Abstract
We have described previously a cell surface channel that is highly selective for nucleic acids. Nucleic acid conductance is 10 pS and the channel is at least 10,000-fold more selective for oligodeoxynucleotides than any anion tested (1). Herein we provide evidence that the nucleic acid-conducting channel (NACh) is a heteromultimeric complex of at least two proteins; a 45-kDa pore-forming subunit (p45) and a 36-kDa regulatory subunit (p36). Reconstitution of p45 in planar lipid bilayers resulted in formation of a channel which gated in the absence of nucleic acid and which was more selective for anions (including oligonucleotide) than cations. This channel exhibited transitions from one level of current to another (or to the closed state); however the incidence of transitions was rare. Channel activity was not observed when p36 was reconstituted alone. Reconstitution of p36 with p45 restored nucleic acid dependence and selectivity to the channel. Protein sequence analysis identified p36 as cytosolic malate dehydrogenase (cMDH). Experiments were performed to prove that cMDH is a regulatory subunit of NACh. Selective activity was observed when p45 was reconstituted with pig heart cMDH but not with mitochondrial MDH. Both the enzyme substrate l-malate and antiserum raised against cMDH block NACh activity. These data demonstrate that a nucleic acid conducting channel is a complex of at least two proteins, p45 and cMDH. Furthermore, these data establish that cMDH confers nucleic acid selectivity of the channel.
In spite of considerable progress toward effective application of molecular-based therapies, many significant barriers to widespread use exist. Antisense oligodeoxynucleotides are an example of agents with a significant therapeutic potential, yet bioavailability and cellular uptake limit their widespread application. It is unlikely that oligodeoxynucleotides (ODN) will reach their full therapeutic potential until these limiting factors have been overcome.
ODNs administered intravenously accumulate primarily in liver and kidney (2–4). Little is known about the uptake of nucleic acids by liver; however, a putative mechanism of nucleic acid transport in renal tissue is emerging. We purified a 45-kDa protein (p45) from renal brush border membrane that bound nucleic acids in electrophoretic mobility shift assays (5). Subsequently, we demonstrated that this protein, when reconstituted in planar lipid bilayers, functions as an ion channel (1). Ion gradient, ion substitution, and radiolabeled ODN studies demonstrated that nucleic acid is the ion conducted by the channel (1). These data indicate that the purified protein functions as a nucleic acid-conducting channel (NACh).
Recently we identified a second protein band with a molecular weight of ≈36 kDa (p36) that copurifies with p45. Studies were initiated to determine the role that this protein plays in nucleic acid channel function.
Methods
Purification of the Nucleic Acid Channel Complex.
The nucleic acid channel complex was purified by first isolating rat-kidney brush border membranes as described (6). Brush border membranes were solublized in detergent and separated by a four-step FPLC protocol. Column eluates were collected in 1-ml fractions and analyzed for NACh activity. The criteria for NACh activity were: nucleic acid dependent channel activity consistent with previously published data, blockade of channel activity by heparan sulfate, and evidence of nucleic acid specificity as indicated by ion substitution and/or radiolabeled ODN-tracer studies. Active fractions were pooled and purified further. The FPLC purification protocol consisted of two sequential ion exchange columns (Mono Q, Amersham Pharmacia) followed by an hydrophobic interaction column (Source 15 PHE, Amersham Pharmacia). The final step was addition of 10 mM DTT and separation by gel filtration chromatography (Sephadex 75, Amersham Pharmacia).
Reconstitution of the Nucleic Acid Channel.
Reconstitution of NACh was performed as previously described (1, 7, 8). In brief, proteoliposomes were prepared by sonicating purified protein (80 kHz for 1 min) with a 1:1 mixture of bovine brain phosphatidylethanolamine (10 mg/ml) and phosphatidylserine (10 mg/ml, Avanti Polar Lipids). A lipid bilayer was formed with a 1:1 mixture of the same lipids and resulted in a high resistance seal between two cups. Except when indicated otherwise, both cups were filled with a buffered solution consisting of 200 mM CsCl, 20 mM Hepes, and 1 mM CaCl2, pH 7.4. When indicated ODN (20-mer homomultimer of deoxythymidine) was added to a concentration of 5–10 μM. The cups were connected to a patch clamp amplifier through a head stage with a 10-gigohm feedback resistor and frequency bandwidth of 10 kHz. The cis chamber was defined as the cup connected to the voltage-holding electrode, and all voltages are referenced to the trans (ground) side. Current output of the patch clamp was filtered at 1 kHz through an eight-pole filter, digitized at 0.05 or 0.25 ms/point, and analyzed with commercial software (pclamp, Version 6.02, Axon Instruments).
