Abstract
Cardiomyocyte apoptosis is present in many cardiac disease states, including heart failure and ischemic heart disease. Apoptosis is associated with the activation of caspases that mediate the cleavage of vital and structural proteins. However, the functional contribution of apoptosis to these conditions is not known. Furthermore, in cardiac myocytes, apoptosis may not be complete, allowing the cells to persist for a prolonged period within the myocardium. Therefore, we examined whether caspase-3 cleaved cardiac myofibrillar proteins and, if so, whether it affects contractile function. The effects of caspase-3 were studied in vitro on individual components of the cardiac myofilament including α-actin, α-actinin, myosin heavy chain, myosin light chain 1/2, tropomyosin, cardiac troponins (T, I, C), and the trimeric troponin complex. Exposure of the myofibrillar protein (listed above) to caspase-3 for 4 h resulted in the cleavage of α-actin and α-actinin, but not myosin heavy chain, myosin light chain 1/2, and tropomyosin, into three fragments (30, 20, and 15 kDa) and one major fragment (45 kDa), respectively. When cTnT, cTnI, and cTnC were incubated individually with caspase-3, there was no detectable cleavage. However, when the recombinant troponin complex was exposed to caspase-3, cTnT was cleaved, resulting in fragments of 25 kDa. Furthermore, rat cardiac myofilaments exposed to caspase-3 exhibited similar patterns of myofibrillar protein cleavage. Treatment with the caspase inhibitor DEVD-CHO or z-VAD-fmk abolished the cleavage. Myofilaments, isolated from adult rat ventricular myocytes after induction of apoptotic pathway by using β-adrenergic stimulation, displayed a similar pattern of actin and TnT cleavage. Exposure of skinned fiber to caspase-3 decreased maximal Ca2+-activated force and myofibrillar ATPase activity. Our results indicate that caspase-3 cleaved myofibrillar proteins, resulting in an impaired force/Ca2+ relationship and myofibrillar ATPase activity. Induction of apoptosis in cardiac cells was associated with similar cleavage of myofilaments. Therefore, activation of apoptotic pathways may lead to contractile dysfunction before cell death.
Cardiomyocyte apoptosis plays an important role in the progression of many cardiovascular disorders including heart failure. Apoptosis is mediated by caspases, specialized cysteine-dependent, aspartate-directed proteases. These proteases cleave major structural elements of the cytoplasm and nucleus in the cells. It is being realized increasingly that apoptosis of myocytes significantly contributes to the progressive loss of ventricular function in congestive heart failure (1, 2). It also has been demonstrated that the process of apoptosis may not be complete in myocytes (1, 3, 4) and may differentially affect cytoplasmic proteins and nuclear substrates (4). Lack of nuclear fragmentation facilitates continuous loss of cytoplasmic proteins and may allow such cells to persist for prolonged periods in myocardium. Recognition of such a process of interrupted apoptosis may offer a window for reversal of myocellular damage and reverse remodeling.
Continued loss of cytoplasmic proteins in the presence of intact nuclear integrity allows formulation of an interesting hypothesis. The bulk of cytoplasmic proteins in the sarcoplasm is composed of contractile proteins. Most of these proteins have amino acid sites amenable to specific caspase-mediated proteolysis. Because caspases are activated abundantly in the failing myocardium (1, 4, 5), it is likely that progressive cleavage of contractile proteins constitutes the basis of inexorable decline of systolic ventricular function. Changes in myofilament calcium responsiveness and calcium-cycling proteins have been hallmarks of experimental and human heart failure (6–10). Reduced calcium sensitivity or decreased cooperation between the thick and thin myofilaments results in reduced contractile activation and force development (6). In human and experimental heart failure, several changes in the myofibrillar proteins have been reported to occur (7–10). These include an isoform shift in troponin T (TnT) and a decrease in myosin light-chain kinase 2 (11). These changes are believed to be partly responsible for a decrease in myofibrillar ATPase activity, a decreased cross-bridge cycling rate, and an altered responsiveness to agents that act on the myofilaments (12).
