Abstract
Purpose
Cancer cells release a multitude of cytokines and growth factors that influence neighboring cells and help establish a favorable environment for tumor development. As part of our studies designed to elucidate the complex cellular interactions within the tumor microenvironment that facilitate tumor development, we investigated cancer cell-induced changes in gene expression in endothelial cells.
Methods
After treatment of human umbilical vein endothelial cells (HUVEC) with conditioned medium (CM) of SNUC5 colon cancer cells, gene expression profile in HUVEC was analyzed using cDNA microarray. Neutralizing antibodies against pro-inflammatory cytokines were used to identify the major effecter in SNUC5 CM.
Results
IL-8 was one of the four genes up-regulated over fourfold, and IL-1α in SNUC5 CM was revealed as a major effecter of IL-8 over-expression and release, which was nearly completely neutralized by anti-IL-1α antibody. Constitutive secretion of IL-1α was confirmed in many other human cancer cells.
Conclusions
IL-1α is constitutively expressed in many human cancer cells and directly induces IL-8 secretion in neighboring endothelial cells.
Keywords: Interleukin-1α, Interleukin-8, Cancer cell, Endothelial cell
Introduction
During the process of carcinoma formation, cancer cells release various growth factors and cytokines into their surroundings, recruit and reprogram various types of other cells to establish a so-called “tumor microenvironment” (Rollins 2006; Werb et al. 2005; Orimo et al. 2005; Elenbaas and Weinberg 2001; Lu et al. 2006). Thus, although more than 90% of human cancers originate from epithelial cells, tumor tissues almost always contain significant amounts of other cell types such as endothelial cells, fibroblasts, and infiltrating inflammatory cells. Investigation of the complex cellular interactions within this tumor microenvironment is essential for understanding the tumor formation, angiogenesis, invasion and metastasis (Elenbaas and Weinberg 2001; Lu et al. 2006; Witz 2006; Kopfstein and Christofori 2006). The challenge in this context is to find genes and/or proteins that play critical roles in the communication between cancer cells and the neighboring cells.
In this study, we focused on the candidate molecules involved in reprogramming adjacent endothelial cells. We monitored changes in gene expression in endothelial cells after treating them with a conditioned medium (CM) of cancer cells. Employing a neutralizing assay with blocking antibodies, we found that IL-1α is constitutively over-expressed and released from many types of cancer cells and directly induces IL-8 over-expression and release from human endothelial cells.
Materials and methods
Antibodies, enzyme-linked immunosorbent assay kits, and reagents
Mouse anti-human IL-1α, IL-1β and TNF-α antibodies, IL-1α and IL-8 ELISA kit were purchased from R&D systems (Minneapolis, MN, USA). LPS was obtained from Sigma (St. Louis, MO, USA).
Cell culture and collection of conditioned media
Human cancer cell lines, SNUC5 (colon), SNU308 (biliary tract), NCI-H290 (lung), SNU682 (cervix), SNU354 (liver), SNU478 (biliary tract), MDA-MB-231 (breast), SNU703 (cervix), SNU5 (stomach), and NCI-H226 (lung), were maintained in RPMI medium supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA) and penicillin/streptomycin (Gibco BRL). Human umbilical vein endothelial cells (HUVEC, Cambrex, Baltimore, MD, USA) were maintained in EGM2 MV endothelial cell media (Cambrex), and cells at passages 8–15 were used for this study. CM of cancer cells was obtained by adding serum-free RPMI medium to cancer cells in 80–90% confluency, incubation at 37°C for 5–14 days and centrifugation at 3,000 rpm for 10 min to remove cells. The CM was filtered, aliquoted and stored at −70°C until use.
Microarray analysis
Total RNAs were isolated from HUVECs incubated for 4 h with serum-free RPMI, SNUC5 CM, or EGM2 MV containing 10% FBS using TRI reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s instructions. Fluorescence-labeled cDNA was prepared by reverse transcription of total RNA in the presence of aminoallyl-dUTP followed by coupling of the Cy3 dye (control) or Cy5 dye (treated samples) (Amersham Pharmacia, Uppsala, Sweden). The labeled cDNAs were applied to Human-7.3 K cDNA TwinChip™ (Digital genomics, Seoul, South Korea) that contains two identical sets of 8,400 human genes. Fluorescence intensities were measured using Axon 4000B scanner (Axon Instruments, Union City, CA, USA). Scanned images were analyzed with GenePix 3.0 software (Axon Instruments, Union City, CA, USA). To minimize the possible differences in efficiencies of hybridization, transformed data were normalized by LOWESS regression (Yang et al. 2002). Each microarray slide contained two duplicate sets of gene fragments. Only genes with statistically significant differences in both sets were further analyzed.
