland et al. 2003). After the administration of infectious or inflammatory agents or certain pro-inflammatory cytokines, reduced food intake and decreased social activities were observed in animals (Dantzer et al. 1998), cytokine antagonists were in some cases able to inhibit these reactions. Similarly, treatment of cancer patients with interferon-α and interleukin-2 was described to impair their mood (Capuron et al. 2001). Notably, cytokine-induced decrease of tryptophan concentrations was found to be more expressed in patients who developed depressive symptoms (Capuron et al. 2003).
Tryptophan depletion as a consequence of immune activation cascades may also be of importance in the pathogenesis of mood disturbances and depression (Iwagaki et al. 1997; Murr et al. 2000; Widner et al. 2002; Myint and Kim 2003). Limiting serotonin biosynthesis, lowered serum tryptophan might increase the susceptibility for depressive episodes/depression and decreased social activities. Findings of this study may provide an explanation for the relationship between impaired QoL and increased fatigue in cancer patients. Associations between tryptophan metabolic changes and QoL and fatigue were rather weak, however, associations between other markers, like hemoglobin (which has been proposed as marker for QoL earlier) and QoL or fatigue were even less expressed, e.g., there was no significant correlation between haemoglobin and QoL or fatigue. Of course, enhanced tryptophan metabolism only represents one among several metabolic changes in cancer patients, but it appears to be also of some clinical relevance: the psychological and maybe also the physical status of patients can be influenced by modification of tryptophan metabolism, e.g., by successful antitumor treatment. Follow-up studies investigating the role of tryptophan regarding QoL, mood, fatigue and also survival analysis in cancer patients under treatment may thus also provide important new insights for future treatment of patients. At least in patients with multiple sclerosis, therapeutic benefit was observed by the use of IDO inhibitors (Platten et al. 2005). However, a potential positive influence of IDO inhibitors on psychological status of patients does not rule out an adverse effect of such compounds on the underlying malignant process.
Acknowledgment
This work was supported by the “Stiftung Propter Homines, Vaduz -Fürstentum Liechtenstein”, by the government of the State of the Austrian Tyrol, and by the European Community, Project #019031 BAMOD “Austrian Cancer Society/Tyrol”. We thank Miss Astrid Haara for excellent technical assistance.
References
- Allen-Mersh TG, Glover C, Fordy C, Henderson DC, Davies M (1998) Relation between depression and circulating immune products in patients with advanced colorectal cancer. J R Soc Med 91:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J E, Ganz P A, Aziz N, Fahey JL (2002) Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 64:604–611 [DOI] [PubMed] [Google Scholar]
- Brown RR, Ozaki Y, Datta S P, Borden EC, Sondel PM, Malone DG (1991) Implications of interferon-induced tryptophan catabolism in cancer auto-immune diseases and AIDS. Adv Exp Med Biol 294:425–435 [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ (2001) Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology 26:797–808 [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH (2003) Interferon-alpha-induced changes in tryptophan metabolism relationship to depression and paroxetine treatment. Biol Psychiatry 54:906–914 [DOI] [PubMed] [Google Scholar]
- Carlin JM, Ozaki Y, Byrne GI, Brown RR, Borden EC (1989) Interferons and indoleamine 2 3-dioxygenase: role in antimicrobial and antitumor effects. Experientia 45:535–541 [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Lee BN (2003) Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer 97:2919–2925 [DOI] [PubMed] [Google Scholar]
- Cruess DG, Petitto JM, Leserman J, Douglas SD, Gettes DR, Ten Have TR, Evans DL. (2003) Depression and HIV infection: impact on immune function and disease progression. CNS Spectr 8:52–58 [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, Kelley KW (1998) Molecular basis of sickness behaviour. Ann NY Acad Sci 856:132–138 [DOI] [PubMed] [Google Scholar]
- Denz H, Fuchs D, Huber H, Nachbaur D, Reibnegger G, Thaler J, Werner ER, Wachter H (1990) Correlation between neopterin interferon-gamma and haemoglobin in patients with haematological disorders. Eur J Haematol 44:186–189 [DOI] [PubMed] [Google Scholar]
- Denz H, Orth B, Weiss G, Herrmann R, Huber P, Wachter H, Fuchs D (1993) Weight loss in patients with hematological neoplasias is associated with immune system stimulation. Clin Investig 71:37–41 [DOI] [PubMed] [Google Scholar]
- Dimeo F, Schmittel A, Fietz T, Schwartz S, Kohler P, Boning D, Thiel E (2004) Physical performance depression immune status and fatigue in patients with hematological malignancies after treatment. Ann Oncol 15:1237–1242 [DOI] [PubMed] [Google Scholar]