Channel Selectivity.
Selectivity of p45 was characterized by creating concentration gradients across the bilayer and then determining reversal potential (Erev). The initial ionic composition of the solution bathing the bilayer consisted of symmetrical 200 mM KCl, 20 mM Hepes, and 1 mM CaCl2, pH 7.4. Current was measured at holding potentials ranging from −75 mV to +75 mV. A 10:1 (cis to trans) gradient for KCl was created by exchanging the trans solution with a solution consisting of 20 mM KCl, 20 mM Hepes, and 1 mM CaCl2, pH 7.4. Determination of the current voltage relationship was repeated. The trans solution was then exchanged with a solution containing 200 mM CsCl, 20 mM Hepes, and 1 mM CaCl2, pH 7.4, and the current-voltage relationship was determined again.
Protein Sequencing.
Nucleic acid channel subunits (p45 and p36) were separated quantitatively by SDS/PAGE and visualized by silver staining (9). The protein bands were excised and individually subjected to in-gel digestion with trypsin (10). Tryptic peptides were analyzed by using a LCQ ion trap mass spectrometer (Thermo-Finnigan, San Jose, CA) fitted with a home-built nano-HPLC microelectrospray ionization (ESI) source.¶ MS/MS analysis of peptides eluting into the mass spectrometer was carried out automatically by using the LCQ's data-dependent data acquisition software. To maximize the number of fragmented peptides, dynamic exclusion and isotope exclusion functions were activated. Following data filtration to remove low quality spectra, the MS/MS spectra were automatically searched against the nonredundant protein database (National Center for Biotechnology Information, Bethesda, MD) by using the sequest search algorithm.
Results
We have shown previously that nucleic acid-affinity chromatography resulted in purification of a highly specific nucleic acid-conducting channel (1). Recent efforts to increase purity and yield have led to the development of a four-step FPLC protocol. Detergent solublized rat-kidney brush border membranes (Fig. 1A, lane 1) were applied to two sequential ion-exchange columns. Fractions with NACh activity were pooled, analyzed by SDS/PAGE (Fig. 1A, Lane 2), and purified further by hydrophobic interaction chromatography. The active peak from this separation contained two protein bands; a 45-kDa band (p45) and a second band at 36-kDa (p36; Fig. 1A, lane 3). The bands were separated by addition of a reducing agent (DTT, 10 mM) and separation by gel filtration chromatography. This separation resulted in two protein peaks as detected by UV spectroscopy (data not shown) that corresponded to p45 (Fig. 1A, lane 4) and p36. Nucleic acid-dependent channel activity was lost by this step of purification, suggesting the nucleic acid channel is a heteromultimer of p45 and p36. Yield of p36 was low in this step of purification and consequently the amount of protein recovered was at the lower limit of detection on silver-stained gels.
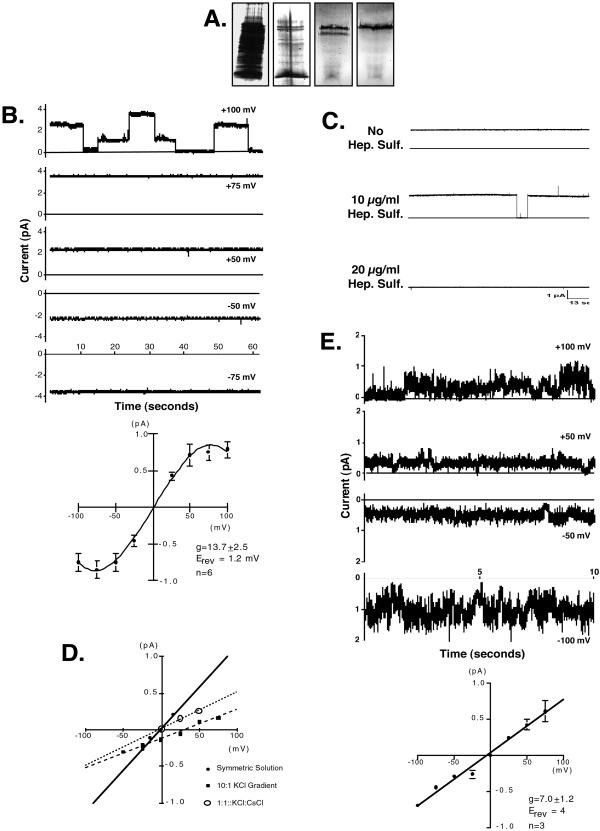
Figure 1.