Methods
In Vitro Caspase-Substrate Assay.
Ten micrograms of purified cardiac myofibrillar proteins from the thick filament myosin heavy chain, myosin light chain 1/2 (from rabbit and bovine muscle, respectively; Sigma), from the thin filament cardiac α-actin in monomer form (bovine cardiac muscle; Cytoskeleton, Denver), tropomyosin (bovine muscle; Sigma), troponins T, I, and C (human cardiac muscle; Advanced Immunological, Long Beach, CA), and from the cytoskeletal structure, α-actinin (rabbit skeletal muscle; Cytoskeleton) were resuspended in caspase assay buffer (50 mM Hepes/2 mM EDTA/0.15 CHAPS/10% sucrose/5 mM DTT and protease inhibitors, pH 7.4). The following proteases were used to prepare the myofilaments: 1 mg/ml aprotinin, 1 mg/ml leupeptin, and 0.1 mM PMSF.
These purified proteins then were incubated in the presence or absence of recombinant human caspase-3 [protein/caspase-3 = 500:1 (wt/wt) for 4 h at room temperature]. In a different set of experiments, troponins, reconstituted in a complexed form (combining recombinant mouse cardiac TnT, TnI, and human TnC in a ratio of 1:1:1.5) (13), were exposed to the buffer assay described above in the presence or absence of caspase-3. Cardiac myofilaments, isolated from male Sprague–Dawley rat left ventricles, also were exposed to the buffer assay described above in the presence or absence of caspase-3. In the experimental set-up, inhibitors of caspase-3, DEVD-CHO (10 μM) or z-VAD-fmk [z-VAD (Z-Val-Ala-Asp(Ome)-FMK), 10 μM] also were added. Poly(ADP-ribose) polymerase was used as an internal control of the activity of caspase-3. Four hours after the addition of caspase-3, the cleavage reaction was stopped by the addition of Laemmli buffer followed by sonication and heating at 95°C for 7 min. The myofilaments were spun down, and then the cleavage reaction was stopped as mentioned above.
SDS/PAGE and Western Blot Analysis.
Samples were separated on a 12% SDS-polyacrylamide gel. Gels were subjected to Coomassie blue or silver staining or transferred to nitrocellulose. Western blot analysis was achieved by using the following antibodies: anti-cTnT that recognized the C-terminal region (clone JLT-12, dilution 1:200; Sigma); two antiactin directed against C-terminal (dilution 1:200; Sigma) and N-terminal of actin, respectively (clone C4, dilution 1:1,000; Chemicon); anti-TnI (clones 10F4 and 6F9; Advanced Immunological); anti-TnC (clones 1A2, 7B9, 12G3; Advanced Immunological); and anti-α-actinin sarcomeric (clone EA-53, dilution 1:800; Sigma). The percentage of myofibrillar protein cleavage was determined by densitometry, using nih image.
Cell Isolation, Culture, and Treatment.
Adult rat ventricular myocytes (ARVM) were isolated by enzymatic dissociation from hearts of adult male Sprague–Dawley rats (240–280 g) as described. The cells were resuspended in DMEM, supplemented with albumin (2 mg/ml), l-carnitine (2 mM), creatine (5 mM), taurine (5 mM), and 0.1% penicillin–streptomycin, and plated on laminin (1 μg/cm2)-coated dishes. ARVM were treated for 6 h with (−) norepinephrine (NE; 10 μM) in the presence of an α1-adrenergic receptor blocker, prazosin (0.1 μM), and ascorbate acid (0.1 mM) (14). In specific experiments, ARVM were pretreated for 4 h with z-VAD-fmk (100 μM). Myofilaments then were isolated as mentioned above, and protein analysis was performed.
Skinned Fibers, Myofibrillar ATPase, and Forces Measurements.
