Introduction
The cyclooxygenases Cox-1 and Cox-2 are the rate-limiting enzymes in the synthesis of all prostanoids from arachidonic acid (Smith et al. 2000). While Cox-1 is expressed constitutively in a subset of cell types, Cox-2 is highly regulated by transcriptional and post-translational mechanisms in response to a plethora of stimuli. Induction of Cox-2 triggers the synthesis of different prostanoids that play essential roles in many physiological processes and responses, such as inflammation, pain, fever, and platelet aggregation. Cyclooxygenases catalyze a two-step reaction that converts arachidonic acid to prostaglandin H2 (PGH2) which in turn serves as the precursor for the synthesis of all biologically active prostanoids: PGD2, PGE2, PGF2, prostacyclin (PGI2), 15-deoxy-Δ12,14-PGJ2, and thromboxane A2 (Fig. 1). Work of the past decade has clearly established Cox-2 and a subset of prostanoids and their receptors as crucial players in oncogenesis that regulate, and are regulated by, pathways with essential functions in oncogenesis. This article will provide a review of the literature covering this area of research.
Fig. 1.
Prostanoid synthesis and signaling receptors. PG prostaglandin, Tx thromboxane, PGJ 2 15-deoxy-Δ12,14-prostaglandin J2. Open boxes enzymes (Cox-1/2 cyclooxygenase-1/2, PGDS, PGES, PGFS, PGIS prostaglandin D2/E2/F2/I2-synthase, PGIS prostacyclin synthase, TxAS thromboxane A2 synthase, PGER prostaglandin E2 9-reductase). Filled boxes uclear receptors for prostanoids (orphan receptors, PPAR peroxisome proliferator-activated receptor). Both PPAR-γ and PPAR-δ interact with PPAR-response elements (PPREs) as heterodimers with the retinoic acid receptor RxR. Filled circles G-protein coupled membrane receptors (DP, EP, FP, IP, TP) for prostanoids
Cox-2 and cancer
Cox-2 is overexpressed in many experimental and human tumors as a consequence of deregulated signaling pathways involved in the control of Cox-2 transcription, steady-state RNA levels, and/or translation (see also Fig. 2). Multiple pathways regulating Cox-2 expression have been described with a predominant role for Ras transmitted signals. Thus, Cox-2 transcription is upregulated through the Ras-Raf-MEK-ERK and Ras-Rac-MEKK1-JNKK-JNK cascades (Araki et al. 2003; Sheng et al. 1998; Subbaramaiah et al. 2002; Xie & Herschman 1995; Xie & Herschman 1996) (M. Kreutzer and R.M., unpubl. observation). While ERK phosphorylates and thereby activates the transcription factors PEA3 (an Ets family member) and C/EPB, JNK targets the transcriptional activator AP-1 (Jun/Fos heterodimers) interacting with an ATF/CRE site in the Cox-2 promoter (Reddy et al. 2000; Subbaramaiah et al. 2002; Xie & Herschman 1995). The Cox-2 promoter is also controlled by other oncogenesis-related mechanisms, including NFκB activation by hypoxia (Ji et al. 1998; Schmedtje et al. 1997), Wnt/Apc-controlled upregulation of the PEA3 and TCF-4 transcription factors (Araki et al. 2003; Howe et al. 2001), p53 (Han et al. 2002; Subbaramaiah et al. 1999) as well as RhoB and cAMP signaling (Shao et al. 2000). Furthermore, regulation of Cox-2 mRNA stability via the Ras-regulated Raf-MEK-ERK and PI3K-PDK-AKT pathways has been reported (Lin et al. 2001; Sheng et al. 2000; Sheng et al. 2001).
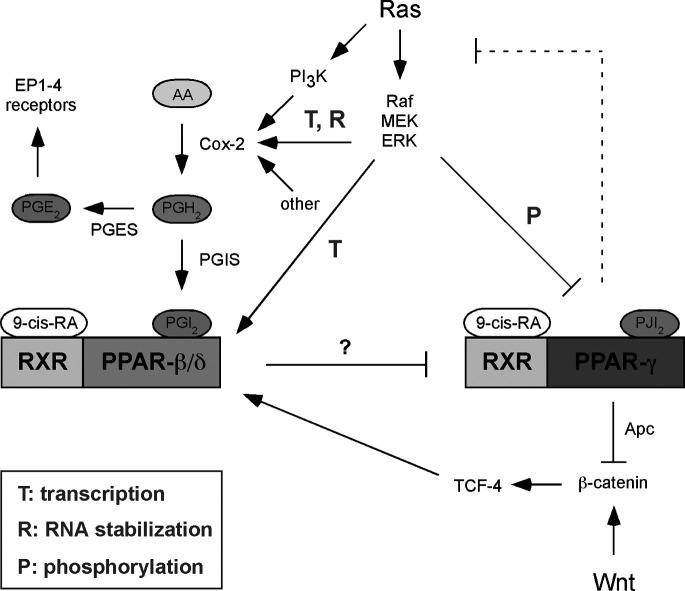
Fig. 2.
Crosstalk of Ras, Wnt, and prostanoid signaling pathways in cancer cells. For details and references see main text. Prostanoid synthesis and receptors are shown in detail in Fig. 1, the Wnt pathway is depicted in Fig. 4
There is an overwhelming body of evidence indicating that the deregulation or overexpression of Cox-2 expression in tumor and/or tumor stroma cells plays a major role in oncogenesis (Dannenberg & Subbaramaiah 2003; Gupta & Dubois 2001; Williams et al. 1999). This conclusion is mainly based on work with non-steroidal anti-inflammatory drugs (NSAIDs) that are known to inhibit either both cyclooxygenases, such as aspirin, or specifically Cox-2, such as the recently developed coxibs (e.g, rofecoxib, celecoxib) (Flower 2003). These studies have clearly demonstrated the potential of Cox-2 inhibiting NSAIDs to interfere with cell cycle progression and to induce apoptosis in tumor cells (Arico et al. 2002; Chen et al. 2003a; Cheng et al. 2002; Denkert et al. 2003; Detjen et al. 2003; Ding et al. 2000; Elder et al. 2000; Elder et al. 2002; Grosch et al. 2001; Hida et al. 2000; Hu et al. 2003; Jendrossek et al. 2003; Kardosh et al. 2004; Kundu et al. 2002; Leahy et al. 2002; Lee et al. 2002; Li et al. 2001; Liu et al. 1998; Minter et al. 2003; Moalic et al. 2001; Nakanishi et al. 2001; Narayanan et al. 2003; Peng et al. 2003; Poon et al. 2001; Richter et al. 2001; Romano & Claria 2003; Sawaoka et al. 1998; Souza et al. 2000; Totzke et al. 2003; Uefuji et al. 2000; Ueta et al. 2001; Wu et al. 2003; Yamazaki et al. 2002). In addition, NSAIDs have been shown to interfere with Cox-2 mediated processes that play essential roles in tumor progression, such as angiogenesis, tumor cell invasion and metastasis. The underlying molecular mechanism include:
Downregulation of cyclin-dependent kinases (Cdks), partly through Rb hyperphosphorylation and upregulation of Cdk inhibitors (Grosch et al. 2001; Kardosh et al. 2004; Nakanishi et al. 2001; Narayanan et al. 2003; Peng et al. 2003; Tseng et al. 2002; Yao et al. 2003; Yao et al. 2000);
Downregulation of anti-apoptotic proteins of the Bcl-2 family (Chen et al. 2003b; Gately & Kerbel 2003; Jendrossek et al. 2003; Lin et al. 2001; Liu et al. 1998; Nzeako et al. 2002; Richter et al. 2001; Uefuji et al. 2000; Ueta et al. 2001);
Modulation of the hyaluronate receptor CD44 and matrix metalloproteinase activities (Attiga et al. 2000; Dohadwala et al. 2002; Dohadwala et al. 2001; Liu et al. 2002; Pai et al. 2002; Pan et al. 2001); and
Inhibition of vascular endothelial growth factor (VEGF) synthesis (Kim et al. 2003; Li et al. 2002; Masferrer et al. 1999; Ogawa et al. 2003; Su et al. 2004; Williams et al. 2000).
It is therefore no surprise that NSAIDs have been shown to be potent suppressors of tumor growth in diverse mouse models, including human xenografts, chemically induced tumors as well as tumors developing in transgenic mice (see Table 1 for summaries and references). The crucial role of Cox-2 in oncogenesis has also been clearly demonstrated in multiple studies making use of mice with targeted disruptions of both alleles of the Cox-2 gene (see Table 2). Remarkably, an anti-tumorigenic effect has also been clearly documented in humans where the long-term use of aspirin appears to reduce the incidence of different types of cancer, in particular the development of colorectal carcinomas in patients with familial polyposis coli (Gupta & Dubois 2001; Markenson 1999; Reddy & Rao 2002; Williams et al. 1999).
Table 1.