RT-PCR
RT-PCR analysis was performed using Qiagen (Valencia, CA, USA) one-step RT-PCR kit. In brief, total RNAs were incubated with reverse transcriptase for 1 h at 50°C, and cDNA was amplified with PCR for 35 cycles, at 94°C for 1 min, at 55°C for 1 min and at 72°C for 3 min. The sense and antisense PCR primer sequences used were: IL-8; 5′-CAGTTTTGCCAAGGAGTGC-3′, 5′-ACTTCTCCACAACCCTCTGC-3′, IL-1α; 5′-TGGCCAAAGTTCCAGACATGTTTG–3′, 5′-GGTTTTCCAGTATCTGAAAGTCAGT-3′, GAPDH; 5′-CCTCAAGATCATCAGCAATGC-3′, 5′-GTTGAAGTCAGAGGAGAC CACC-3′. The PCR products were analyzed by electrophoresis on 1.5–2% agarose gel.
Neutralization and depletion assay
HUVECs were treated with serum-free RPMI, SNUC5 CM, and SNUC5 CM pre-incubated with 0.01 μg/ml TNF-α-, 0.15 μg/ml IL-1α-, or 0.1 μg/ml IL-1β-specific neutralizing antibody at their neutralization dose50 (ND50). After 24 h incubation, CM of HUVEC was collected and its IL-8 content was measured using the ELISA kit. For IL-1α or IL-1β depletion assay, SNUC5 CM was incubated at 37°C for 1 h in the wells of ELISA plate coated with 5 μg/ml of IL-1α or IL-1β antibody and blocked with 10% FBS in RPMI, and then the supernatants were collected. HUVECs were treated with IL-1α- or IL-1β-depleted SNUC5 CM for 24 h, and IL-8 in the CM of HUVEC was measured using the ELISA kit.
Results
IL-8 expression in HUVECs was induced by conditioned medium of SNUC5
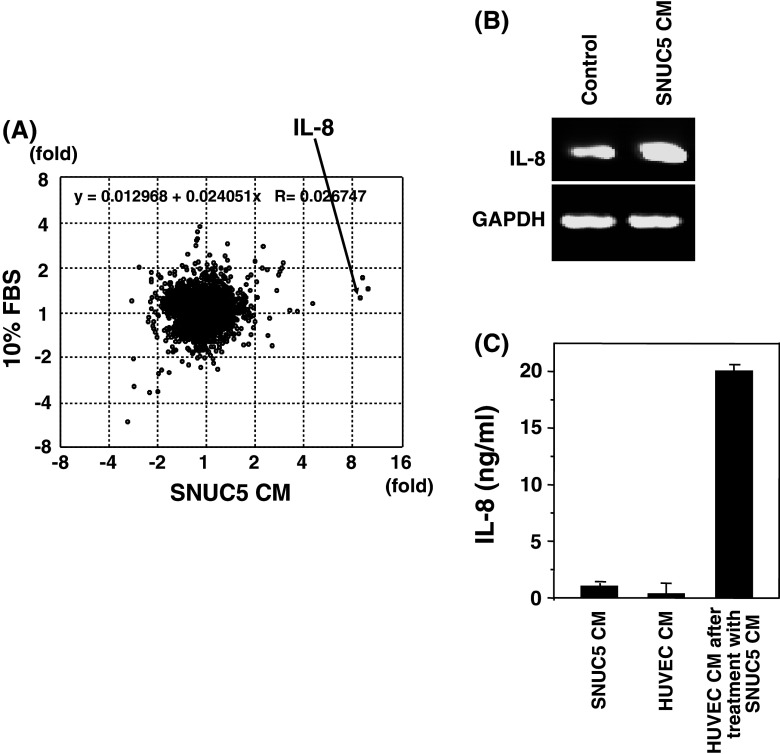
We determined the effect of secretions from SNUC5 human colon cancer cells on HUVEC, by treating HUVECs with CM of SNUC5 cells for 4 h, and subjecting the total RNA prepared from HUVECs to cDNA microarray analysis. The mRNAs prepared from HUVECs exposed to serum-free RPMI and EGM2 MV medium containing 10% FBS were, respectively, used as the reference and control. Among 8,400 genes tested, the expression levels of four genes; superoxide dismutase-2 (SOD-2), intercellular adhesion molecule-1 (ICAM-1), gamma-amino butyric acid A receptor, alpha-6 (GABAR A6), and IL-8, increased more than fourfold (Cy5/Cy3 ratio > 2.0) by treatment with SNUC5 CM, but not with EGM2 MV containing 10% FBS (Fig. 1a). IL-8 over-expression and release from HUVECs induced by SNUC5 CM were confirmed both in RT-PCR and in ELISA (Fig. 1b, c).