Force/Ca2+ relationships and myofibrillar ATPase activity were measured simultaneously in skinned fibers by using a system described before (15, 16). In these experiments, fibers obtained from trabeculae of mice hearts were skinned overnight in a relaxing buffer (150 mM KCl/20 mM Mops/10 mM EGTA/1 mM free Mg2/5 mM ATP/12 mM creatine phosphate/10 units/ml creatine kinase/0.5 mM DTT and protease inhibitors) and 1% Triton X-100. The fibers were attached to a displacement generator at one end and a force transducer at the other end. The resting sarcomere length was adjusted to 2.3 μm on the basis of a laser diffraction pattern. The fiber width, height, and length were measured, and the cross-sectional area was estimated based on an elliptical model. Tension was expressed as force/cross-sectional area. To determine the relationship of free Ca2+ concentration to tension, the fibers were bathed sequentially in solutions (with pCa ranging from pCa 8 to 4.5, at 20°C), the corresponding force was measured, and the data were stored with an analog-to-digital converter to a computer. Fiber ATPase activity was measured as follows: ATP regeneration from ADP was coupled to the breakdown of phosphoenolpyruvate to pyruvate, and ATP was catalyzed by pyruvate kinase, which was linked to the synthesis of lactate catalyzed by lactate dehydrogenase. The breakdown of NADH, which is proportional to the amount of ATP consumed, was measured by using a UV light that was projected through the quartz window of the bath and recorded at 340 nm. The ratio of light intensity at 340 nm to a signal reference at 410 nm was obtained by the mean of an analog divider. After each recording, the UV absorbance of NADH was calibrated by injections of ADP in the bathing solution. Data were analyzed with labview (National Instruments, Austin, TX). Data were linearized by using the Hill transformation, and the force/pCa was fitted to the Hill equation with nonlinear regression analysis to derive the pCa50 and Hill coefficient (sigmaplot, Chicago). The protocol consisted of two series of measurements: a first measurement at t = 0 min under control conditions, followed by a second measurement after exposure of the fibers to caspase-3 [ratio of protein/caspase = 1:50 (wt/wt)] after 45 min. In sham experiments, the fibers were bathed in the caspase buffer in the absence of caspase-3. The protocol was repeated in the presence of the inhibitor of caspase-3, DEVD-CHO (10 μM). The data were calculated as percentages compared with the maximum control values obtained at t = 0 min. Cardiac myofibrillar proteins isolated from these same fibers also were tested for breakdown of troponins.
Statistical Analysis.
Data are presented as mean ± SE and were analyzed with a one-way ANOVA, with statistical differences identified at P < 0.05.
Results
Caspase-3 Activation and Myofilament Cleavage.
To determine whether caspase-3 cleaves the myofilaments, we treated myofibrillar proteins individually and/or in complexed form with highly purified human recombinant caspase-3 for 4 h and analyzed the cleavage products by SDS/PAGE and Western blot analysis. Fig. 1 demonstrates that caspase-3 induced a robust cleavage of cTnT into fragments of ≈25 kDa only when cTnT was in complexed form with other troponins (% of cleavage = 37 ± 4%, P < 0.01, as compared with control; Fig. 1b) or in the myofilament (% of cleavage = 57 ± 5%, P < 0.01, as compared with control; Fig. 1c) but not individually (Fig. 1a). Of note, myosin, myosin light chains, tropomyosin, TnI, and TnC were not cleaved by caspase-3.
Figure 1.
Cleavage of cTnT by human recombinant caspase-3. (a) Western blot, demonstrating the absence of any effect of caspase-3 on purified cTnT. (b) Western blot, demonstrating a reduction of the intact, 39-kDa cTnT and the appearance of a fragment at ≈25 kDa after caspase-3 treatment in the troponin complex. Bar graph represents mean ± SE of the percentage of the 25-kDa fragment; *, P < 0.01, caspase-3-treated compared with untreated. (c) Western blot, demonstrating a reduction of the intact, 39-kDa cTnT and the appearance of a fragment at ≈25 kDa after caspase-3 treatment when cTnT is in myofilaments. Bar graph represents mean ± SE of the percentage of the 25-kDa fragment; *, P < 0.01, caspase-3-treated compared with untreated.