Silver-stained SDS/PAGE analysis of the FPLC purification of NACh and electrophysiologic characterization of p45. (A) Fractions from each step of FPLC purification that had NACh activity were pooled and analyzed by SDS/PAGE. Lane 1, Total renal brush border membrane protein; lane 2, protein from pooled fractions after two steps of ion-exchange chromatography; lane 3, hydrophobicity chromatography resulted in a single active peak consisting of two protein bands (36 kDa and 45 kDa); and lane 4, the purification of a single 45-kDa protein band (p45) by gel filtration chromatography. (B) Current output traces and mean current-voltage plot (n = 6) when p45 was reconstituted in lipid bilayers and ODN was not present. (Upper) Current traces (1-min) collected at the indicated holding potentials; the solid horizontal line indicates zero current. Current was observed in the absence of ODN. (Lower) Mean current-voltage relationship (IV-plot; n = 6). (C) Current traces (2-min) showing blockade of the p45 channel with heparan sulfate, a blocker of NACh activity. (D) Ion substitution studies to determine the ion selectivity of p45. ●, Symmetrical buffered solutions; ■, 10:1 gradient (cis to trans) for potassium and chloride; and ○, elimination of Cl− gradient. (E) Reconstitution of a single FPLC fraction containing both the 36-kDa protein band (p36) and p45. Current was observed only when ODN was present. Gating kinetics (Upper) and conductance (Lower) were comparable to that previously reported for NACh (1, 7).
To test the hypothesis that the nucleic acid channel is a heteromultimer of p45 and p36, fractions containing either p36 or p45 were used to form proteoliposomes and channel activity was assessed in planar lipid bilayers. These experiments are summarized in Fig. 1 B–E. Fig. 1B depicts current traces from a single experiment (Upper) and the mean current-voltage plot (IV-plot) from six experiments (Lower) when p45 was reconstituted alone and ODN was not present. Under these conditions, significant current was observed in the absence of ODN indicating that p45 forms an ion-conducting channel, which conducts ion(s) other than ODN. Furthermore, transitions in current level were rare. This is exemplified in Fig. 1B Upper where transitions were observed only in the trace collected at a holding potential of +100 mV. It is unclear whether these intermediate levels of current represent subconductive states of a single channel or whether multiple channels are present in the bilayer. In analyzing the data from each experiment, we have assumed they result from the presence of multiple channels in the bilayer, and therefore, the transitions represent single channel gating, and unitary conductance can be determined from these transitions. Consequently, if transitions were observed in any of the traces in an experiment, these transitions were used to define single channel current. All of the traces from that experiment were then factored by this single channel current, and unitary conductance was determined. The data in Fig. 1B (Lower), which have been adjusted in this way, shows that the current-voltage relationship of p45 was linear from −50 to +50 and reached a plateau at holding potentials above +50 mV and below −50 mV, suggesting saturation of the channel. Channel conductance (slope conductance) between −50 mV and +50 mV was 13.7 ± 2.5 pS (n = 6).
In several experiments, p45 was reconstituted, channel activity was recorded, ODN was then added to both solution chambers, and channel activity was again recorded. In these experiments, addition of ODN resulted in small fluctuations in current (data not shown) indicating some form of interaction between ODN and p45.