- Earlam S, Glover C, Fordy C, Burke D, Allen-Mersh TG (1996) Relation between tumor size quality of life and survival in patients with colorectal liver metastases. J Clin Oncol 14:171–175 [DOI] [PubMed] [Google Scholar]
- Fuchs D, Moeller AA, Reibnegger G, Werner ER, Werner-Felmayer G, Dierich MP, Wachter H (1991) Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett 28:207–211 [DOI] [PubMed] [Google Scholar]
- Giusti RM, Maloney EM, Hanchard B, Morgan OS, Steinberg SM, Wachter H, Williams E, Cranston B, Fuchs D, Manns A (1996) Differential patterns of serum biomarkers of immune activation in human T-cell lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis and adult T-cell leukemia/lymphoma. Cancer Epidemiol Biomarkers Prev 5:699–704 [PubMed] [Google Scholar]
- Huang A, Fuchs D, Widner B, Glover C, Henderson DC, Allen-Mersh TG (2002) Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br J Cancer 86:1691–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwagaki H, Hizuta A, Tanaka N, Orita K (1995) Decreased serum tryptophan in patients with cancer cachexia correlates with increased serum neopterin. Immunol Invest 24:467–478 [DOI] [PubMed] [Google Scholar]
- Iwagaki H, Hizuta A, Uomoto M, Takeuchi Y, Saito S, Tanaka N (1997) Cancer cachexia and depressive states: a neuro-endocrine-immunological disease? Acta Med Okayama 51:233–236 [DOI] [PubMed] [Google Scholar]
- Kronberger P, Weiss G, Tschmelitsch J, Fuchs D, Salzer GM, Wachter H, Reibnegger G (1995) Predictive value of urinary neopterin in patients with lung cancer. Eur J Clin Chem Clin Biochem 33:831–837 [DOI] [PubMed] [Google Scholar]
- Kurzrock R (2001) The role of cytokines in cancer-related fatigue. Cancer 92:1684–1688 [DOI] [PubMed] [Google Scholar]
- Lewenhaupt A, Ekman P, Eneroth P, Eriksson A, Nilsson B, Nordstrom L (1986) Serum levels of neopterin as related to the prognosis of human prostatic carcinoma. Eur Urol 12:422–425 [DOI] [PubMed] [Google Scholar]
- Mahmoud FA, Rivera NI (2002) The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 4:250–255 [DOI] [PubMed] [Google Scholar]
- Murr C, Bergant A, Widschwendter M, Heim K, Schrocksnadel H, Fuchs D (1999) Neopterin is an independent prognostic variable in females with breast cancer. Clin Chem 45:1998–2004 [PubMed] [Google Scholar]
- Murr C, Widner B, Sperner-Unterweger B, Ledochowski M, Schubert C, Fuchs D (2000) Immune reaction links disease progression in cancer patients with depression. Med Hypotheses 55:137–140 [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK (2003) Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses 61:519–525 [DOI] [PubMed] [Google Scholar]
- Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L (2005) Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science 310:850–855 [DOI] [PubMed] [Google Scholar]
- Reibnegger G J, Bichler A H, Dapunt O, Fuchs DN, Fuith LC, Hausen A, Hetzel HM, Lutz H, Werner ER, Wachter H (1986) Neopterin as a prognostic indicator in patients with carcinoma of the uterine cervix. Cancer Res 46:950–955 [PubMed] [Google Scholar]
- Reibnegger G, Hetzel H, Fuchs D, Fuith LC, Hausen A, Werner ER, Wachter H (1987) Clinical significance of neopterin for prognosis and follow-up in ovarian cancer. Cancer Res 47:4977–4981 [PubMed] [Google Scholar]
- Schroecksnadel K, Winkler C, Fuith L C, Fuchs D (2005) Tryptophan degradation in patients with gynecological cancer correlates with immune activation. Cancer Lett 223:323–299 [DOI] [PubMed] [Google Scholar]
- Taylor MW, Feng GS (1991) Relationship between interferon-gamma indoleamine 2 3-dioxygenase and tryptophan catabolism. FASEB J 5:2516–2522 [PubMed] [Google Scholar]
- UICC (1997) TNM-Klassifikation maligner Tumoren 5th edition Wittekind CH, Wagner G (eds) Springer, Berlin
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274 [DOI] [PubMed] [Google Scholar]
- Weiss G, Kronberger P, Conrad F, Bodner E, Wachter H, Reibnegger G (1993) Neopterin and prognosis in patients with adenocarcinoma of the colon. Cancer Res 53:260–265 [PubMed] [Google Scholar]
- Werner E R, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Wachter H (1989) Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2 3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J 262:861–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Werner ER, Schennach H, Wachter H, Fuchs D (1997) Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem 43:2424–2426 [PubMed] [Google Scholar]
- Widner B, Sepp N, Kowald E, Ortner U, Wirleitner B, Fritsch P, Baier-Bitterlich G, Fuchs D (2000) Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology 201:621–630 [DOI] [PubMed] [Google Scholar]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D (2002) Neopterin production tryptophan degradation and mental depression—what is the link? Brain Behav Immun 16:590–595 [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D (2003) Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem 10:1581–1591 [DOI] [PubMed] [Google Scholar]