Fig. 1.
Induction of IL-8 in HUVEC by conditioned medium (CM) of SNUC5. HUVECs were treated with SNUC5 CM or EGM2 MV containing 10% FBS for 4 h. a Using a cDNA microarray, their gene expression patterns were analyzed and compared, which revealed that IL-8 over-expression is specifically induced by SNUC5 CM. b The over-expression of IL-8 induced by SNUC5 CM was confirmed in RT-PCR analysis. c The level of IL-8 in SNUC5 CM, and HUVEC CM that were obtained after incubation of HUVEC cells with either EGM2 MV containing 10% FBS or SNUC5 CM was determined by an ELISA. CM conditioned medium
IL-1α in conditioned medium of SNUC5 directly induced over-expression and release of IL-8 in HUVECs
Proinflammatory cytokines, IL-1α, IL-1β and TNF-α have been reported to induce IL-8 over-expression (Matsushima and Oppenheim 1989; Pang et al. 1994). In order to identify the molecule inducing IL-8 over-expression and release from HUVECs, we performed a neutralization assay using blocking antibodies against IL-1α, IL-1β and TNF-α. IL-8 release from HUVECs caused by SNUC5 CM was reduced almost to the basal level when a neutralizing antibody against IL-1α was added to SNUC5 CM at a final concentration of 0.15 μg/ml (Fig. 2a). On the other hand, blocking antibodies against TNF-α and IL-1β did not show any significant inhibitory effect on SNUC5 CM-induced IL-8 over-expression in HUVECs at concentrations of 0.01 and 0.1 μg/ml, their respective ND50s. To confirm again that IL-8 over-expression in HUVEC depends on IL-1α in SNUC5 CM, we depleted IL-1α from the SNUC5 CM by incubating the CM on an ELISA plate coated with anti-IL-1α antibody and treated HUVECs with the IL-1α-depleted CM. Induction of IL-8 in HUVEC was significantly reduced after IL-1α depletion, whereas IL-1β depletion exerted no significant effect (Fig. 2b). These two independent experiments demonstrated that IL-1α released from SNUC5 directly induced IL-8 over-expression in HUVEC. In addition, both over-expression of IL-8 induced by SNUC5 CM and its inhibition by an IL-1α neutralizing antibody were also confirmed in human micro-vascular endothelial cell derived from Lung (HMVEC-L) (data not shown).
Fig. 2.