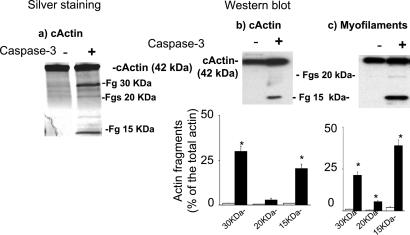
We then examined whether caspase-3 targets actin. Using silver staining and Western blot analyses with two different types of antiactin antibodies, directed against the either the N- or C-terminal region, we found that caspase-3 cleaved cardiac actin in its monomeric form in two major fragments (≈30 and ≈15 kDa) and two minor fragments (both ≈20 kDa) (Fig. 2 a and b). Caspase-3 also cleaved cardiac actin when polymerized in the lattice of the myofilaments (Fig. 2c). The generation of the ≈20-kDa fragments was more evident in the myofilament.
Figure 2.
Cleavage of cardiac α-actin by human recombinant caspase-3. (a) Silver staining of SDS/12% polyacrylamide gel, demonstrating the appearance of three bands when the monomeric purified form of α-actin is treated with caspase-3. (b) Western blot, using antibodies directed against the C terminus of α-actin, demonstrating a reduction of the intact, 42-kDa α-actin and the appearance of a major fragment at ≈15 kDa and a minor fragment at 20 kDa after caspase-3 treatment. Bar graph represents mean ± SE of the percentage of 15- and 20-kDa fragments; *, P < 0.01, caspase-3-treated compared with untreated. (c) Western blot, demonstrating a reduction of the intact, 42-kDa α-actin and the appearance of two fragments at ≈15 and 20 kDa after caspase-3 treatment of the myofilaments. Bar graph represents mean ± SE of the percentage of 15- and 20-kDa fragments; *, P < 0.01, caspase-3-treated compared with untreated.
In addition, Coomassie blue staining demonstrated that caspase-3 cleaved the cytoskeletal element α-actinin individually in fragments ≈45 kDa. This was blocked by the addition of caspase inhibitor z-VAD (Fig. 3a). Furthermore, caspase-3 also cleaved α-actinin in the lattice of the myofilaments. A fragment of ≈25 kDa was detected by using Western blot analysis (Fig. 3b). The addition of capase-3 inhibitors also blocked the cleavage of cTnT, cardiac α-actin (data not shown).
Figure 3.
Cleavage of cardiac α-actinin by human recombinant caspase-3. (a) Coomassie blue staining of SDS/7.5% polyacrylamide gel, demonstrating the appearance of one band at ≈45 kDa when the individual form of α-actinin (100 kDa) is treated with caspase-3. The other bands are contaminant proteins. (b) Western blot, demonstrating a reduction of the intact, 100-kDa α-actinin and the appearance of fragments at ≈25 kDa after caspase-3 treatment of the myofilaments.
Effect of β-Adrenergic Stimulation on Myofilament Breakdown.
We have shown that β-adrenergic receptor stimulation induces apoptosis in adult rat ventricular myocytes, and this was associated with the early activation of the mitochondrial pathway and activation of caspase-3. Therefore, ARVM were exposed to β-adrenergic stimulation for 6 h and myofilaments then were isolated during the early phase of apoptosis when no major DNA breakdown (terminal deoxynucleotidyltransferase-mediated UTP end labeling-positive cells) is found. β-Adrenergic receptor stimulation induced similar cleavage of cTnT (≈25 kDa) in ARVM (Fig. 4). The antibodies against the N-terminal of actin (residues 23–24) and C-terminal (last 11 residues) recognized, in addition to the intact actin, a similar, major fragment of ≈20 kDa. Cleavages of cTnT and actin were reduced after pretreatment with VAD-fmk (Fig. 4), demonstrating that β-adrenergic receptor-stimulated myofilament cleavage occurred mainly via caspase activation.
Figure 4.
Generation of cTnT and c-actin fragments during an early phase of β-adrenergic-stimulated apoptosis in ARVM. ARVM were treated with norepinephrine (NE, 10 μM) in the presence of α1-adrenergic receptor blocker prazosin (PZ, 0.1 μM) for 6 h. Where indicated, ARVM were pretreated with z-VAD-fmk (z-VAD, 100 μM). Myofilaments were isolated and analyzed by Western blotting, using antibodies anti-cTnT (a) and antibodies directed against the C-terminal region of actin (b).