Effects of cyclooxygenase inhibitors in mouse models of tumorigenesis
| Drug | Experimental system | Effect | Reference |
|---|---|---|---|
| Gastrointestinal tumors | |||
| MF-tricyclic (Cox-2 inhibitor) or Sulindac | Intestinal adenoma (polyps) | Suppression of tumor formation, but less inhibition by sulindac | Oshima et al. 1996 |
| (ApcΔ716 mouse) | |||
| Sulindac | Intestinal adenoma | Suppression of tumorigenesis | Boolbol et al. 1996 |
| (ApcΜιν mouse) | |||
| Celecoxib | Intestinal adenoma | Prevention of tumorigenesis, partial regression of established tumors | Jacoby et al. 2000 |
| (ApcΜιν mouse) | |||
| Rofecoxib | Intestinal adenoma (polyps) | Suppression of tumor formation | Oshima et al. 2001 |
| (ApcΔ716 mouse) | |||
| MF-tricyclic (Cox-2 inhibitor) or Sulindac | Intestinal adenoma | Suppression of tumor formation, but less inhibition by sulindac | Lal et al. 2001 |
| (ApcΜιν x Msh2-/- mouse) | |||
| JTE-522 (Cox-2 inhibitor) | Human colon cancer xenograft (LM-H3) | Reduced liver metastases, reduced expression of PDGF and MMP-2, but not VEGF | Nagatsuka et al. 2002 |
| Rofecoxib | MC26 colon carcinoma (splenic transplant) | Reduced tumor growth and metasteses, decreased cyclin D1 and VEGF expresion | Yao et al. 2003 |
| JTE-522 (Cox-2 inhibitor) | Colon cancer xenografts | No effect on growth of primary tumors, but reduced metastasis (HAT-29), decreased VEGF expression | Yamauchi et al. 2003 |
| (HT-29, COLO205) | |||
| NS-398 (Cox-2 inhibitor) or Indometahcine | Gastric cancer xenograft (MKN45) | Reduced tumor growth, increased tumor cell apoptosis | Sawaoka et al. 1998 |
| Lung tumors | |||
| Cox-1/2 inhibitors (sulindac or ASS) | Chemically induced lung adenoma (NKK) | Reduced lung tumor multiplicity | Duperron & Castonguay 1997 |
| NS-398 (Cox-2 inhibitor) or Aspirin | Chemically induced lung adenoma (NKK) | Reduced lung tumor multiplicity, induction of apoptosis | Rioux & Castonguay 1998 |
| Yao et al. 2000 | |||
| Indomethacine | Chemically induced lung adenoma (uretahne) | Reduced lung tumor multiplicity | Moody et al. 2001 |
| Indomethacine | Lewis lung carcinoma (s.c. xenograft, lung ‘metastases’) | Delayed and attenuated tumor growth | Eli et al. 2001 |
| JTE-522 (Cox-2 inhibitor) | Human lung adenocarcinoma xenograft (ACC-LC-319) | Reduced tumor growth | Hida et al. 2002 |
| Celecoxib or Aspirin | Chemically induced lung adenoma (MCA/BHT) | No reduction of lung tumor growth, but reduction of both PGE2 synthesis and inflammation (celecoxib) | Kisley et al. 2002 |
| Celecoxib | Chemically induced lung adenoma (urethane) | ||
| Other carcinomas | |||
| JTE-522 (Cox-2 inhibitor) | Human head & neck cancer xenograft (KB) | Reduced tumor growth | Nishimura et al. 1999 |
| SC-560 (Cox-1 inhibitor) | Human head & neck cancer xenograft (1483 cells) | Reduced tumor growth, decreased PGE2 synthesis | Zweifel et al. 2002 |
| Celecoxib | No effect on tumor growth | ||
| Celecoxib | Oral carcinoma intradermal transplant | Suppression of tumor growth, decreased vascular density | Wang et al. 2002 |
| Celecoxib | Chemically induced urinary bladder cancer (nitrosamine) | Inhibition of tumorigenesis | Grubbs et al. 2000 |
| Rofecoxib | Human pancreatic carcinoma orthotopic xenograft (PaCa-2) | Reduced tumor growth, reduced cyclin D1 and increased p21 expression | Tseng et al. 2002 |
| Celecoxib | MMTV-HER2/neu transgenic mouse (mammary carcinoma) | Reduced tumor growth, decreased PGE2 synthesis | Howe et al. 2002 |
| Sarcomas | |||
| Indomethacine | Sarcoma (MCG-101) and colon cancer (HT-29) xenografts | Reduced tumor growth, increased tumor ell apoptosis, deceased teleomerase | Lonnroth et al. 2001 |
| NS-398 or JTE-522 (Cox-2 inhibitors) or Aspirin | S-180 sarcoma s.c. transplant | Reduction of tumor growth, reduced angiogenesis | Yoshida et al. 2003 |
| Mofezolac (Cox-1 inhibitor) | No effect on tumor growth | ||
Table 2.
Tumor models in mice with targeted alterations in prostanoid signaling
| Genetic alteration | Tumor model | Effect | Reference |
|---|---|---|---|
| Gastrointestinal tumors | |||
| Cox-2 disruption | Intestinal adenoma (polyps) | Suppression of tumor formation | Oshima et al. 1996 |
| (ApcΔ716 mouse) | |||
| Cox-1 disruption | Intestinal adenoma (polyps) | Suppression of tumor formation | Chulada et al. 2000 |
| (ApcΜιν mouse) | |||
| Cox-2 disruption | |||
| EP4 disruption | Chemically induced polyps (azoxymethane) | Reduced polyp formation | Mutoh et al. 2002 |
| EP2 disruption | Intestinal adenoma (polyps) | Reduced polyp incidence and size, reduced tumor cell proliferation | Sonoshita et al. 2001 |
| (ApcΔ716 mouse) | |||
| EP3 or EP4 disruption | No effect | ||
| EP2 or Cox-2 disruption | Intestinal adenoma (polyps) and carcinoma | Reduced tumor growth and decreased microvessel density | Seno et al. 2002 |
| (ApcΔ716 and ApcΔ716 x Smad4 mouse) | |||
| EP1 or EP3 disruption | No effect | ||
| PPAR-γ disruption (heterozygous) | Intestinal adenoma (polyps) | Increased polyp formation | Girnun et al. 2002 |
| (ApcΜιν mouse) | |||
| Chemically induced polyps in rats (azoxymethane) | No effect of PPAR-γ status | ||
| PPAR-δ disruption | HCT116 colon cancer cells with PPAR-δ null allels | Decreased tumorigenicity (xenotransplant) | Park et al. 2001 |
| PPAR-δ disruption | Intestinal adenoma (polyps) | Reduced number of polyps larger than 1 mm | Barak et al. 2002 |
| (ApcΜιν mouse) | |||
| Lung tumors | |||
| Cox-2 disruption | Lewis lung carcinoma (syngeneic s.c. transplant) | Reduced tumor growth, angiogenesis and VEGF production by fibroblasts in null mice | Williams et al. 2000 |
| EP2 disruption | Lewis lung carcinoma and C26 colon carcinoma (syngeneic s.c. transplant) | Reduced tumor growth, increased anti-tumor CTL response | Yang et al. 2003 |
| PGIS driven by the alveolar-specific SP-C promoter | Chemically induced lung adenoma (urethane or MCA/BHT) | Reduced tumor growth in transgenic mice | Keith et al. 2002 |
| Other tumors | |||
| Cox-1 or Cox-2 disruption | Chemically induced skin tumors (DMBA) | Reduced skin tumorigenesis | Tiano et al. 2002 |
| Cox-2 driven by keratin-5 promoter | Chemically induced skin tumors (DMBA) | Enhanced formation of sqamous cell carcinomas | Muller-Decker et al. 2002 |
| Cox-2 driven by keratin-14 promoter | Chemically induced skin tumors (DMBA/TPA) | Decreased (!) tumor formation | Bol et al. 2002 |
| EP3 disruption | Sarcoma-180 | Reduced tumor growth and tumor-associated angiogenesis, reduced VEGF expression by stromal fibroblasts | Amano et al. 2003 |
| (syngeneic s.c. transplant) | |||
| Targeted PPAR-γ disruption in mammary epithelium | Spontaneous mammary tumors | No changein tumor incidence | Cui et al. 2002 |
Prostanoid signaling and oncogenesis
PGD2, PGE2, PGF2, prostacyclin, and thromboxane A2 interact with G-protein coupled membrane receptors, termed DP, EP-1 through EP-4, FP, IP and TP, respectively (Fig. 1). These receptors trigger multiple second messenger generating systems, including adenylate cyclase and phospholipase C (Versteeg et al. 1999). 15-deoxy-Δ12,14-PGJ2 also binds to and stimulates the nuclear “orphan” receptor and transcription factor “peroxisome proliferator-activated receptor-γ” (PPAR-γ), while the related PPAR-δ, also known as PPAR-β, interacts with prostacyclin (Fig. 1) (Berger & Moller 2002; Forman et al. 1996; Forman et al. 1995; Giguere 1999; Kliewer et al. 1999; Lim & Dey 2002; Michalik et al. 2004). Both PPARs form heterodimers with the retinoic acid receptor RxR and interact with composite DNA elements comprised of RxR and PPAR recognizing half-sites (Gearing et al. 1993; Kliewer et al. 1992; Marcus et al. 1993; Tontonoz et al. 1994).
Several specific components of the prostanoid signaling network have also been associated with oncogenesis, in particular PGE2 and its membrane receptors EP-2, EP-3, and EP-4 as well as PPAR-γ and PPAR-δ. While PGE2 signaling seems to play a predominant role in promoting tumor angiogenesis through upregulation of pro-angiogenic growth factors (Kurie & Dubois 2001), PPAR-δ and its ligand prostacyclin as well as PPAR-γ and its natural agonist 15-deoxy-Δ12,14-prostaglandin J2 have also been implicated in the regulation of tumor cell proliferation and apoptosis with opposing effects: while PPAR-γ activation results in anti-oncogenic effects, PPAR-δ seems to promote oncogenesis (Michalik et al. 2004). The published work addressing these issues is discussed in detail in the following sections. The only other Cox-2 product implicated in oncogenesis is TxA2. This is suggested by the observation that the ectopic expression of TxAS increases the tumorigenicity of colon-26 cells in syngeneic mice (Pradono et al. 2002). Clearly, more experimental work is required to clarify a possible role of TxA2 in oncogenesis.
Prostaglandin E2 signaling in oncogenesis
Three of the four identified G-protein coupled PGE2 receptors have been associated with cancer in different mouse models of oncogenesis (summarized in Table 2). Disruption of both EP2 alleles has been reported to inhibit tumor cell proliferation and tumor growth in ApcΔ716 mice (Seno et al. 2002; Sonoshita et al. 2001). These mice harbor a mutant allele of the Apc tumor suppressor gene (see below) and develop multiple intestinal adenomas with high penetrance. In mice with an additional mutation in the Smad-4 gene, which codes for a component of TGF-β signaling cascade, adenomas progress to adenocarcinomas (Seno et al. 2002). Disruption of EP2 led to reduced polyp incidence and size and inhibited carcinoma growth beyond a small size. These tumors showed decreased microvessel density indicating a critical role for EP2 in tumor angiogenesis (Seno et al. 2002). In contrast, genetic inactivation of EP1, EP3 or EP4 had no effect on tumorigenesis (see Table 3) in ApcΔ716 mice pointing to a specific role for EP2 in this model (Seno et al. 2002; Sonoshita et al. 2001). EP2 disruption has also been reported to inhibit the growth of syngeneic s.c. transplants of Lewis lung carcinoma and C26 colon carcinoma, but in these cases a major determinant of tumor growth inhibition was an increased anti-tumor CTL response (Yang et al. 2003).