Over-expression and release of IL-8 in HUVECs induced by IL-1α in SNUC5 CM. a HUVECs were treated with SNUC5 CM and SNUC5 CM pre-incubated with 0.01 μg/ml TNF-α-, 0.15 μg/ml IL-1α-, or 0.1 μg/ml IL-1β-specific neutralizing antibody for 24 h. b HUVECs were treated with IL-1α-or IL-1β-depleted SNUC5 CM for 24 h. IL-8 level in HUVEC CM was determined by an ELISA. Values are presented as means ± SE
Over-expression and release of IL-1α from human cancer cell lines
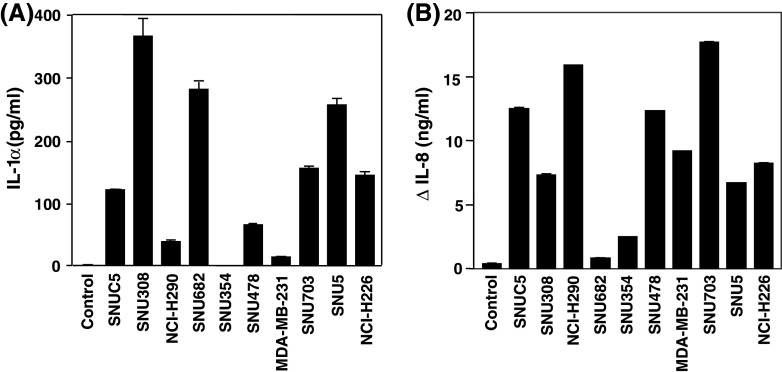
We tested ten human cancer cell lines: SNUC5, SNU308, NCI-H290, SNU682, SNU354, SNU478, MDA-MB-231, SNU703, SNU5, NCI-H226 to determine whether the secretion of IL-1α is a general phenomenon in cancer cells. After cancer cells were incubated in serum-free media for 5–14 days, IL-1α in the collected CM of each cell line was determined using ELISA. IL-1α ranging from 10 to 360 pg/ml was detected in the CM of all cancer cell lines except SNU354 hepatocellular carcinoma cell line. The concentration of IL-1α in the CM of five cancer cell lines was higher than that in SNUC5 CM (Fig. 3a). After HUVEC cells were treated with CM of each cell line with or without IL-1α-specific neutralizing antibody at a final concentration of 0.15 μg/ml for 24 h, the concentrations of IL-8 in HUVEC CM were measured using ELISA. The difference in concentrations of IL-8 in the presence and absence of IL-1α-specific neutralizing antibody was calculated and plotted (Fig. 3b). IL-1α-specific neutralizing antibody significantly reduced IL-8 release induced by the CM of many cancer cell lines.
Fig. 3.
Release of IL-1α from various cancer cells and induction of IL-8 by IL-1α in HUVECs. a After ten human cancer cell lines were cultured in serum-free medium for 5–14 days, the concentration of IL-1α in CM was determined by an ELISA. b HUVEC cells were treated with CM of each cell line with or without IL-1α-specific neutralizing antibody. The difference between the concentrations of IL-8 in HUVEC CM in the presence and absence of IL-1α-specific neutralizing antibody was plotted. Values are presented as means ± SE
IL-1α is constitutively over-expressed in SNUC5
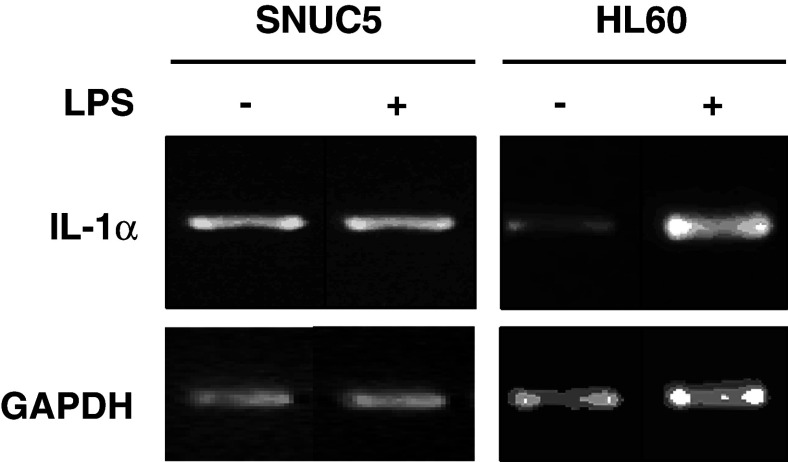
We checked whether the over-expression of IL-1α in SNUC5 is constitutive or enhanced further by external stimulus such as LPS, reported to induce IL-1α production in monocytes and macrophages (Nathan 1987). SNUC5 cells were treated with 1 μg/ml LPS for 2 h, and mRNAs were prepared and subjected to RT-PCR analysis using a primer set specific to IL-1α. As shown in Fig. 4, LPS had no effect on the mRNA level of IL-1α in SNUC5 cells although it dramatically induced IL-1α over-expression in HL60 cells.
Fig. 4.