Cleavage Site on TnT.
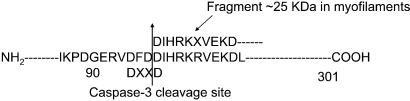
To determine the major site of caspase-3 cleavage of cTnT, caspase-3-generated cTnT fragments from the troponin complex were isolated from a polyvinylidene membrane, and partial amino acid sequences were obtained by Edman degradation and HPLC. The fragment that migrated near 25 kDa displayed an N sequence of DIHRKXVEKD, indicating that it did, in fact, result from cTnT cleavage between Asp-96 and Asp-97 as shown in Fig. 5. This finding points to the cleavage site for caspase-3 as being DFDD. The consensus caspase-3 cleavage site is usually DEVD. The two aspartic acid residues are absolutely required, but the glutamic acid and valine residues may vary. Comparison between cardiac TnT from mice, rat, and human revealed that this site is highly conserved.
Figure 5.
The target site for caspase-3 on TnT. The fragment that migrates near 25 kDa displayed an N sequence of DIHRKXVEKD, indicating that it results from cTnT cleavage between Asp-96 and Asp-97. Therefore, the cleavage site for caspase-3 is DFDD. The consensus caspase-3 cleavage site usually is DEVD. The two aspartic acid residues absolutely are required, but the glutamic acid and valine residues may vary.
Because we had no access to antibodies directed against the N-term of cTnT, we treated a troponin complex (cMyc attached to cTnT) with caspase-3, and, after treatment, we isolated the troponin complex and probed with antibodies directed against cMyc. We could not find the cMyc fragment (N-terminal fragment), suggesting that only the C terminus remains in the complex or in the lattice of the myofilament. This N-terminal region has been shown to be important for the cardiac function. Indeed, the truncation of the N-terminal region of cTnT impairs the force/Ca2+ relationship and decreases myofibrillar ATPase activity in skinned fiber.
Caspase Activation and Contractile Activation.
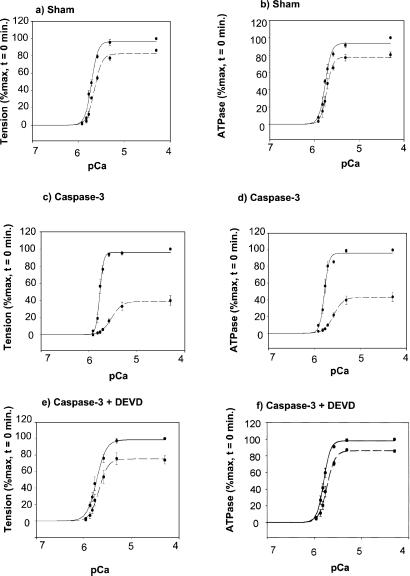
To examine whether the activation of caspase-3 and the concomitant breakdown of cardiac TnT, α-actin, and α-actinin result in functional effects, we measured the effect of caspase-3 treatment on force/Ca2+ relationship and simultaneous myofibrillar ATPase activity in skinned fibers isolated from the mice hearts. Fig. 6 illustrates the force/Ca2+ relationship and simultaneous myofibrillar ATPase before (t = 0) and after 45 min of caspase-3 treatment. After 45 min of caspase-3 treatment, there was a significant decrease in maximal Ca2+-activated force and in Ca2+ sensitivity (Fig. 6c) as compared with sham fibers that were treated with buffer solution (Fig. 6a). There was a parallel decrease in maximal Ca2+-activated myofibrillar ATPase activity (Fig. 6d) as compared with sham fiber (Fig. 6b). The addition of caspase-3 inhibitor DEVD-CHO prevented the functional changes mediated by caspase-3 (Fig. 6 e and f) but DEVD-CHO had no independent effects on myofilament activation (data not shown).
Figure 6.