Because we have reported previously that NACh activity is blocked by heparan sulfate (1), we examined possible interactions between p45 and heparan sulfate (Fig. 1C). Under control conditions, channel open probability of p45 in the absence of ODN was 1.0 (Fig. 1C, top trace). Addition of 10-μg/ml heparan sulfate decreased open probability to 0.88 (Fig. 1C, middle trace) and 20 μg/ml blocked channel activity completely (Fig. 1C, bottom trace). This dose-response is identical to that previously reported (1) and indicates that p45 is a component of NACh. Furthermore, these data suggest that heparan sulfate blocks nucleic acid channel activity in this system by binding to p45.
Ion gradient and ion substitution studies were performed to identify the ion(s) conducted by p45. In symmetrical solution of 200 mM KCl, 1 mM CaCl2, and 10 mM Hepes, pH 7.4 (Fig. 1D, ●), channel conductance was 11 pS with a reversal potential (Erev) of ≈0 mV. When a 10:1 gradient for KCl was created (cis to trans), Erev was +32 mV (Fig. 1D, ▪). The Goldman-Hodgkin-Katz equation predicts the channel is five times more permeable to anions than cations, indicating that an anion (i.e., Cl−) is preferentially conducted through the channel. When the buffered solution in the trans chamber was changed to 200 mM CsCl, thus eliminating the Cl− gradient, Erev shifted back to zero. Together, these data indicate that although p45 conducts several ions, it is somewhat more selective for anions than cations.
When a single fraction containing both p45 and p36 was reconstituted (Fig. 1E), channel activity was seen only when ODN was present. The channel characteristics (conductance and kinetics) were identical to the previously reported characteristics of NACh (1, 7).
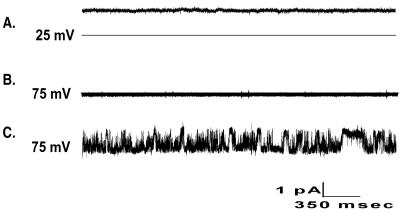
To characterize the functional role of p36 in NACh activity, p36 was reconstituted alone or in combination with p45. Current was not seen when p36 was reconstituted alone in either the absence or presence of ODN (data not shown), suggesting that p36 alone does not form a channel. When p45-containing proteoliposomes were reconstituted, the expected ODN-independent activity was observed (Fig. 2A). The channel closed on addition of p36-containing proteoliposomes (Fig. 2B), and current was not observed until ODN was added (Fig. 2C). Channel activity observed after reconstitution of p36-proteoliposomes with p45-proteoliposomes was comparable to activity observed after reconstitution of a single FPLC fraction, which contained both proteins (Fig. 1E), and to the activity of the channel complex as previously described (1). These data support the hypothesis that the nucleic acid channel is a heteromultimeric protein complex consisting of at least two proteins, p45 and p36. P45 forms a nonselective transmembrane pore that is more permeable to anions than cations. The 36-kDa subunit, which does not have channel activity when reconstituted alone, is a regulatory subunit that interacts with p45 to form the nucleic acid specific channel.
Figure 2.
The functional reconstitution of nucleic acid channel subunits. Ten-second current traces when channel subunits were reconstituted. (Top) Reconstitution of p45 alone and in the absence of ODN. (Middle) Channel reconstituted with proteoliposomes containing p45 and proteoliposomes containing p36; ODN was not present. (Bottom) The same conditions as B but in the presence of 10 μM ODN.
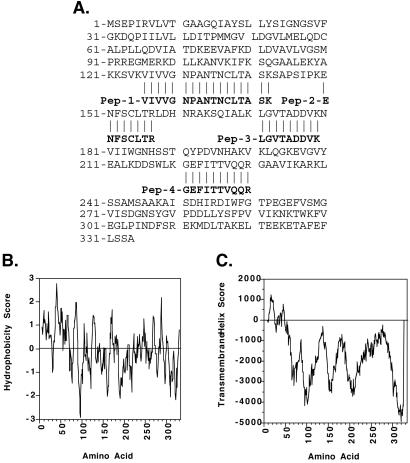
To identify p45 and p36, the proteins were purified by two-dimensional gel electrophoresis and sequenced by MS. These efforts resulted in several peptide sequences each from p45 and p36. The sequences from p45 do not match any sequence in searchable databases; whereas, the sequences from p36 are identical to rat cytosolic malate dehydrogenase (cMDH). Alignment of p36 sequences with rat cMDH is shown in Fig. 3A. We used hydropathy analysis (11) and tmpred software (12), which is an algorithm that predicts membrane-spanning domains, to assess the potential that cMDH is an integral membrane protein. Hydropathy analysis demonstrates highly hydrophobic domains from amino acids 8–21 and 37–53 (Fig. 3B) and tmpred predicts putative transmembrane-spanning domains from amino acids 7–25 and 35–53 (Fig. 3C). These computational analyses suggest that cMDH may be an integral membrane protein and as such we would hypothesize that cMDH interacts with p45 to form the nucleic acid channel.