Constitutive expression of IL-1α in SNUC5 cells. After SNUC5 and HL60 cells were treated with 1 μg/ml LPS for 2 h, the level of IL-1α mRNA was determined by RT-PCR. GAPDH mRNA was used as a control
Discussion
As part of a study in tumor–host interactions, we tested changes in gene expression in human endothelial cells treated with CM of cancer cells. Using DNA microarray we compared the differences in gene expression profiles of HUVECs induced by CM of two human colon cancer cells, SNUC1 and SNUC5, with those induced by EGM2 MV containing 10% FBS. Interestingly, EGM2 MV containing 10% FBS- and SNUC1 CM-treated HUVECs showed relatively similar gene expression patterns (R 2 = 0.488) (data not shown). However, SNUC5 CM induced a distinctive gene expression pattern compared to that induced by EGM2 MV containing 10% FBS (R 2 = 0.027) (Fig. 1a). These results suggest that although the repertoire of molecules released from some cancer cells could be quite similar to that of transcription-inducing molecules in serum, other cancer cells might release a different set of molecules that could induce a directional change of gene expression in HUVEC, and possibly play a critical role in establishment of tumor microenvironment.
Among the 8,400 genes tested, the expression level of four genes, IL-8, ICAM-1, SOD-2, and GABAR A6, increased more than fourfold. Proinflammatory cytokines, IL-1α, IL-1β and TNF-α were reported to induce IL-8, ICAM-1 and SOD-2 in various cells (Pang et al. 1994; Nakata et al. 1993; Ono et al. 1992; Chokki et al. 2006). In our study, IL-8 and ICAM-1 induction caused by IL-1α in SNUC5 CM was confirmed in neutralization and depletion assays with an antibody specific to IL-1α (Fig. 2 and data not shown, respectively). Thus, IL-1α appears to be the major effecter in the CM of SNUC5 cancer cells on gene expression of endothelial cells. As shown in Figs. 3 and 4, most of the cancer cell lines we tested showed constitutive secretion of IL-1α. But, there was no exact correlation between the quantity of IL-1α in cancer cell CM and the quantity of IL-8 reduced by IL-1α-specific neutralizing antibody in endothelial cell CM. Relevant to this is our finding that the cancer cell CM also contains IL-8 which could induce IL-8 secretion from endothelial cells (data not shown). Other cytokines, such as IL-1β and IL-1R antagonist cytokine (IL-1Ra) (Dinarello 1996), which can bind to the same receptors might also exist in the cancer cell CM and affect IL-8 release.
Clinically, the over-expression of IL-1α and IL-8 has been observed in many types of cancer tissues (Portier et al. 1993; Chen et al. 1999; Cohen et al. 1995; Ueda et al. 1994). IL-1α has been linked with increases of tumor invasiveness and metastasis in various experimental models (Chirivi et al. 1993; Vidal-Vanaclocha et al. 1994, 1996; Chirivi et al. 1996). Release of IL-1α from cancer cells and the subsequent induction of IL-8 release from the cancer cells themselves by an autocrine process or from neighboring fibroblasts were reported previously (Nozaki et al. 2000). An opposite mechanism, secretion of IL-1 from macrophages in tumor tissue and induction of IL-8 release from cancer cells, was also reported. (Liss et al. 2001; Torisu et al. 2000).
This study suggests that some cancer cells can release IL-1α in quantities sufficient to induce IL-8 release from endothelial cells, and that cancer cells have a mechanism to express and release IL-1α constitutively.
Acknowledgment
This work was supported by the Korea Science and Engineering Foundation (KOSEF) (R11-2002-097-07001-0) through the Center for Ageing and Apoptosis Research at Seoul National University.
Contributor Information
Kum-Joo Shin, Phone: +82-2-36687439, FAX: +82-2-7475769, Email: kumjoo@snu.ac.kr.
Junho Chung, Phone: +82-2-36687440, FAX: +82-2-7475769, Email: jjhchung@snu.ac.kr.