Normalized pCa tension and pCa-ATPase relation in detergent-skinned fiber bundle were measured after caspase-3 treatment. Resting tension was fixed at 2.3 μm. (a) pCa tension of untreated fiber at t = 0 (solid line) and 45 min (dashed line); maximum tensions at pCa 4.5 at t = 0 and t = 45 min were 39.6 ± 4.4 mN/mm2 and 34.0 ± 4.0 mN/mm2, respectively. (b) pCa-ATPase of untreated fiber at t = 0 and 45 min; maximum ATP consumptions at pCa 4.5 at t = 0 and t = 45 min were 335 ± 29 pmol/μl per sec and 289 ± 24 pmol/μl per sec, respectively. (c) pCa-tension of fibers after t = 0 and 45 min of caspase-3 treatment; maximum tensions at pCa 4.5 at t = 0 and t = 45 min were 37.6 ± 5.0 mN/mm2 and 16.6 ± 2.7 mN/mm2, respectively. (d) pCa-ATPase of fibers after t = 0 and 45 min of caspase-3 treatment; maximum ATP consumptions at pCa 4.5 at t = 0 and t = 45 min were 297 ± 15 pmol/μl per sec and 149 ± 15 pmol/μl per sec, respectively. (e) pCa-tension of fibers after t = 0 and 45 min of caspase-3 and DEVD-CHO (10 μM) treatments; maximum tensions at pCa 4.5 at t = 0 and t = 45 min were 36.6 ± 6.9 mN/mm2 and 27.2.0 ± 3.2 mN/mm2, respectively. (f) pCa-ATPase of fibers after t = 0 and 45 min of caspase-3 and DEVD-CHO treatments; maximum ATP consumptions at pCa 4.5 at t = 0 and t = 45 min were 312 ± 8 pmol/μl per sec and 273 ± 3 pmol/μl per sec, respectively. Graph represents mean ± SE of percent change from t = 0 min from three to four fibers, from three to four different hearts for each case. After 45 min, caspase-3 treatment decreases maximal Ca2+-activated tension as compared with sham (respectively, at pCa 4.5, caspase-3 = 39.6 ± 6.3%* vs. Sham = 82.9 ± 0.7%, P < 0.01, n = 3). Furthermore, after 45 min, caspase-3 treatment decreases maximal Ca2+-activated ATPase as compared with sham (respectively, caspase-3 = 48.4 ± 6.0%* vs. Sham = 77.9 ± 2.0%, P < 0.05, n = 3). However, there was no change in pCa50.
To ascertain that these fibers, which were used for the mechanical studies, indeed had breakdown of cardiac TnT, actin, and α-actinin, we analyzed these fibers by Western blotting. As in the myofilaments, we found one detectable cleavage product for cTnT (at 25 kDa), two detectable products for actin (20 and 15 kDa), and one cleavage product for α-actinin (at 25 kDa) (data not shown).
Discussion
Our results demonstrate that caspase-3 activation directly targets three components of the myofilaments, namely, α-actin, α-actinin, and TnT. Interestingly, TnT was cleaved only when it was combined with the other troponins. Furthermore, pro-apoptotic stimuli such as β-adrenergic stimulation induce similar breakdowns of myofilaments. Finally, the cleavage of these proteins by caspase-3 had direct functional effects on myofilament activation and contractile function.
In cardiac tissue, apoptosis is a form of cell death that has been described as distinct from necrotic cell death. It has been proposed that ventricular dilatation and neurohormonal activation during heart failure lead to up-regulation of transcription factors, induce myocyte hypertrophy, and prepare the cell for entry into the cell cycle. However, terminally differentiated myocytes cannot divide, and, instead, they undergo apoptosis. Initiation of apoptosis is associated with activation of upstream cascades, including the release of cytochrome c from mitochondria to cytoplasm and the processing of proteolytic caspases. The activation of caspases leads to fragmentation of various cytoplasmic proteins. However, the nuclear fragmentation and condensation is completed only rarely. The balance between nuclear fragmentation and protein cleavage is variable in myocytes (1, 3, 4). When this balance is shifted toward the continuous loss of cytoplasmic proteins, cells persist but, in the case of cardiomyocytes, are in a “Zombie” state with little or no contraction. When this balance is shifted toward nuclear fragmentation, the cells die. This state of interrupted apoptosis, where cardiac myocytes are dysfunctional (without nuclear fragmentation), may offer a window for reversal of myocellular damage and reverse remodeling.