Figure 3.
Sequence alignment of p36 peptides with rat cMDH, hydropathy analysis (Kyte–Doolittle) of rat cMDH, and the prediction of transmembrane helixes in rat cMDH. (A) Alignment of four tryptic p36 peptides (sequenced by MS) with rat cMDH. (B) Hydropathy plot [Kyte–Doolittle (12)] of rat cMDH. Hydrophobicity scores above zero indicate hydrophobic domains. (C) Prediction of transmembrane-spanning domains in rat cMDH by using tmpred algorithms (13). Transmembrane helix scores >500 predict transmembrane-spanning domains with high probability.
Experiments were performed to test this hypothesis and confirm that cMDH is a subunit of the nucleic acid channel. First, we explored whether l-malate alters channel function (Fig. 4A). Reconstitution of a single fraction containing both p45 and p36 resulted in ODN-dependent channel activity (Fig. 4A Top). At least four channels are present in the trace shown. Addition of 20 μM l-malate to both solution chambers resulted in a decrease in channel open probability from 0.71 to 0.60 (Fig. 4A Middle). Increasing l-malate concentration to 25 μM resulted in a further decrease in open probability to 0.43 (Fig. 4A Bottom). Open probability increased when l-malate was washed out (data not shown). In four experiments, 20 μM l-malate resulted in a mean decrease in open probability of 79 ± 11% (P < 0.05 vs. control). To validate the l-malate blockade, these open probability data were determined from 3-min current traces. Solution pH did not change after the addition of l-malate. To ensure the effect of l-malate was specific to NACh activity (i.e., specific to the activity of the p45-p36 channel complex), l-malate effects on p45 were examined (n = 4). In the representative experiment shown in Fig. 4B, symmetrical l-malate at concentrations as high as 30 μM had no effect on open probability of p45-alone or its conductance (Fig. 4B Bottom) when compared to zero malate control (Fig. 4B Top). These data demonstrate that NACh activity is inhibited by l-malate and that this inhibition requires the presence of the 36-kDa subunit.
Figure 4.
Blockade of the nucleic acid channel by l-malate. Ten-second current traces and the corresponding open probability from an experiment investigating the ability of l-malate to block nucleic acid channel activity (A) or p45 alone activity (B). Membrane potential was held at +50 mV for all traces. (A) Reconstitution of a single fraction containing p45 and p36 in the presence of the indicated amount of l-malate. Open probability was 0.71, 0.60, and 0.43 in the presence of 0, 20, and 25 μM l-malate, respectively. (B) A fraction containing only p45 was reconstituted and l-malate was added as indicated. l-Malate did not alter channel activity under these conditions.
We generated a polyclonal antiserum against pig heart cMDH (Sigma) in a mouse at the Mount Sinai School of Medicine Hybridoma Core Facility by using previously publish methodology (13). As seen in Fig. 5A, the animal mounted a strong immune response against cMDH as indicated by a titer of >1:10,000 by ELISA. In Western blots of pig heart cMDH, this serum primarily recognized the 36-kDa pig heart cMDH as well as two larger molecular weight products present in the commercial cMDH preparation (Fig. 5B, lane 1). The serum was immunoreactive with only a single protein that comigrated with a cMDH control when blots of whole kidney lysate were probed (Fig. 5B, lane 2). We then tested the ability of the antiserum to alter NACh activity. Antiserum at a dilution of 1:200 caused a mean decrease in channel open probability of 80 ± 13% (P < 0.05 vs. control, n = 6). In these, open probability data also were determined from an entire 3-min current trace. Normal serum, at the same dilutions, had no effect on channel open probability (data not shown). The results from a single experiment are depicted in Fig. 5C.