Table 3.
Tumorigenesis-related effects of modulated prostacyclin levels
| Compound | Experimental system | Effect | Reference |
|---|---|---|---|
| Prostacyclin | Perfused rat lung | Increased VEGF synthesis | Hoper et al. 1997 |
| Prostacyclin | Renal medullary interstitial cells | Inhibition of stress-induced apoptosis via PPAR-δ | Hao et al. 2002 |
| Prostacyclin (stromal fibroblast-derived) | HCA-7 human colon cancer cells | Inhibition of apoptosis probably via PPAR-δ | Cutler et al. 2003 |
| PGIS antisense | HUVECs | Reduced capillary-like tube formation in vitro | Spisni et al. 2001 |
| PGIS | HEK293 cells | Induction of apoptosis probably via PPAR-δ | Hatae et al. 2001 |
| PGIS | C26 mouse colon cancer cells (retrovirally transduced) | Inhibition of tumorigenicity | Pradono et al. 2002 |
In contrast to the lack of any effect of EP3 disruption on polyp formation in ApcΔ716 mice (see above), its homozygous deletion has been reported to inhibit the growth of syngeneic s.c. transplants of Sarcoma-180, concomitant with reduced tumor-associated angiogenesis and reduced VEGF expression by stromal fibroblasts (Amano et al. 2003).
The role of EP4 has been studied in another mouse model of intestinal oncogenesis, i.e., the formation of polyps (aberrant crypt foci) in mice treated with the chemical carcinogen azoxymethane (Mutoh et al. 2002). Genetic inactivation of EP4 led to a 44% reduction in the numbers of polyps, while no significant effect on tumor growth was seen when other prostanoids receptor genes (EP2, DP, FP, IP or TP) were genetically inactivated. An inhibitory effect in the same animal model was also seen with a selective EP4 antagonist (Mutoh et al. 2002). In this case, the therapeutic target may be the tumor cell itself rather than tumor angiogenesis supporting stromal cells.
A direct effect of PGE2 on tumor cells has also been described in another study using colon and gastric cancer cells as a model (Pai et al. 2002). In this study, PGE2 has been found to transactivate the EGF receptor (EGFR), which requires activation of the tyrosine protein kinase c-Src, metalloproteinase activity and release of the EGFR ligand TGF-α. As a consequence of EGFR activation, the Erk signaling pathway is induced potentially leading to increased cell proliferation and promoting oncogenesis.
Prostacyclin and PPAR-δ signaling
PPAR-δ is a nuclear receptor that binds to, and is activated by, prostacyclin. However, a number of other natural fatty acids, including arachidonic acid and eicosapentaenoic acid, also interact with PPAR-δ, albeit with an apparently lower activation potential (Forman et al. 1997; Xu et al. 1999). It remains therefore unclear whether prostacyclin is the only physiologically relevant PPAR-δ ligand, but so far the role of other fatty acids or derivatives in PPAR-δ mediated oncogenesis-related processes has not been investigated. PPAR-δ heterodimerizes with the retinoic acid receptor RxR and interacts with a number of transcriptional cofactors and other nuclear proteins (Fig. 3), but their precise function in transcriptional regulation by PPAR-δ is unclear.
Fig. 3.
Interaction of PPARγ and PPAR-δ with RxR, transcriptional coactivators and corepressors, and regulation by natural and synthetic agonistic and antagonistic ligands. Agonists are thought to switch the interaction of PPARs with corepressors recruiting histone deacetylases (HDACs) to an interaction with coactivators with histone acetylase activity (e.g., p300, CBP). Ligand-induced alterations of chromatin structure therefore to play an essential role in the regulation of PPAR activity. An important target of PPAR-γ in intestinal tumor cells is the Wnt signaling pathway. PPAR-γ induces increased levels of ß-catenin by an as yet unknown mechanism, and thus increases the transcriptional activity of TCF-4 and thereby the expression of cyclin D1 and presumably other genes with functions in tumorigenesis. PPAR-δ targets several genes encoding proteins of the PI3K pathway in keratinocytes (upreguation of PDK1 and ILK, downregulation of PTEN) and thereby increases apoptotic resistance. See main text for further details and references
A role for PPAR-δ in tumorigenesis has been shown in intestinal cells where the Apc tumor suppressor gene product normally inhibits the TCF-4 mediated transcriptional induction of PPAR-δ (He et al. 1999), a transcriptional target of the Wnt pathway (Willert & Nusse 1998) (see also Fig. 4). In colorectal polyposis, Apc is genetically inactivated and transcription of PPAR-δ is increased due to deregulated TCF-4 activity. That this increase in PPAR-δ expression is functionally relevant is suggested by two independent observations. First, the disruption of both PPAR-δ alleles in the human colorectal carcinoma cell line HCT116 has been reported to inhibit tumor growth in immune-deficient mice (Park et al. 2001). The second piece of evidence has been obtained with ApcMin mice, which harbor a dominant mutant Apc allele and develop intestinal adenomas with 100% penetrance. Even though the homozygous deletion of PPAR-δ in these mice does not lead to a lower overall tumor incidence, the growth of tumors beyond a diameter of approximately 2 mm is dramatically reduced (Barak et al. 2002), suggesting a role for PPAR-δ in tumor progression rather than initiation. In agreement with these findings is the recent observation that treatment of ApcMin mice with the PPAR-δ agonist GW501516 led to a fivefold increase in the number of polyps larger than 2 mm, clearly implicating PPAR-δ in the regulation of intestinal adenoma growth (Gupta et al. 2004).
Fig. 4.
Wnt signaling, Apc and PPARs. In Apc wild-type cells, glycogen synthase kinase-3 (GSK-3) phosphorylates components of a cytoplasmic multi-protein complex containing the tumor suppressor protein Apc, β-catenin, and axins. This results in the proteolytic ubiquitin-dependent degradation of β-catenin in the proteasome and thereby its exclusion from the nucleus. The transcription factors TCF-4 is inctive and its target genes are repressed by a histone deacetylase (HDAC). In cells cells lacking sufficient amounts of functional Apc, no cytoplasmic muti-protein complex is formed, β-catenin is not phosphprylated by GSK-3 and can enter the nucleus to stimulate TCF-4 dependent transcription of multiple target genes in concert with other transcription factors and coactivators. The induced genes include c-Myc, cyclin D1, and PPAR-δ
In line with a pro-oncogenic potential of prostacyclin is the observation that prostacyclin released by human colon carcinoma stromal fibroblasts promotes the survival of the tumor cells (Cutler et al. 2003), that keratinocytes from PPAR-δ null mice show an increased rate of apoptosis (Tan et al. 2001) and that apoptosis in mesenchymal renal medullary interstitial cells is inhibited by prostacyclin and PPAR-δ (Hao et al. 2002). Furthermore, the 15-lipoxygenase-1 product 13(S)-HODE, a PPAR-γ agonist (Nagy et al. 1998) and inhibitor of PPAR-δ expression (Shureiqi et al. 2003), induces apoptosis in colon cancer cells in a PPAR-δ dependent fashion (Shureiqi et al. 2003), confirming a crucial role for PPAR-δ in the 13(S)-HODE induced inhibition of proliferation.
Prostacyclin probably also plays a role in angiogenesis. Female Cox-2 null mice are unable to produce litters due to a failure in blastocyst implantation and decidualization (Dinchuk et al. 1995; Lim et al. 1997). These mice synthesize approximately 50% lower levels of prostacyclin, PGE2, and TxB2 at the implantation site (Lim et al. 1999). In addition, expression of the VEGF receptor Flk-1 at the implantation site is reduced. The implantation defect can be partially rescued by treatment with prostacyclin, which in this scenario probably acts through PPAR-δ. Interestingly, treatment with prostacyclin and PGE2 also induces Flk1 expression at the implantation site (Lim et al. 1999). Collectively, these observations point to an involvement of prostacyclin-PPAR-δ signaling in angiogenesis during decidualization. A pro-angiogenic function or prostacyclin is also suggested by two other observations: perfusion of rat lung tissue with prostacyclin (or PGE2) induces the synthesis of VEGF (Hoper et al. 1997), and the antisense-mediated inhibition of PGIS has been reported to interfere with capillary-like tube formation in HUVEC cultures (Spisni et al. 2001).
In apparent contrast to the observations summarized above, the ectopic expression of PGIS has been reported to inhibit chemically induced lung tumorigenesis in mice (Keith et al. 2002) and to promote apoptosis in the human embryonic kidney cell line 293 (Hatae et al. 2001). These apparent discrepancies may be due to different reasons. It is possible that intracellularly synthesized prostacyclin (by PGIS overexpression) and prostacyclin acting in a paracrine fashion have fundamentally different biological effects due to differences in subcellular distribution or interactions with other cellular components, such as fatty acid binding proteins (FABPs) serving as transport vehicles for nuclear translocation and modifiers of the transcriptional and biological response (Tan et al. 2002). Furthermore, cell type-specific differences in the effects of prostacyclin and PPAR-δ cannot be ruled out. In this context, the availability of other proteins interacting with, and modulating the function of, PPAR-δ, such as the retinoic acid receptor RxR or FABPs, may be crucial. Finally, PPAR-δ ligands other than prostacyclin most likely exist (Forman et al. 1997; Shaw et al. 2003; Xu et al. 1999), so that a functional link between prostacyclin and PPAR-δ is not obligatory. Clearly, more work is required to clarify the role of prostacyclin and PPAR-δ mediated signaling in tumorigenesis.
To date, only few target genes of PPAR-δ are known. Of particular interest with respect to tumorigenesis is the agonist-dependent PPAR-δ mediated upregulation of the Akt pathway in keratinocytes through the transcriptional induction of PDK-1 and ILK and the repression of the phosphatidylinositolphosphate phosphatase and tumor suppressor gene PTEN (Di-Poi et al. 2002). In vascular smooth muscle cells, PPAR-δ modulates the expression several cell cycle regulators (cyclin A, cdk2, p57Kip) (Zhang et al. 2002) but this was not observed in skeletal muscle cells (Dressel et al. 2003). It is currently not known whether the regulation of Cdk activity by PPAR-δ plays a role in tumor cell proliferation. Finally, as PPAR-δ appears to be able to repress the transcriptional activity of PPAR-γ (Shi et al. 2002), targets of PPAR-γ (see below) might also represent indirect targets for PPAR-δ.