Our results would suggest that the activation of caspases indeed induce the breakdown of myofibrillar proteins, which leads to a decrease in ATPase activity and force development. This decrease in systolic function therefore could precede any breakdown of DNA. The cleavage of the myofilaments clearly results in a reduction of systolic function, which can be detrimental for the overall function of the heart. Interestingly, in a model of ischemia reperfusion, blockade of apoptosis by gene transfer of akt improved ventricular wall shortening. However, it also could serve to decrease energy utilization while the cell is attempting to actively repair DNA, a costly endeavor for the cell from an energy standpoint.
Structurally, cardiac troponin T connects tropomyosin to the TnC–TnI complex and confers calcium sensitivity and cooperativity to the activity of the TnC–TnI complex on actomyosin ATPase (6, 15–18). The C terminus of TnT is necessary for submaximal activation whereas the N terminus is essential for maximal activation. In our studies, cleavage of TnT occurred only when it was complexed, probably because the cleavage site is exposed in that complex. A number of TnT mutations (missense and base pair deletions) have been associated with hypertrophic cardiomyopathies (19, 20). Functionally, these TnT mutations result in decreases in maximal activated force, with variable effects on calcium sensitivity. Interestingly, our results revealed that the maximal Ca2+ activation force was affected without much change in calcium sensitivity. A recent study showed that after ischemia/reperfusion, caspase inhibition blocked the breakdown of TnI (21). These results would seem at odds with our data, wherein we did not observe breakdown of TnI in the presence of caspase-3. However, in their study, Ruetten et al. (21) found that the breakdown of TnI depended directly on calpain activation, which was not the case with our preparations. Furthermore, stunning in ischemia reperfusion specifically induces TnI (22), which may not be preponderant in other cardiovascular conditions and diseases in which cardiac apoptosis is present.
Another target of caspase-3 was α-actinin, which is a main component of the Z band and is responsible for linking the thin filaments together (23). The role of α-actinin has been proposed to keep the thin filaments in a cooperative mode during contraction and relaxation. In ischemia, the loss of α-actinin has been thought to decrease maximal force of contraction by altering the transmission of force from the actin–myosin reaction and by altering interfilament spacing. These effects would cause a decrease in the affinity of actin for α-actinin and disrupt parallel interfilament formation.
Interestingly, we did not find any breakdown of the other troponins with activation of caspase-3. Furthermore, myosin molecule also was unaffected by the activation of caspase-3. This could be because there is a small amount of degradation and because our methods of detection are not sensitive enough.
These results show an important interrelationship between caspase activation and functional reserve in cardiomyocytes. Targeting caspases in cardiomyocytes may be beneficial not only for cardiomyocyte survival but also function.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health [HL 57623 to R.J.H.; R01-63704 to P.d.T.; and PO1-HL62426 (project 1 to R.J.S. and M.S. and project 4 to P.d.T.)]; and a Doris Duke Charitable Foundation Clinician Scientist Award (to R.J.H.). R.J.H. is a Beeson Scholar of the American Federation of Aging Research. C.C. was supported in part by Grant HL61557 to Dr. Anthony Rosenzweig. M.S. is supported in part by an Individual National Research Service Award (F32HL10409–02).