Figure 5.
Characterization of antiserum raised against cMDH. (A) ELISA of serum from a mouse immunized with cMDH (Sigma). The animal mounted a strong immune response to cMDH as indicated by a titer of >1:10,000. (B) Serum was then tested for specificity in Western blots of either pig heart cMDH (lane 1) or whole kidney lysate from rat (lane 2). This serum recognized both the commercial cMDH and a single protein in whole kidney lysates that comigrates with control cMDH. (C) Ten-second current traces and the corresponding open probability from an experiment in which antiserum against cMDH was used to block nucleic acid channel activity. Membrane potential was held at +50 mV for all traces. (Top) Control activity (no antiserum). Open probability under these conditions was 0.34. (Middle) The addition of 2 μl of anti-cMDH antiserum (1:500 dilution) reduced open probability to 0.02. (Bottom) Anti-cMDH antiserum and a dilution of 1:250 resulted in nearly complete blockade of channel function. (D) Immunolocalization of cMDH in a cell line derived from pig kidney proximal tubule (LLC-PK cells). As indicated by the arrowheads, anti-cMDH antiserum was immunoreactive with cell membranes, supporting the hypothesis of plasma membrane localization of cMDH. Preimmune sera were not immunoreactive with LLC-PK cells (data not shown).
These data suggest that cMDH is the regulatory subunit of the nucleic acid channel. To provide direct evidence for this hypothesis, proteoliposomes were formed with either p45 or pig heart cMDH (Sigma), fused with lipid bilayers either individually or together, and channel activity was characterized. To form cMDH proteoliposomes, ≈20 μg of cMDH (Sigma) was sonicated with a 1:1 mixture of phosphatidylethanolamine and phosphatidylserine in a volume of 25 μl. Five to ten microliters was then added to the trans solution cup and allowed to fuse with the preformed lipid bilayer. Reconstitution with p45 alone resulted in the expected nucleic acid-independent channel activity (Fig. 6A) previously described in Fig. 2. Channel activity was not observed after reconstitution of pig heart cMDH alone either in the presence (Fig. 6B) or absence (data not shown) of ODN. Channel activity was not seen when p45 and commercial cMDH were reconstituted together in the absence of ODN (Fig. 6C). When 10 μM ODN was added, however, channel gating was observed (Fig. 6D). Channel conductance of reconstituted p45 and pig heart cMDH was 12.4 ± 3.9 pS (n = 11), which is comparable to the conductance of the ODN-gated channel published previously (1). In control experiments, proteoliposomes containing mitochondrial MDH did not restore nucleic acid selectivity to p45 (data not shown). These data provide direct evidence that cMDH is the regulatory subunit of the nucleic acid channel.
Figure 6.
Functional reconstitution of nucleic acid channel activity with purified p45 and pig heart cMDH. To provide direct evidence that cMDH is the regulatory subunit of the nucleic acid channel, proteoliposomes were formed with either pure p45 or pig heart cMDH and then fused either individually or together with planar lipid bilayers. Current traces (1-min) from a representative experiment are shown. Membrane potential was held at +50 mV for each trace. (A) Reconstitution of pure p45 in the absence of ODN. (B) Reconstitution of pig heart cMDH in the absence of ODN. (C) Reconstitution of pure p45 and pig heart cMDH in the absence of ODN. (D) Reconstitution of pure p45 and pig heart cMDH in the presence of 10 μM ODN. Reconstitution of p45 and commercially available pig heart cMDH recapitulated the activity of the nucleic acid channel purified form rat-kidney brush border membrane. These data provide direct evidence that cMDH is the regulatory subunit of the nucleic acid channel.