References
- Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, McCullagh L, Mousa S, Quezado M, Herscher LL, Van Waes C (1999) Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res 5:1369–1379 [PubMed] [Google Scholar]
- Chirivi RG, Garofalo A, Padura IM, Mantovani A, Giavazzi R (1993) Interleukin-1 receptor antagonist inhibits the augmentation of metastasis induced by interleukin 1 or lipopolysaccharide in a human melanoma/nude mouse system. Cancer Res 53:5051–5054 [PubMed] [Google Scholar]
- Chirivi RG., Chiodoni C, Musiani P, Garofalo A, Bernasconi S, Colombo MP, Giavazzi R (1996) IL-1alpha gene-transfected human melanoma cells increase tumor-cell adhesion to endothelial cells and their retention in the lung of nude mice. Int J Cancer 67:856–863 [DOI] [PubMed] [Google Scholar]
- Chokki M, Mitsuhashi H, Kamimura T (2006) Metalloprotease-dependent amphiregulin release mediates tumor necrosis factor-alpha-induced IL-8 secretion in the human airway epithelial cell line NCI-H292. Life Sci 78:3051–3057 [DOI] [PubMed] [Google Scholar]
- Cohen RF, Contrino J, Spiro JD, Mann EA, Chen LL, Kreutzer DL (1995) Interleukin-8 expression by head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 121:202–209 [DOI] [PubMed] [Google Scholar]
- Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87:2095–2147 [PubMed] [Google Scholar]
- Elenbaas B, Weinberg RA (2001) Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res 264:169–184 [DOI] [PubMed] [Google Scholar]
- Kopfstein L, Christofori G (2006) Metastasis: cell-autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci 63:449–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss C, Fekete MJ, Hasina R, Lam CD, Lingen MW (2001) Paracrine angiogenic loop between head-and neck squamous-cell carcinomas and macrophages. Int J Cancer 93:781–785 [DOI] [PubMed] [Google Scholar]
- Lu H, Ouyang W, Huang C (2006) Inflammation, a key event in cancer development. Mol Cancer Res 4:221–233 [DOI] [PubMed] [Google Scholar]
- Matsushima K, Oppenheim JJ (1989) Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL-1 and TNF. Cytokine 1:2–13 [DOI] [PubMed] [Google Scholar]
- Nakata T, Suzuki K, Fujii J, Ishikawa M, Taniguchi N (1993) Induction and release of manganese superoxide dismutase from mitochondria of human umbilical vein endothelial cells by tumor necrosis factor-alpha and interleukin-1 alpha. Int J Cancer 55:646–650 [DOI] [PubMed] [Google Scholar]
- Nathan CF (1987) Secretory products of macrophages. J Clin Invest 79:319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki S, Sledge Jr G.W, Nakshatri H (2000) Cancer cell-derived interleukin-1 alpha contributes to autocrine and paracrine induction of pro-metastatic genes in breast cancer. Biochem Biophys Res Commun 275:60–62 [DOI] [PubMed] [Google Scholar]
- Ono M, Kohda H, Kawaguchi T, Ohhira M, Sekiya C, Namiki M, Takeyasu A, Taniguchi N (1992) Induction of Mn-superoxide dismutase by tumor necrosis factor, interleukin-1 and interleukin-6 in human hepatoma cells. Biochem Biophys Res Commun 182:1100–1107 [DOI] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348 [DOI] [PubMed] [Google Scholar]
- Pang G, Couch L, Batey R, Clancy R, Cripps A (1994) GM-CSF, IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1 alpha and TNF-alpha. Clin Exp Immunol 96:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier M, Zhang XG, Ursule E, Lees D, Jourdan M, Bataille R, Klein B (1993) Cytokine gene expression in human multiple myeloma. Br J Haematol 85:514–520 [DOI] [PubMed] [Google Scholar]
- Rollins BJ (2006) Inflammatory chemokines in cancer growth and progression. Eur J Cancer 42:760–767 [DOI] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M (2000) Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNF-alpha and IL-1 alpha. Int J Cancer 85:182–188 [PubMed] [Google Scholar]
- Ueda T, Shimada E, Urakawa T (1994) Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol 29:423–429 [DOI] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Amezaga C, Asumendi A, Kaplanski G, Dinarello CA (1994) Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res 54:2667–2672 [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA (1996) Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst 88:198–205 [DOI] [PubMed] [Google Scholar]
- Werb Z, Egeblad M, Littlepage LE (2005) Coevolution of cancer and stromal cellular responses. Cancer Cell 7:499–500 [DOI] [PubMed] [Google Scholar]
- Witz IP (2006) Tumor-microenvironment interactions: the selectin-selectin ligand axis in tumor-endothelium cross talk. Cancer Treat Res 130:125–140 [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30:e15 [DOI] [PMC free article] [PubMed] [Google Scholar]