Abbreviations
- TnC
TnI, and TnT, troponin C, troponin I, and troponin T, respectively
- z-VAD
Z-Val-Ala-Asp(Ome)-FMK
- ARVM
adult rat ventricular myocytes
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Narula J, Haider N, Virmani R, DiSalvo T G, Kolodgie F D, Hajjar R J, Schmidt U, Semigran M J, Dec G W, Khaw B A. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara J A, Quaini E, Di Loreto C, Beltrami C A, Krajewski S, et al. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 3.Kanoh M, Takemura G, Misao J, Hayakawa Y, Aoyama T, Nishigaki K, Noda T, Fujiwara T, Fukuda K, Minatoguchi S, et al. Circulation. 1999;99:2757–2764. doi: 10.1161/01.cir.99.21.2757. [DOI] [PubMed] [Google Scholar]
- 4.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie F D, Dal Bello B, Semigran M J, Bielsa-Masdeu A, Dec G W, et al. Proc Natl Acad Sci USA. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G. N Engl J Med. 1996;335:1190–1196. doi: 10.1056/NEJM199610173351604. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar R J, Schwinger R H, Schmidt U, Kim C S, Lebeche D, Doye A A, Gwathmey J K. Circulation. 2000;101:1679–1685. doi: 10.1161/01.cir.101.14.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munch G, Bolck B, Sugaru A, Brixius K, Bloch W, Schwinger R H. Circulation. 2001;103:2739–2744. doi: 10.1161/01.cir.103.22.2739. [DOI] [PubMed] [Google Scholar]
- 8.Brixius K, Mehlhorn U, Bloch W, Schwinger R H. J Pharmacol Exp Ther. 2000;295:1284–1290. [PubMed] [Google Scholar]
- 9.Munch G, Bolck B, Brixius K, Reuter H, Mehlhorn U, Bloch W, Schwinger R H. Am J Physiol. 2000;278:H1924–H1932. doi: 10.1152/ajpheart.2000.278.6.H1924. [DOI] [PubMed] [Google Scholar]
- 10.Schwinger R H, Bohm M, Schmidt U, Karczewski P, Bavendiek U, Flesch M, Krause E G, Erdmann E. Circulation. 1995;92:3220–3228. doi: 10.1161/01.cir.92.11.3220. [DOI] [PubMed] [Google Scholar]
- 11.Margossian S S, White H D, Caulfield J B, Norton P, Taylor S, Slayter H S. Circulation. 1992;85:1720–1733. doi: 10.1161/01.cir.85.5.1720. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar R J, Gwathmey J K. Circulation. 1992;86:1819–1826. doi: 10.1161/01.cir.86.6.1819. [DOI] [PubMed] [Google Scholar]
- 13.Potter J D. Methods Enzymol. 1982;85:241–263. doi: 10.1016/0076-6879(82)85024-6. [DOI] [PubMed] [Google Scholar]
- 14.Communal C, Singh K, Pimentel D R, Colucci W S. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 15.de Tombe P P, Stienen G J. Circ Res. 1995;76:734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- 16.Van Eyk J E, Powers F, Law W, Larue C, Hodges R S, Solaro R J. Circ Res. 1998;82:261–271. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Dalloz F, Osinska H, Robbins J. J Mol Cell Cardiol. 2001;33:9–25. doi: 10.1006/jmcc.2000.1289. [DOI] [PubMed] [Google Scholar]
- 18.Papp Z, van der Velden J, Stienen G J. Cardiovasc Res. 2000;45:981–993. doi: 10.1016/s0008-6363(99)00374-0. [DOI] [PubMed] [Google Scholar]
- 19.Solaro R J. Circ Res. 1999;84:122–124. doi: 10.1161/01.res.84.1.122. [DOI] [PubMed] [Google Scholar]
- 20.Seidman C E, Seidman J G. Basic Res Cardiol. 1998;93:13–16. doi: 10.1007/s003950050196. [DOI] [PubMed] [Google Scholar]
- 21.Ruetten H, Badorff C, Ihling C, Zeiher A M, Dimmeler S. J Am Coll Cardiol. 2001;38:2063–2070. doi: 10.1016/s0735-1097(01)01670-9. [DOI] [PubMed] [Google Scholar]
- 22.Murphy A M, Kogler H, Marban E. Mol Med Today. 2000;6:330–331. doi: 10.1016/s1357-4310(00)01732-9. [DOI] [PubMed] [Google Scholar]
- 23.Hein S, Kostin S, Heling A, Maeno Y, Schaper J. Cardiovasc Res. 2000;45:273–278. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]