Finally, cMDH has previously been described as a globular cytosolic protein and has not been considered to be associated with cell membranes (14). To function as the subunit of a plasma membrane channel complex, cMDH must interact with p45 and be in close proximity to the cell membrane. To demonstrate proximity of cMDH with cell membranes, anti-cMDH antiserum was used to immunolocalize cMDH in LLC-PK cells, a cell line derived from pig kidney proximal tubule. We have recently purified NACh from this cell line (data not shown). LLC-PK cells were grown on acid-washed, collagen-coated coverslip, fixed, permeabilized, immunostained, and viewed by confocal microscopy. As seen in Fig. 5D, the pattern of immunoreactivity is reticular, suggesting intracellular membrane association. In addition, staining on the margin of cells (arrowheads) is also detected, which is consistent with localization of cMDH to the plasma membrane. Preimmune sera were not immunoreactive with LLC-PK cells (data not shown). These data, together with the computational analyses shown in Fig. 3 B and C, support the hypothesis that cMDH associates with p45 at the cell membrane to regulate channel activity.
Discussion
The data presented herein demonstrate that the nucleic acid conducting channel is a complex of at least two proteins. One of these proteins has been identified as cMDH, whereas the second protein, p45, has yet to be identified. These data indicate that p45 and cMDH must both be present to reconstitute nucleic acid channel function. When we first identified and characterized the nucleic acid channel (1), p36 (i.e., cMDH) was not detected and therefore NACh activity had been attributed to a single protein, p45. Subsequent to a significant scale-up of the purification methodology, p36 was seen to copurify with p45. These experiments were initiated to determine whether p36 was an artifact of purification or whether it participates in nucleic acid channel function. As detailed above, the data support the hypothesis that p36 is a critical component of the channel. We would contend, based on the striking difference between the channel activity of p45 alone and that of the p45-cMDH channel complex, that p36 was present, although not detected on gels, in the original affinity-purified samples.
MDH is a highly conserved, ubiquitous enzyme present in bacteria, plants, and animals. In mammalian cells there are two isoforms; a mitochondrial isoform (mMDH) and a “cytosolic isoform” (cMDH). Both isoforms are NAD-dependent enzymes that catalyze l-malate to oxaloacetate. Despite similar enzymatic function, the isoforms share little homology at the amino acid level (≈20% sequence identity and <35% homology (15). Sequence analysis of mdh genes from several species indicate that the cytosolic and mitochondrial isoforms resulted from a gene duplication before the evolution of the eukaryotic cell (14, 16). Although the biochemical role of mitochondrial MDH is well documented, the functional significance of cMDH remains unclear. The genetic divergence of the two genes, however, would suggest that they might have distinct biochemical or physiologic functions. The studies detailed herein provide compelling evidence for an additional, novel function of cMDH.
It is also interesting to note that the enzymatic reaction catalyzed by MDH is thought to be an obligate-ordered reaction, which requires NAD interaction with the enzyme before malate can bind. In our experiments, l-malate blocks channel activity in the absence of NAD suggesting that p45 results in a conformational change in cMDH, which opens the malate binding site. Conversely, it is possible that NAD purifies with cMDH as has been suggested for the β-subunit of the voltage-dependent K+ channel (17).
Because p45, when reconstituted alone, is nonselective and essentially constitutively open, its presence in the plasma membrane would lead to a dissipation of the charge separation across the membrane, and as such would be incompatible with cell viability. We would hypothesize, therefore, that p45 cannot exist in the plasma membrane alone and must be in complex with a regulatory subunit. Our data suggest that cMDH plays this regulatory role in the nucleic acid channel complex.
In summary, we have provided evidence for an important function of cMDH. We have shown that the nucleic acid channel is a heteromultimer consisting of a 45-kDa subunit and a 36-kDa subunit; p45 forms a nonselective transmembrane pore which is more permeable to anions than cations whereas p36, which has no channel activity when reconstituted alone, is a regulatory subunit that regulates channel selectivity and gating kinetics. Protein sequence data and functional reconstitution studies have shown that cMDH is the regulatory subunit of NACh. Finally, we have localized cMDH to cell membranes by immunofluorescence. The cellular function of cMDH is not completely understood, and its involvement as a component of a plasma membrane transport complex has not, until now, been described. Although such a role for cMDH has not been described, other enzymes have been shown to participate in channel function. Two notable examples are a recent report that the beta subunit of voltage-dependent potassium channels is a putative oxido-reductase enzyme (17) and the observation that a splice variant of the NADPH oxidase homolog-1 gene (NOH-1) forms an H+ channel when expressed in HEK-293 cells (18). The current observations together with the findings of these other studies suggest an emerging duality of function for some metabolic enzymes and it is intriguing that these newly described functions are related to regulation of cell membrane permeability.