PPAR-γ signaling
PPAR-γ is another nuclear receptor that interacts with fatty acid derivatives. PPAR-γ shows similar protein-protein interactions as PPAR-δ (see above and Fig. 3), but is activated by different ligands, including the natural prostanoid 15-PGJ2 and a number of isoform-specific synthetic agonists (thiazolidinedione anti-diabetic drugs), such as rosiglitazone (BRL 49653), troglitazone, ciglitizone and pioglitazone (Fig. 3). In addition, PPAR-γ specific antagonists have been identified, e.g., GW9662 and BADGE. A large body of evidence suggests that PPAR-γ has anti-oncogenic properties. Activation of PPAR-γ by 15-PGJ2 or synthetic ligands inhibits tumor cell proliferation in vitro, suppresses tumor growth in various mouse models and induces tumor cell apoptosis in vivo and in vitro (see Table 4 for details and references). In addition, it has been reported that PPAR-γ is expressed at high levels in tumor endothelial cells, and that PPAR-γ ligands inhibit endothelial cells proliferation and interfere with tumor angiogenesis in mouse models (Panigrahy et al. 2002; Xin et al. 1999).
Table 4.
Biological effects of PPAR-γ agonists in experimental tumors and cultured cells
| Compound | Experimental system | Effect | Reference |
|---|---|---|---|
| Gastrointestinal tumors | |||
| Troglitazone | Human colon cancer cells | Reduction of clonogenic growth, reversal of cancer-specific gene expression patterns, tumor growth inhibition in vivo (xenotransplant) | Sarraf et al. 1998 |
| Rosiglitazone, Troglitazone | Intestinal adenoma (polyps) | Enhanced colonic polyp formation | Lefebvre et al. 1998 |
| (ApcΜιν mouse) | Saez et al. 1998 | ||
| Troglitazone | Chemically induced polyps in rats (azoxymethane) | Reduced polyp formation and cell proliferation | Tanaka et al. 2001 |
| Rosiglitazone | Caco2 human colon cancer cells | Reduced proliferation; upregulation of PTEN | Patel et al. 2001 |
| 15-PGJ2, Ciglitizone | HT29 human colon cancer cells | Induction of apoptosis, reduced levels of Bcl-2 and NFκB | Chen et al. 2002 |
| Troglitazone | HT29 human colon cancer cells | Inhibition of proliferation; reduced adhesion to extracellular matrix; reduced MMP-7 expression | Sunami et al. 2002 |
| Rosiglitazone, Troglitazone, Pioglitazone | Chemically induced intestinal polyps (azoxymethane) | Suppression of tumor growth | Osawa et al. 2003 |
| 15-PGJ2 | MCG-803 human gastric carcinoma cells | Inhibition of cell proliferation, induction of apoptosis | Chen et al. 2003b |
| Lung tumors | |||
| Ciglitizone,15-PGJ2 | Human NSCLC cells | Induction of differentiation and apoptosis, decreased expression of cyclin D1, hypophosphorylation of Rb | Chang & Szabo 2000 |
| PPAR-γ agonist or PPAR-γ overexpression | Human NSCLC cells | Inhibition of proliferation | Wick et al. 2002 |
| Ciglitizone | Lewis lung carcinoma and other tumor cell lines | Inhibition of tumor growth and metastasis, suppression of angiogenesis | Panigrahy et al. 2002 |
| Other carcinomas | |||
| Troglitazone | Human breast cancer cells | Inhibition of proliferation and induction of apoptosis in vitro and in vivo (xenotransplant) | Elstner et al. 1998 |
| Mueller et al. 1998 | |||
| Rosiglitazone | MCF7 human breatst cancer cells | Reduced proliferation; upregulation of PTEN | Patel et al. 2001 |
| Troglitazone | Human hepatoma cells | Inhibition of proliferation (G1 arrest); increased expression of Cdk inhibitors p18, p21, p27 | Koga et al. 2001 |
| Troglitazone | Human pancreatic carcinoma cells | Inhibition of proliferation (G1 arrest); reduction of cyclin D1 expression; synergy with 9-cis-retinoic acid | Toyota et al. 2002 |
| Troglitazone, Pioglitazone | Human NSCLC cells | Inhibition of proliferation and induction of apoptosis; induction of GADD153 gene | Satoh et al. 2002 |
| 15-PGJ2 | MCF7 human breast cancer cells | Cyclin D1 repression/degradation | Wang et al. 2001 |
| Qin et al. 2003 | |||
| Rosiglitazone | Human prostate carcinoma cells | Inhibition of proliferation | Xu et al. 2003 |
| Other tumors | |||
| Rosiglitazone, TroglitazonePPAR-γ agonists | Human pituatary tumor cells | Inhibition of proliferation and induction of apoptosis in vitro and in vivo (xenotransplant) | Heaney et al. 2002 |
| Heaney et al. 2003 | |||
| Ciglitizone, Troglitazone | Human osteosarcoma cells | Induction of differentiation and apoptosis | Haydon et al. 2002 |
| Ciglitizone, Troglitazone,15-PGJ2 | Uterine leiomyoma | Inhibition of proliferation | Houston et al. 2003 |
| Endothelial cells | |||
| 15-PGJ2, Ciglitizone, BRL49653 | HUVECs | Inhibition of differentiation and proliferation, inhibition of angiogenesis in rat cornea | Xin et al. 1999 |
| Ciglitizone | HUVECs | Inhibition of proliferation, suppression of angiogenesis in chick chorioallantois, cornea and tumors | Panigrahy et al. 2002 |
In ApcMin mice, PPAR-γ agonists paradoxically enhance the formation of intestinal polyps (Lefebvre et al. 1998; Saez et al. 1998) whereas the growth of chemically induced polyps is inhibited (Osawa et al. 2003; Tanaka et al. 2001). A possible explanation is suggested by the observation that the heterozygous disruption of PPAR-γ leads to an increase in the level of β-catenin (Girnun et al. 2002), a central component of the Wnt-Apc pathway (see Fig. 4) (Willert & Nusse 1998). This induction of β-catenin depends on the presence of two intact Apc alleles, possibly because PPAR-γ targets one of the components acting upstream of β-catenin, such as axins, GSK-3 or Apc itself (Girnun et al. 2002). In analogy, ligand-activated PPAR-γ should be able to suppress β-catenin levels only in an Apc wildtype situation (Fig. 3), which would explain the discrepancies seen in different animal models of intestinal tumorigenesis.
The PPAR-γ mediated downregulation of β-catenin described above (Girnun et al. 2002) results in decreased transcriptional activity of TCF-4 and suppression of c-myc and cyclin D1 as target genes of the Wnt pathway (He et al. 1998; Tetsu & McCormick 1999; Yamakawa-Karakida et al. 2002). PPAR-γ thus impinges on oncogenesis by modulating the expression of essential components of the cell cycle control machinery. Intriguingly, β-catenin/TCF-4 signaling induces PPAR-δ in colorectal carcinoma cells (He et al. 1999). It is therefore tempting to speculate that PPAR-γ agonists exert their anti-tumorigenic effect in apart by downregulating PPAR-δ expression (Fig. 2 and Fig. 4). This may also explain the anti-proliferative and proapoptotic effect of the PPAR-γ agonist 13(S)-HODE described above (Shureiqi et al. 2003).
The microarray-based search for PPAR-γ target genes has provided additional hints how PPAR-γ might exert its inhibitory function during oncogenesis. Microarray studies with colon carcinoma cells have led to the identification of a number of bona fide PPAR-γ target genes with potential functions in growth, differentiation, and adhesion (Gupta et al. 2001) (see also Fig. 4). Although the role of these genes in colon tumorigenesis has not been addressed directly, one of them, RegIA, appears to be of particular interest. RegIA encodes a secreted protein that is overexpressed in colon carcinomas and has oncogenic properties in transgenic mice. It is thus conceivable that the observed downregulation of RegIA by PPAR-γ agonists might contribute to the inhibitory effect of these drugs on intestinal tumorigenesis.
PPAR-γ agonists have also been reported to modulate the expression and/or activity of a number of other cell cycle regulators and suppressors of apoptosis, including inhibition of cyclin D1 expression (Kitamura et al. 2001; Qin et al. 2003; Toyota et al. 2002; Wang et al. 2001), Rb hyperphosphorylation (Wakino et al. 2000), and inhibition of E2F activity (Altiok et al. 1997), induction of the Cdk inhibitors p18ink4C, p21WAF1 and p27Kip1 (Koga et al. 2001; Morrison & Farmer 1999), upregulation of the growth inhibitory GADD153 gene (Satoh et al. 2002) and the TGF-β target gene TSC22 (Gupta et al. 2003), induction of the tumor suppressor and PI3 K antagonist PTEN (Farrow & Evers 2003; Patel et al. 2001), and inhibition of NfkB and Bcl-2 (Chen et al. 2002). Induction of ubiquitin-dependent protein degradation by PPAR-γ ligands has been described as an additional mechanism leading to reduced cyclin D1 (Qin et al. 2003) and cFLIP protein levels (Kim et al. 2002).
PPAR-γ have also been reported to suppress VEGF expression by tumor cells (Panigrahy et al. 2002), to inhibit the expression of the VEGF receptor genes Flt-1 and Flk-1 by endothelial cells (Xin et al. 1999), to downregulate matrix metalloproteinase (MMP) activity secreted by tumor cells and induce MMP inhibitors (Liu et al. 2003; Panigrahy et al. 2002; Sunami et al. 2002), supporting the notion that PPAR-γ plays a negative regulatory role in tumor metastasis and angiogenesis.