Acknowledgments
We thank Drs. Ruth Abramson, Leslie Bruggeman, and Diomedes Logothetis for critical evaluation of this manuscript. Confocal laser scanning microscopy was performed at the Mt. Sinai School of Medicine-Confocal Laser Beaming Microscopy core facility, supported with funding from an National Institutes of Health shared instrumentation grant and a National Science Foundation Major Research Instrumentation grant. Research was supported by a U.S. Public Health Service Grant (to D.F.H.) and an National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant (to P.E.K.).
Abbreviations
- NACh
nucleic acid-conducting channel
- cMDH
cytosolic malate dehydrogenase
- ODN
oligodeoxynucleotides
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Shabanowitz, J., Settlage, R. E., Marto, J. A., Christian, R. E., White, F. M., Russo, P. S., Martin, S. E. & Hunt, D. F., Fourth Symposium on Mass Spectrometry in the Life and Health Sciences, Aug. 25–29, 1998, San Francisco, CA, pp. 163–177.
References
- 1.Hanss B, Leal-Pinto E, Bruggeman L A, Copeland T D, Klotman P E. Proc Natl Acad Sci USA. 1998;95:1921–1926. doi: 10.1073/pnas.95.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal S, Temsamani J, Tang J Y. Proc Natl Acad Sci USA. 1991;88:7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cossum P A, Sasmor H, Dellinger D, Truong L, Cummins L, Owens S R, Markham P M, Shea J P, Crooke S. J Pharmacol Exp Ther. 1993;267:1181–1190. [PubMed] [Google Scholar]
- 4.Goodarzi G, Watabe M, Watabe K. Biopharm Drug Dispos. 1992;13:221–227. doi: 10.1002/bdd.2510130308. [DOI] [PubMed] [Google Scholar]
- 5.Rappaport J, Hanss B, Kopp J B, Copeland T D, Bruggeman L A, Coffman T M, Klotman P E. Kidney Int. 1995;47:1462–1469. doi: 10.1038/ki.1995.205. [DOI] [PubMed] [Google Scholar]
- 6.Leal-Pinto E. Acta Cientifica Venezuela Bioquimica. 1987;38:157–163. [PubMed] [Google Scholar]
- 7.Leal-Pinto E, Hanss B, Klotman P E. Kidney Int Suppl. 1996;57:S4–S10. [PubMed] [Google Scholar]
- 8.Leal-Pinto E, Tao W, Rappaport J, Richardson M, Knorr B A, Abramson R G. J Biol Chem. 1997;272:617–625. doi: 10.1074/jbc.272.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Rabilloud T. Electrophoresis. 1992;13:429–439. doi: 10.1002/elps.1150130190. [DOI] [PubMed] [Google Scholar]
- 10.Shevchenko A, Wilm M, Vorm O, Mann M. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 11.Kyte J, Doolittle R F. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann K, Stoffel W. Biol Chem Hoppe-Seyler. 1993;347:166. doi: 10.1515/bchm3.1992.373.1.187. [DOI] [PubMed] [Google Scholar]
- 13.Wang B S, Lumanglas A L, Bona C A, Moran T M. Mol Immunol. 1996;33:1197–1202. doi: 10.1016/s0161-5890(96)00055-7. [DOI] [PubMed] [Google Scholar]
- 14.Musrati R A, Kollarova M, Mernik N, Mikulasova D. Gen Physiol Biophys. 1998;17:193–210. [PubMed] [Google Scholar]
- 15.Joh T, Takeshima H, Tsuzuki T, Setoyama C, Shimada K, Tanase S, Kuramitsu S, Kagamiyama H, Morino Y. J Biol Chem. 1987;262:15127–15131. [PubMed] [Google Scholar]
- 16.Shimada K, Joh T, Ding S H, Choudhury B K, Setoyama C. Prog Clin Biol Res. 1990;344:139–158. [PubMed] [Google Scholar]
- 17.Gulbis J M, Mann S, MacKinnon R. Cell. 1999;97:943–952. doi: 10.1016/s0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 18.Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause K H. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]