The results summarized above suggest that PPAR-γ agonists modulate the expression and activity of a large number of genes and proteins, and thereby affect multiple biological processes that are directly relevant to tumorigenesis. It is, however, unlikely that all these effects reflect bona fide functions of PPAR-γ. Thus, PPAR-γ independent effects by PPAR-γ agonists on gene expression, cell proliferation, and apoptosis have been described in several cases (Baek et al. 2004; Baek et al. 2003; Chawla et al. 2001; Clay et al. 2002; Laurora et al. 2003; Nosjean & Boutin 2002; Palakurthi et al. 2001). Of particular relevance in this context is the observation that thiazolidinediones inhibit translation initiation through inactivation of eukaryotic initiation factor 2 (eIF2) (Palakurthi et al. 2001) which in analogy to inhibitors of the mTOR kinase (like rapamycin) (Mills et al. 2001) might explain many of the anti-oncogenic effects of this class of PPAR-γ agonists. Even though these observations cast some doubt on the mechanistic interpretation of a number of published studies (including some of those listed in Table 4), the anti-proliferative, pro-apoptotic, and tumor suppressive effects of thiazolidinedione agonists, and thus their potential as anti-cancer drugs, are out of the question.
Observations made with human tumors support the notion that PPAR-γ has an inhibitory effect on oncogenesis. Thus, the PPAR-γ gene has been found to be altered by loss-of-function mutations in a fraction of human colon carcinomas (Sarraf et al. 1999). In human thyroid follicular carcinomas, the frequent t(2;3)(q13;p25) translocation results in fusion of the DNA binding domains of the thyroid transcription factor PAX8 to domains A to F of PPAR-γ (Kroll et al. 2000). The PAX8-PPAR-γ fusion protein inhibits thiazolidinedione-induced transactivation by PPAR-γ in a dominant negative manner. Furthermore, PPAR-γ transcriptional activity is down-regulated by oncogenic Ras signaling through Erk-mediated phosphorylation of a regulatory serine residue (Adams et al. 1997; Hu et al. 1996; Mueller et al. 1998). Thus, genetic alterations leading to a deregulated Ras pathway, such as constitutive receptor tyrosine kinase activation or Ras mutations, would potentially inhibit the tumor suppressive function of PPAR-γ (Fig. 2). In contrast, PPAR activity is inhibited by protein kinase A (PKA) mediated phosphorylation (Hansen et al. 2001; Lazennec et al. 2000). PKA can antagonize the Ras-Erk pathway and is generally associated with anti-oncogenic properties (Hafner et al. 1994). Opposing effects of Erk and PKA on the function of PPAR-γ are therefore conceivable in view of a tumor suppressor function of PPAR-γ.
On the basis of the observation that agonists for PPAR-γ induce terminal differentiation in normal preadipocytes and human liposarcoma cells in vitro (Tontonoz et al. 1997), three patients with advanced liposarcoma were treated with troglitazone (Demetri et al. 1999). Tumor biopsies showed that terminal adipocytic differentiation concomitant with a marked reduction of cell proliferation was induced in these tumors by troglitazone. Although larger clinical trials have to be carried out before any definitive statement regarding the benefit of troglitazone in the treatment of liposarcoma is possible, the encouraging pilot study by Demetri and colleagues suggests that PPAR-γ agonists may indeed turn out as suitable drugs for the treatment of a subset of human cancers.
Acknowledgements
Work in the author’s laboratory is supported by grants from the DFG, the Dr. Mildred Scheel Stiftung and the Wilhelm Sander Stiftung.
References
- Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK (1997) Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272:5128–5132 [DOI] [PubMed] [Google Scholar]
- Altiok S, Xu M, Spiegelman BM (1997) PPARgamma induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via down-regulation of PP2A. Genes Dev 11:1987–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto Y, Murata T, Yoshimura H, Narumiya S, Majima M (2003) Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med 197:221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, Miura K, Harris CC (2003) Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res 63:728–734 [PubMed] [Google Scholar]
- Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E (2002) Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem 277:27613–27621 [DOI] [PubMed] [Google Scholar]
- Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR (2000) Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res 60:4629–4637 [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, Hsi LC, Eling TE (2003) Troglitazone a peroxisome proliferator-activated receptor gamma (PPAR gamma ) ligand selectively induces the early growth response-1 gene independently of PPAR gamma A novel mechanism for its anti-tumorigenic activity. J Biol Chem 278:5845–5853 [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE (2004) Expression of NAG-1 a transforming growth factor-beta superfamily member by troglitazone requires the early growth response gene EGR-1. J Biol Chem 279:6883–6892 [DOI] [PubMed] [Google Scholar]
- Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM (2002) Effects of peroxisome proliferator-activated receptor delta on placentation adiposity and colorectal cancer. Proc Natl Acad Sci USA 99:303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435 [DOI] [PubMed] [Google Scholar]
- Bol DK, Rowley RB, Ho CP, Pilz B, Dell J, Swerdel M, Kiguchi K, Muga S, Klein R, Fischer SM (2002) Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res 62:2516–2521 [PubMed] [Google Scholar]
- Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ, Bertagnolli MM (1996) Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res 56:2556–2560 [PubMed] [Google Scholar]
- Chang TH, Szabo E (2000) Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res 60:1129–1138 [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM (2001) PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7:48–52 [DOI] [PubMed] [Google Scholar]
- Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY (2002) Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci 70:2631–2646 [DOI] [PubMed] [Google Scholar]
- Chen WS, Liu JH, Wei SJ, Liu JM, Hong CY, Yang WK (2003a) Colon cancer cells with high invasive potential are susceptible to induction of apoptosis by a selective COX-2 inhibitor. Cancer Sci 94:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YX, Zhong XY, Qin YF, Bing W, He LZ (2003b) 15d-PGJ2 inhibits cell growth and induces apoptosis of MCG-803 human gastric cancer cell line. World J Gastroenterol 9:2149–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Imanishi H, Amuro Y, Hada T (2002) NS-398 a selective cyclooxygenase 2 inhibitor inhibited cell growth and induced cell cycle arrest in human hepatocellular carcinoma cell lines. Int J Cancer 99:755–761 [DOI] [PubMed] [Google Scholar]
- Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R (2000) Genetic disruption of Ptgs-1 as well as Ptgs-2 reduces intestinal tumorigenesis in Min mice. Cancer Res 60:4705–4708 [PubMed] [Google Scholar]
- Clay CE, Monjazeb A, Thorburn J, Chilton FH, High KP (2002) 15-Deoxy-delta1214-prostaglandin J2-induced apoptosis does not require PPARgamma in breast cancer cells. J Lipid Res 43:1818–1828 [DOI] [PubMed] [Google Scholar]
- Cui Y, Miyoshi K, Claudio E, Siebenlist UK, Gonzalez FJ, Flaws J, Wagner KU, Hennighausen L (2002) Loss of the peroxisome proliferation-activated receptor gamma (PPARgamma ) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J Biol Chem 277:17830–17835 [DOI] [PubMed] [Google Scholar]
- Cutler NS, Graves-Deal R, LaFleur BJ, Gao Z, Boman BM, Whitehead RH, Terry E, Morrow JD, Coffey RJ (2003) Stromal production of prostacyclin confers an antiapoptotic effect to colonic epithelial cells. Cancer Res 63:1748–1751 [PubMed] [Google Scholar]
- Dannenberg AJ, Subbaramaiah K (2003) Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 4:431–436 [DOI] [PubMed] [Google Scholar]
- Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S (1999) Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA 96:3951–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Furstenberg A, Daniel PT, Koch I, Kobel M, Weichert W, Siegert A, Hauptmann S (2003) Induction of G0/G1 cell cycle arrest in ovarian carcinoma cells by the anti-inflammatory drug NS-398 but not by COX-2-specific RNA interference. Oncogene 22:8653–8661 [DOI] [PubMed] [Google Scholar]
- Detjen KM, Welzel M, Wiedenmann B, Rosewicz S (2003) Nonsteroidal anti-inflammatory drugs inhibit growth of human neuroendocrine tumor cells via G1 cell-cycle arrest. Int J Cancer 107:844–853 [DOI] [PubMed] [Google Scholar]
- Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B (2002) Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell 10:721–733 [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, et al (1995) Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378:406–409 [DOI] [PubMed] [Google Scholar]
- Ding XZ, Tong WG, Adrian TE (2000) Blockade of cyclooxygenase-2 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Anticancer Res 20:2625–2631 [PubMed] [Google Scholar]
- Dohadwala M, Luo J, Zhu L, Lin Y, Dougherty GJ, Sharma S, Huang M, Pold M, Batra RK, Dubinett SM (2001) Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem 276:20809–20812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, Dubinett SM (2002) Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem 277:50828–50833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE (2003) The peroxisome proliferator-activated receptor beta/delta agonist GW501516 regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol 17:2477–2493 [DOI] [PubMed] [Google Scholar]
- Duperron C, Castonguay A (1997) Chemopreventive efficacies of aspirin and sulindac against lung tumorigenesis in A/J mice. Carcinogenesis 18:1001–1006 [DOI] [PubMed] [Google Scholar]
- Elder DJ, Halton DE, Crew TE, Paraskeva C (2000) Apoptosis induction and cyclooxygenase-2 regulation in human colorectal adenoma and carcinoma cell lines by the cyclooxygenase-2-selective non-steroidal anti-inflammatory drug NS-398. Int J Cancer 86:553–560 [DOI] [PubMed] [Google Scholar]
- Elder DJ, Halton DE, Playle LC, Paraskeva C (2002) The MEK/ERK pathway mediates COX-2-selective NSAID-induced apoptosis and induced COX-2 protein expression in colorectal carcinoma cells. Int J Cancer 99:323–327 [DOI] [PubMed] [Google Scholar]
- Eli Y, Przedecki F, Levin G, Kariv N, Raz A (2001) Comparative effects of indomethacin on cell proliferation and cell cycle progression in tumor cells grown in vitro and in vivo. Biochem Pharmacol 61:565–571 [DOI] [PubMed] [Google Scholar]
- Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP (1998) Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA 95:8806–8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow B, Evers BM (2003) Activation of PPARgamma increases PTEN expression in pancreatic cancer cells. Biochem Biophys Res Commun 301:50–53 [DOI] [PubMed] [Google Scholar]
- Flower RJ (2003) The development of COX2 inhibitors. Nat Rev Drug Discov 2:179–191 [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM (1995) 15-Deoxy-delta 12 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83:803–812 [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM (1996) The peroxisome proliferator-activated receptors: ligands and activators. Ann N Y Acad Sci 804:266–275 [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs polyunsaturated fatty acids and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 94:4312–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately S, Kerbel R (2003) Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis. Prog Exp Tumor Res 37:179–192 [DOI] [PubMed] [Google Scholar]
- Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson JA (1993) Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci USA 90:1440–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V (1999) Orphan nuclear receptors: from gene to function. Endocr Rev 20:689–725 [DOI] [PubMed] [Google Scholar]
- Girnun GD, Smith WM, Drori S, Sarraf P, Mueller E, Eng C, Nambiar P, Rosenberg DW, Bronson RT, Edelmann W, Kucherlapati R, Gonzalez FJ, Spiegelman BM (2002) APC-dependent suppression of colon carcinogenesis by PPARgamma. Proc Natl Acad Sci USA 99:13771–13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G (2001) COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J 15:2742–2744 [DOI] [PubMed] [Google Scholar]
- Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K (2000) Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res 60:5599–5602 [PubMed] [Google Scholar]
- Gupta RA, Dubois RN (2001) Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 1:11–21 [DOI] [PubMed] [Google Scholar]
- Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN (2001) Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J Biol Chem 276:29681–29687 [DOI] [PubMed] [Google Scholar]
- Gupta RA, Sarraf P, Brockman JA, Shappell SB, Raftery LA, Willson TM, DuBois RN (2003) Peroxisome proliferator-activated receptor gamma and transforming growth factor-beta pathways inhibit intestinal epithelial cell growth by regulating levels of TSC-22. J Biol Chem 278:7431–7438 [DOI] [PubMed] [Google Scholar]
- Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN (2004) Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-delta accelerates intestinal adenoma growth. Nat Med [DOI] [PubMed]
- Hafner S, Adler HS, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W (1994) Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol 14:6696–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JA, Kim JI, Ongusaha PP, Hwang DH, Ballou LR, Mahale A, Aaronson SA, Lee SW (2002) P53-mediated induction of Cox-2 counteracts p53- or genotoxic stress-induced apoptosis. EMBO J 21:5635–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JB, Zhang H, Rasmussen TH, Petersen RK, Flindt EN, Kristiansen K (2001) Peroxisome proliferator-activated receptor delta (PPARdelta )-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J Biol Chem 276:3175–3182 [DOI] [PubMed] [Google Scholar]
- Hao CM, Redha R, Morrow J, Breyer MD (2002) Peroxisome proliferator-activated receptor delta activation promotes cell survival following hypertonic stress. J Biol Chem 277:21341–21345 [DOI] [PubMed] [Google Scholar]
- Hatae T, Wada M, Yokoyama C, Shimonishi M, Tanabe T (2001) Prostacyclin-dependent apoptosis mediated by PPAR delta. J Biol Chem 276:46260–46267 [DOI] [PubMed] [Google Scholar]
- Haydon RC, Zhou L, Feng T, Breyer B, Cheng H, Jiang W, Ishikawa A, Peabody T, Montag A, Simon MA, He TC (2002) Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clin Cancer Res 8:1288–1294 [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281:1509–1512 [DOI] [PubMed] [Google Scholar]
- He TC, Chan TA, Vogelstein B, Kinzler KW (1999) PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney AP, Fernando M, Yong WH, Melmed S (2002) Functional PPAR-gamma receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med 8:1281–1287 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Fernando M, Melmed S (2003) PPAR-gamma receptor ligands: novel therapy for pituitary adenomas. J Clin Invest 111:1381–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T (2000) Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res 6:2006–2011 [PubMed] [Google Scholar]
- Hida T, Kozaki K, Ito H, Miyaishi O, Tatematsu Y, Suzuki T, Matsuo K, Sugiura T, Ogawa M, Takahashi T (2002) Significant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase 2 inhibitor JTE-522 and conventional anticancer agents. Clin Cancer Res 8:2443–2447 [PubMed] [Google Scholar]
- Hoper MM, Voelkel NF, Bates TO, Allard JD, Horan M, Shepherd D, Tuder RM (1997) Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am J Respir Cell Mol Biol 17:748–756 [DOI] [PubMed] [Google Scholar]
- Houston KD, Copland JA, Broaddus RR, Gottardis MM, Fischer SM, Walker CL (2003) Inhibition of proliferation and estrogen receptor signaling by peroxisome proliferator-activated receptor gamma ligands in uterine leiomyoma. Cancer Res 63:1221–1227 [PubMed] [Google Scholar]
- Howe LR, Crawford HC, Subbaramaiah K, Hassell JA, Dannenberg AJ, Brown AM (2001) PEA3 is up-regulated in response to Wnt1 and activates the expression of cyclooxygenase-2. J Biol Chem 276:20108–20115 [DOI] [PubMed] [Google Scholar]
- Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, Thaler HT, Muller WJ, Du B, Brown AM, Dannenberg AJ (2002) Celecoxib a selective cyclooxygenase 2 inhibitor protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res 62:5405–5407 [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM (1996) Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 274:2100–2103 [DOI] [PubMed] [Google Scholar]
- Hu KQ, Yu CH, Mineyama Y, McCracken JD, Hillebrand DJ, Hasan M (2003) Inhibited proliferation of cyclooxygenase-2 expressing human hepatoma cells by NS-398 a selective COX-2 inhibitor. Int J Oncol 22:757–763 [PubMed] [Google Scholar]
- Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA (2000) The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res 60:5040–5044 [PubMed] [Google Scholar]
- Jendrossek V, Handrick R, Belka C (2003) Celecoxib activates a novel mitochondrial apoptosis signaling pathway. FASEB J 17:1547–1549 [DOI] [PubMed] [Google Scholar]
- Ji YS, Xu Q, Schmedtje JF, Jr (1998) Hypoxia induces high-mobility-group protein I(Y) and transcription of the cyclooxygenase-2 gene in human vascular endothelium. Circ Res 83:295–304 [DOI] [PubMed] [Google Scholar]
- Kardosh A, Blumenthal M, Wang WJ, Chen TC, Schonthal AH (2004) Differential effects of selective COX-2 inhibitors on cell cycle regulation and proliferation of glioblastoma cell lines. Cancer Biol Ther 3 [DOI] [PubMed]
- Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, Malkinson AM, Golpon HA, Nemenoff RA, Geraci MW (2002) Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res 62:734–740 [PubMed] [Google Scholar]
- Kim HS, Youm HR, Lee JS, Min KW, Chung JH, Park CS (2003) Correlation between cyclooxygenase-2 and tumor angiogenesis in non-small cell lung cancer. Lung Cancer 42:163–170 [DOI] [PubMed] [Google Scholar]
- Kim Y, Suh N, Sporn M, Reed JC (2002) An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem 277:22320–22329 [DOI] [PubMed] [Google Scholar]
- Kisley LR, Barrett BS, Dwyer-Nield LD, Bauer AK, Thompson DC, Malkinson AM (2002) Celecoxib reduces pulmonary inflammation but not lung tumorigenesis in mice. Carcinogenesis 23:1653–1660 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Miyazaki Y, Hiraoka S, Nagasawa Y, Toyota M, Takakura R, Kiyohara T, Shinomura Y, Matsuzawa Y (2001) PPARgamma agonists inhibit cell growth and suppress the expression of cyclin D1 and EGF-like growth factors in ras-transformed rat intestinal epithelial cells. Int J Cancer 94:335–342 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM (1992) Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 358:771–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lehmann JM, Willson TM (1999) Orphan nuclear receptors: shifting endocrinology into reverse. Science 284:757–760 [DOI] [PubMed] [Google Scholar]
- Koga H, Sakisaka S, Harada M, Takagi T, Hanada S, Taniguchi E, Kawaguchi T, Sasatomi K, Kimura R, Hashimoto O, Ueno T, Yano H, Kojiro M, Sata M (2001) Involvement of p21(WAF1/Cip1) p27(Kip1) and p18(INK4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology 33:1087–1097 [DOI] [PubMed] [Google Scholar]
- Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA (2000) PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science 289:1357–1360 [DOI] [PubMed] [Google Scholar]
- Kundu N, Smyth MJ, Samsel L, Fulton AM (2002) Cyclooxygenase inhibitors block cell growth increase ceramide and inhibit cell cycle. Breast Cancer Res Treat 76:57–64 [DOI] [PubMed] [Google Scholar]
- Kurie JM, Dubois RN (2001) Prostaglandin E synthase: another enzyme in the cyclooxygenase pathway driving epithelial cancer? Clin Cancer Res 7:2608–2610 [PubMed] [Google Scholar]
- Lal G, Ash C, Hay K, Redston M, Kwong E, Hancock B, Mak T, Kargman S, Evans JF, Gallinger S (2001) Suppression of intestinal polyps in Msh2-deficient and non-Msh2-deficient multiple intestinal neoplasia mice by a specific cyclooxygenase-2 inhibitor and by a dual cyclooxygenase-1/2 inhibitor. Cancer Res 61:6131–6136 [PubMed] [Google Scholar]
- Laurora S, Pizzimenti S, Briatore F, Fraioli A, Maggio M, Reffo P, Ferretti C, Dianzani MU, Barrera G (2003) Peroxisome proliferator-activated receptor ligands affect growth-related gene expression in human leukemic cells. J Pharmacol Exp Ther 305:932–942 [DOI] [PubMed] [Google Scholar]
- Lazennec G, Canaple L, Saugy D, Wahli W (2000) Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol Endocrinol 14:1962–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL (2002) Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res 62:625–631 [PubMed] [Google Scholar]
- Lee DW, Sung MW, Park SW, Seong WJ, Roh JL, Park B, Heo DS, Kim KH (2002) Increased cyclooxygenase-2 expression in human squamous cell carcinomas of the head and neck and inhibition of proliferation by nonsteroidal anti-inflammatory drugs. Anticancer Res 22:2089–2096 [PubMed] [Google Scholar]
- Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J (1998) Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med 4:1053–1057 [DOI] [PubMed] [Google Scholar]
- Li G, Yang T, Yan J (2002) Cyclooxygenase-2 increased the angiogenic and metastatic potential of tumor cells. Biochem Biophys Res Commun 299:886–890 [DOI] [PubMed] [Google Scholar]
- Li M, Wu X, Xu XC (2001) Induction of apoptosis in colon cancer cells by cyclooxygenase-2 inhibitor NS398 through a cytochrome c-dependent pathway. Clin Cancer Res 7:1010–1016 [PubMed] [Google Scholar]
- Lim H, Dey SK (2002) A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology 143:3207–3210 [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK (1997) Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91:197–208 [DOI] [PubMed] [Google Scholar]
- Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK (1999) Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev 13:1561–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML (2001) Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL10 cells Involvement of phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem 276:48997–49002 [DOI] [PubMed] [Google Scholar]
- Liu H, Zang C, Fenner MH, Possinger K, Elstner E (2003) PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat 79:63–74 [DOI] [PubMed] [Google Scholar]
- Liu LT, Chang HC, Chiang LC, Hung WC (2002) Induction of RECK by nonsteroidal anti-inflammatory drugs in lung cancer cells. Oncogene 21:8347–8350 [DOI] [PubMed] [Google Scholar]
- Liu XH, Yao S, Kirschenbaum A, Levine AC (1998) NS398 a selective cyclooxygenase-2 inhibitor induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res 58:4245–4249 [PubMed] [Google Scholar]
- Lonnroth C, Andersson M, Lundholm K (2001) Indomethacin and telomerase activity in tumor growth retardation. Int J Oncol 18:929–937 [PubMed] [Google Scholar]
- Marcus SL, Miyata KS, Zhang B, Subramani S, Rachubinski RA, Capone JP (1993) Diverse peroxisome proliferator-activated receptors bind to the peroxisome proliferator-responsive elements of the rat hydratase/dehydrogenase and fatty acyl-CoA oxidase genes but differentially induce expression. Proc Natl Acad Sci USA 90:5723–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markenson J (1999) Clinical implications of cyclooxygenase enzymes COX-1/COX-2: role of the new NSAIDs Cancer Control 6 (Suppl): http://www.moffitt.usf.edu/pubs/ccj/v6ns/article5.htm [DOI] [PubMed]
- Masferrer JL, Koki A, Seibert K (1999) COX-2 inhibitors A new class of antiangiogenic agents. Ann NY Acad Sci 889:84–86 [DOI] [PubMed] [Google Scholar]
- Michalik L, Desvergne B, Wahli W (2004) Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer 4:61–70 [DOI] [PubMed] [Google Scholar]
- Mills GB, Lu Y, Kohn EC (2001) Linking molecular therapeutics to molecular diagnostics: inhibition of the FRAP/RAFT/TOR component of the PI3 K pathway preferentially blocks PTEN mutant cells in vitro and in vivo. Proc Natl Acad Sci USA 98:10031–10033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter HA, Eveson JW, Huntley S, Elder DJ, Hague A (2003) The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin Cancer Res 9:1885–1897 [PubMed] [Google Scholar]
- Moalic S, Liagre B, Le Bail JC, Beneytout JL (2001) Dose-dependent modulation of apoptosis and cyclooxygenase-2 expression in human 1547 osteosarcoma cells by NS-398 a selective cyclooxygenase-2 inhibitor. Int J Oncol 18:533–540 [DOI] [PubMed] [Google Scholar]
- Moody TW, Leyton J, Zakowicz H, Hida T, Kang Y, Jakowlew S, You L, Ozbun L, Zia H, Youngberg J, Malkinson A (2001) Indomethacin reduces lung adenoma number in A/J mice. Anticancer Res 21:1749–1755 [PubMed] [Google Scholar]
- Morrison RF, Farmer SR (1999) Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors p18(INK4c) and p21(Waf1/Cip1) during adipogenesis. J Biol Chem 274:17088–17097 [DOI] [PubMed] [Google Scholar]
- Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, Fletcher C, Singer S, Spiegelman BM (1998) Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell 1:465–470 [DOI] [PubMed] [Google Scholar]
- Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G (2002) Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA 99:12483–12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, Tani K, Kobayashi M, Maruyama T, Kobayashi K, Ohuchida S, Sugimoto Y, Narumiya S, Sugimura T, Wakabayashi K (2002) Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res 62:28–32 [PubMed] [Google Scholar]
- Nagatsuka I, Yamada N, Shimizu S, Ohira M, Nishino H, Seki S, Hirakawa K (2002) Inhibitory effect of a selective cyclooxygenase-2 inhibitor on liver metastasis of colon cancer. Int J Cancer 100:515–519 [DOI] [PubMed] [Google Scholar]
- Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93:229–240 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Kamijo R, Takizawa K, Hatori M, Nagumo M (2001) Inhibitors of cyclooxygenase-2 (COX-2) suppressed the proliferation and differentiation of human leukaemia cell lines. Eur J Cancer 37:1570–1578 [DOI] [PubMed] [Google Scholar]
- Narayanan BA, Condon MS, Bosland MC, Narayanan NK, Reddy BS (2003) Suppression of N-methyl-N-nitrosourea/testosterone-induced rat prostate cancer growth by celecoxib: effects on cyclooxygenase-2 cell cycle regulation and apoptosis mechanism(s). Clin Cancer Res 9:3503–3513 [PubMed] [Google Scholar]
- Nishimura G, Yanoma S, Mizuno H, Kawakami K, Tsukuda M (1999) A selective cyclooxygenase-2 inhibitor suppresses tumor growth in nude mouse xenografted with human head and neck squamous carcinoma cells. Jpn J Cancer Res 90:1152–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosjean O, Boutin JA (2002) Natural ligands of PPARgamma: are prostaglandin J(2) derivatives really playing the part? Cell Signal 14:573–583 [DOI] [PubMed] [Google Scholar]
- Nzeako UC, Guicciardi ME, Yoon JH, Bronk SF, Gores GJ (2002) COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology 35:552–559 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Rafiee P, Fisher PJ, Johnson NA, Otterson MF, Binion DG (2003) Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition. FEBS Lett 554:88–94 [DOI] [PubMed] [Google Scholar]
- Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H, Nakagama H (2003) Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology 124:361–367 [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87:803–809 [DOI] [PubMed] [Google Scholar]
- Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF (2001) Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib a specific cyclooxygenase-2 inhibitor. Cancer Res 61:1733–1740 [PubMed] [Google Scholar]
- Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS (2002) Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8:289–293 [DOI] [PubMed] [Google Scholar]
- Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA (2001) Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor gamma and mediated by inhibition of translation initiation. Cancer Res 61:6213–6218 [PubMed] [Google Scholar]
- Pan MR, Chuang LY, Hung WC (2001) Non-steroidal anti-inflammatory drugs inhibit matrix metalloproteinase-2 expression via repression of transcription in lung cancer cells. FEBS Lett 508:365–368 [DOI] [PubMed] [Google Scholar]
- Panigrahy D, Singer S, Shen LQ, Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing S, Fletcher C, Fletcher JA, Hlatky L, Hahnfeldt P, Folkman J, Kaipainen A (2002) PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest 110:923–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BH, Vogelstein B, Kinzler KW (2001) Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA 98:2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH (2001) Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol 11:764–768 [DOI] [PubMed] [Google Scholar]
- Peng JP, Liu LT, Chang HC, Hung WC (2003) Enhancement of chemotherapeutic drug-induced apoptosis by a cyclooxygenase-2 inhibitor in hypopharyngeal carcinoma cells. Cancer Lett 201:157–163 [DOI] [PubMed] [Google Scholar]
- Poon R, Smits R, Li C, Jagmohan-Changur S, Kong M, Cheon S, Yu C, Fodde R, Alman BA (2001) Cyclooxygenase-two (COX-2) modulates proliferation in aggressive fibromatosis (desmoid tumor). Oncogene 20:451–460 [DOI] [PubMed] [Google Scholar]
- Pradono P, Tazawa R, Maemondo M, Tanaka M, Usui K, Saijo Y, Hagiwara K, Nukiwa T (2002) Gene transfer of thromboxane A(2) synthase and prostaglandin I(2) synthase antithetically altered tumor angiogenesis and tumor growth. Cancer Res 62:63–66 [PubMed] [Google Scholar]
- Qin C, Burghardt R, Smith R, Wormke M, Stewart J, Safe S (2003) Peroxisome proliferator-activated receptor gamma agonists induce proteasome-dependent degradation of cyclin D1 and estrogen receptor alpha in MCF-7 breast cancer cells. Cancer Res 63:958–964 [PubMed] [Google Scholar]
- Reddy BS, Rao CV (2002) Novel approaches for colon cancer prevention by cyclooxygenase-2 inhibitors J Environ Pathol Toxicol Oncol 21:155–164 [PubMed] [Google Scholar]
- Reddy ST, Wadleigh DJ, Herschman HR (2000) Transcriptional regulation of the cyclooxygenase-2 gene in activated mast cells. J Biol Chem 275:3107–3113 [DOI] [PubMed] [Google Scholar]
- Richter M, Weiss M, Weinberger I, Furstenberger G, Marian B (2001) Growth inhibition and induction of apoptosis in colorectal tumor cells by cyclooxygenase inhibitors. Carcinogenesis 22:17–25 [DOI] [PubMed] [Google Scholar]
- Rioux N, Castonguay A (1998) Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res 58:5354–5360 [PubMed] [Google Scholar]
- Romano M, Claria J (2003) Cyclooxygenase-2 and 5-lipoxygenase converging functions on cell proliferation and tumor angiogenesis: implications for cancer therapy. FASEB J 17:1986–1995 [DOI] [PubMed] [Google Scholar]
- Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM (1998) Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med 4:1058–1061 [DOI] [PubMed] [Google Scholar]
- Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM (1998) Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med 4:1046–1052 [DOI] [PubMed] [Google Scholar]
- Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, Aaltonen LA, de la Chapelle A, Spiegelman BM, Eng C (1999) Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol Cell 3:799–804 [DOI] [PubMed] [Google Scholar]
- Satoh T, Toyoda M, Hoshino H, Monden T, Yamada M, Shimizu H, Miyamoto K, Mori M (2002) Activation of peroxisome proliferator-activated receptor-gamma stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene 21:2171–2180 [DOI] [PubMed] [Google Scholar]
- Sawaoka H, Kawano S, Tsuji S, Tsujii M, Gunawan ES, Takei Y, Nagano K, Hori M (1998) Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol 274:G1061–1067 [DOI] [PubMed] [Google Scholar]
- Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS (1997) Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem 272:601–608 [DOI] [PubMed] [Google Scholar]
- Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM (2002) Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res 62:506–511 [PubMed] [Google Scholar]
- Shao J, Sheng H, Inoue H, Morrow JD, DuBois RN (2000) Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J Biol Chem 275:33951–33956 [DOI] [PubMed] [Google Scholar]
- Shaw N, Elholm M, Noy N (2003) Retinoic acid is a high affinity selective ligand for PPAR-β/δ. J Biol Chem [DOI] [PubMed]
- Sheng H, Williams CS, Shao J, Liang P, DuBois RN, Beauchamp RD (1998) Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem 273:22120–22127 [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, Beauchamp RD (2000) Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem 275:6628–6635 [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Dubois RN (2001) K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves activation of the protein kinase B1. Cancer Res 61:2670–2675 [PubMed] [Google Scholar]
- Shi Y, Hon M, Evans RM (2002) The peroxisome proliferator-activated receptor delta an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci USA 99:2613–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shureiqi I, Jiang W, Zuo X, Wu Y, Stimmel JB, Leesnitzer LM, Morris JS, Fan HZ, Fischer SM, Lippman SM (2003) The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci USA 100:9968–9973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural cellular and molecular biology. Annu Rev Biochem 69:145–182 [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM (2001) Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med 7:1048–1051 [DOI] [PubMed] [Google Scholar]
- Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ (2000) Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res 60:5767–5772 [PubMed] [Google Scholar]
- Spisni E, Griffoni C, Santi S, Riccio M, Marulli R, Bartolini G, Toni M, Ullrich V, Tomasi V (2001) Colocalization prostacyclin (PGI2) synthase--caveolin-1 in endothelial cells and new roles for PGI2 in angiogenesis. Exp Cell Res 266:31–43 [DOI] [PubMed] [Google Scholar]
- Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, Wei LH, Yang PC, Kuo ML (2004) Cyclooxygenase-2 Induces EP(1)- and HER-2/Neu-Dependent vascular endothelial growth factor-c up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res 64:554–564 [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ (1999) Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem 274:10911–10915 [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ (2002) Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem 277:18649–18657 [DOI] [PubMed] [Google Scholar]
- Sunami E, Tsuno NH, Kitayama J, Saito S, Osada T, Yamaguchi H, Tomozawa S, Tsuruo T, Shibata Y, Nagawa H (2002) Decreased synthesis of matrix metalloproteinase-7 and adhesion to the extracellular matrix proteins of human colon cancer cells treated with troglitazone. Surg Today 32:343–350 [DOI] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, Fluhmann B, Desvergne B, Wahli W (2001) Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev 15:3263–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, Wahli W, Noy N (2002) Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol 22:5114–5127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Yoshitani S, Takashima S, Okumura A, Murakami A, Hosokawa M (2001) Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res 61:2424–2428 [PubMed] [Google Scholar]
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426 [DOI] [PubMed] [Google Scholar]
- Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, Dunson DB, Rogan EG, Morham SG, Smart RC, Langenbach R (2002) Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res 62:3395–3401 [PubMed] [Google Scholar]
- Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM (1994) Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors PPAR gamma and RXR alpha. Nucleic Acids Res 22:5628–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, Brun RP, Mueller E, Altiok S, Oppenheim H, Evans RM, Spiegelman BM (1997) Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci USA 94:237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totzke G, Schulze-Osthoff K, Janicke RU (2003) Cyclooxygenase-2 (COX-2) inhibitors sensitize tumor cells specifically to death receptor-induced apoptosis independently of COX-2 inhibition. Oncogene 22:8021–8030 [DOI] [PubMed] [Google Scholar]
- Toyota M, Miyazaki Y, Kitamura S, Nagasawa Y, Kiyohara T, Shinomura Y, Matsuzawa Y (2002) Peroxisome proliferator-activated receptor gamma reduces the growth rate of pancreatic cancer cells through the reduction of cyclin D1. Life Sci 70:1565–1575 [DOI] [PubMed] [Google Scholar]
- Tseng WW, Deganutti A, Chen MN, Saxton RE, Liu CD (2002) Selective cyclooxygenase-2 inhibitor rofecoxib (Vioxx) induces expression of cell cycle arrest genes and slows tumor growth in human pancreatic cancer. J Gastrointest Surg 6:838–843 [DOI] [PubMed] [Google Scholar]
- Uefuji K, Ichikura T, Shinomiya N, Mochizuki H (2000) Induction of apoptosis by JTE-522 a specific cyclooxygenase-2 inhibitor in human gastric cancer cell lines. Anticancer Res 20:4279–4284 [PubMed] [Google Scholar]
- Ueta E, Yoneda K, Kimura T, Tatemoto Y, Doi S, Yamamoto T, Osaki T (2001) Mn-SOD antisense upregulates in vivo apoptosis of squamous cell carcinoma cells by anticancer drugs and gamma-rays regulating expression of the BCL-2 family proteins COX-2 and p21. Int J Cancer 94:545–550 [DOI] [PubMed] [Google Scholar]
- Versteeg HH, van Bergen en Henegouwen PM, van Deventer SJ, Peppelenbosch MP (1999) Cyclooxygenase-dependent signalling: molecular events and consequences. FEBS Lett 445:1–5 [DOI] [PubMed] [Google Scholar]
- Wakino S, Kintscher U, Kim S, Yin F, Hsueh WA, Law RE (2000) Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1→S transition in vascular smooth muscle cells. J Biol Chem 275:22435–22441 [DOI] [PubMed] [Google Scholar]
- Wang C, Fu M, D’Amico M, Albanese C, Zhou JN, Brownlee M, Lisanti MP, Chatterjee VK, Lazar MA, Pestell RG (2001) Inhibition of cellular proliferation through IkappaB kinase-independent and peroxisome proliferator-activated receptor gamma-dependent repression of cyclin D1. Mol Cell Biol 21:3057–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fuentes CF, Shapshay SM (2002) Antiangiogenic and chemopreventive activities of celecoxib in oral carcinoma cell. Laryngoscope 112:839–843 [DOI] [PubMed] [Google Scholar]
- Wick M, Hurteau G, Dessev C, Chan D, Geraci MW, Winn RA, Heasley LE, Nemenoff RA (2002) Peroxisome proliferator-activated receptor-gamma is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol Pharmacol 62:1207–1214 [DOI] [PubMed] [Google Scholar]
- Willert K, Nusse R (1998) Beta-catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev 8:95–102 [DOI] [PubMed] [Google Scholar]
- Williams CS, Mann M, DuBois RN (1999) The role of cyclooxygenases in inflammation cancer and development. Oncogene 18:7908–7916 [DOI] [PubMed] [Google Scholar]
- Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN (2000) Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest 105:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xia HH, Tu SP, Fan DM, Lin MC, Kung HF, Lam SK, Wong BC (2003) 15-Lipoxygenase-1 mediates cyclooxygenase-2 inhibitor-induced apoptosis in gastric cancer. Carcinogenesis 24:243–247 [DOI] [PubMed] [Google Scholar]
- Xie W, Herschman HR (1995) v-src induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase and the c-Jun transcription factor. J Biol Chem 270:27622–27628 [DOI] [PubMed] [Google Scholar]
- Xie W, Herschman HR (1996) Transcriptional regulation of prostaglandin synthase 2 gene expression by platelet-derived growth factor and serum. J Biol Chem 271:31742–31748 [DOI] [PubMed] [Google Scholar]
- Xin X, Yang S, Kowalski J, Gerritsen ME (1999) Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 274:9116–9121 [DOI] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV (1999) Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell 3:397–403 [DOI] [PubMed] [Google Scholar]
- Xu Y, Iyengar S, Roberts RL, Shappell SB, Peehl DM (2003) Primary culture model of peroxisome proliferator-activated receptor gamma activity in prostate cancer cells. J Cell Physiol 196:131–143 [DOI] [PubMed] [Google Scholar]
- Yamakawa-Karakida N, Sugita K, Inukai T, Goi K, Nakamura M, Uno K, Sato H, Kagami K, Barker N, Nakazawa S (2002) Ligand activation of peroxisome proliferator-activated receptor gamma induces apoptosis of leukemia cells by down-regulating the c-myc gene expression via blockade of the Tcf-4 activity. Cell Death Differ 9:513–526 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Watanabe M, Hasegawa H, Nishibori H, Ishii Y, Tatematsu H, Yamamoto K, Kubota T, Kitajima M (2003) The potential for a selective cyclooxygenase-2 inhibitor in the prevention of liver metastasis in human colorectal cancer. Anticancer Res 23:245–249 [PubMed] [Google Scholar]
- Yamazaki R, Kusunoki N, Matsuzaki T, Hashimoto S, Kawai S (2002) Selective cyclooxygenase-2 inhibitors show a differential ability to inhibit proliferation and induce apoptosis of colon adenocarcinoma cells. FEBS Lett 531:278–284 [DOI] [PubMed] [Google Scholar]
- Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM (2003) Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest 111:727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Kargman S, Lam EC, Kelly CR, Zheng Y, Luk P, Kwong E, Evans JF, Wolfe MM (2003) Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res 63:586–592 [PubMed] [Google Scholar]
- Yao R, Rioux N, Castonguay A, You M (2000) Inhibition of COX-2 and induction of apoptosis: two determinants of nonsteroidal anti-inflammatory drugs’ chemopreventive efficacies in mouse lung tumorigenesis. Exp Lung Res 26:731–742 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Amano H, Hayashi I, Kitasato H, Kamata M, Inukai M, Yoshimura H, Majima M (2003) COX-2/VEGF-dependent facilitation of tumor-associated angiogenesis and tumor growth in vivo. Lab Invest 83:1385–1394 [DOI] [PubMed] [Google Scholar]
- Zhang J, Fu M, Zhu X, Xiao Y, Mou Y, Zheng H, Akinbami MA, Wang Q, Chen YE (2002) Peroxisome proliferator-activated receptor delta is up-regulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J Biol Chem 277:11505–11512 [DOI] [PubMed] [Google Scholar]
- Zweifel BS, Davis TW, Ornberg RL, Masferrer JL (2002) Direct evidence for a role of cyclooxygenase 2-derived prostaglandin E2 in human head and neck xenograft tumors. Cancer Res 62:6706–6711 [PubMed] [Google Scholar]