Abstract
The ATP-binding cassette transporter 1 (ABCA1) has recently been identified as a key regulator of high-density lipoprotein (HDL) metabolism, which is defective in familial HDL-deficiency syndromes such as Tangier disease. ABCA1 functions as a facilitator of cellular cholesterol and phospholipid efflux, and its expression is induced during cholesterol uptake in macrophages. To assess the role of macrophage ABCA1 in atherosclerosis, we generated low-density lipoprotein (LDL) receptor knockout (LDLr−/−) mice that are selectively deficient in leukocyte ABCA1 (ABCA1−/−) by using bone marrow transfer (ABCA1−/− → LDLr−/−). Here we demonstrate that ABCA1−/− → LDLr−/− chimeras develop significantly larger and more advanced atherosclerotic lesions compared with chimeric LDLr−/− mice with functional ABCA1 in hematopoietic cells. Targeted disruption of leukocyte ABCA1 function did not affect plasma HDL cholesterol levels. The amount of macrophages in liver and spleen and peripheral blood leukocyte counts is increased in the ABCA1−/− → LDLr−/− chimeras. Our results provide evidence that leukocyte ABCA1 plays a critical role in the protection against atherosclerosis, and we identify ABCA1 as a leukocyte factor that controls the recruitment of inflammatory cells.
ATP-binding cassette (ABC) transporters constitute a large family of evolutionary conserved transmembrane proteins that translocate a wide variety of substrates across cellular membranes (1–5). Work from our laboratory and from others provided evidence that mutations in the human ABC transporter 1 (ABCA1) gene are the underlying molecular defect in familial high-density lipoprotein (HDL)-deficiency syndromes such as Tangier disease (TD) (6–8). TD is an autosomal recessive disorder that is characterized by severe HDL deficiency, deposition of cholesteryl esters in cells of the reticuloendothelial system, and premature development of coronary artery disease in a subgroup of patients (9–11). ABCA1 is a multispan transmembrane molecule with high expression levels in the adrenal gland, liver, lung, intestine, placenta, and fetal tissues (2, 12). A recent study demonstrated that it functions as a facilitator of cholesterol and phospholipid export at the plasma membrane (13). Expression of ABCA1 is induced during monocyte differentiation into macrophages and up-regulated by cholesterol influx (12), suggesting a role for this ABC lipid transporter in reverse cholesterol transport, a process by which excess cholesterol is removed from the periphery and transported to the liver (14). Recent data showed that ABCA1 is directly involved in cAMP-inducible (15) and peroxisome proliferator-activated receptor (PPAR)-α and -γ mediated removal of cholesterol from macrophage foam cells in vitro (16). A role for ABCA1 and, in particular, macrophage ABCA1 in atherogenesis is therefore conceivable.
Materials and Methods
Mice.
ABCA1 knockout (ABCA1−/−) mice, previously generated by the R.W. Johnson Pharmaceutical Research Institute (17), and nontransgenic littermates were used. Homozygous low-density lipoprotein (LDL) receptor knockout (LDLr −/−) mice (18, 19) were obtained from The Jackson Laboratory as mating pairs and bred at the Gaubius Laboratory, Leiden, The Netherlands. Mice were housed in sterilized filter-top cages and given unlimited access to food and water. They were maintained on sterilized regular chow (SRM-A), containing 5.7% (wt/wt) fat and no cholesterol (Hope Farms, Woerden, The Netherlands), or were fed a semisynthetic high-cholesterol Western-type diet [15% (wt/wt) cacao butter, 0.25% (wt/wt) cholesterol, 20% (wt/wt) casein, 10% (wt/wt) corn starch, and 41% (wt/wt) sucrose], composed according to Nishina et al. (20). Drinking water was supplied with antibiotics (83 mg/liter of ciprofloxacin and 67 mg/liter of polymyxin B sulfate) and 6.5 g/liter of sucrose.
Bone Marrow Transplantation.
To induce bone marrow aplasia, female LDLr −/− mice (age 9–11 weeks) were exposed to a single dose of 13 Gy (0.28 Gy/min, 200 kV, 4 mA) total body irradiation, as described (21). Irradiated recipients were transplanted with 0.5 × 107 bone marrow cells, isolated from female ABCA1−/− mice (age 14–19 weeks), or the nontransgenic ABCA1+/+ littermates (age 14–19 weeks).
Assessment of Chimerism.
The hematologic chimerism of the LDLr−/− mice was determined in genomic DNA from bone marrow by PCR at 20 weeks posttransplant. ABC3057 (5′-GAGCACATCTGGTTCTATGC-3′), NEO1455 (5′-CGCTTCCTCGCTTTACGGTAT-3′), and ABC210R (5′-AAGACACGGTGCTGCTACTGTT-3′) were used for PCR amplification to detect both the wild-type and the null mutant ABCA1 gene simultaneously, as described by Christiansen-Weber et al. (17).
Macrophage Cholesterol Efflux Studies.
Thioglycollate-elicited peritoneal macrophages, harvested from mice transplanted with ABCA1+/+ and ABCA1−/− bone marrow, were loaded with 0.5 μCi/ml 3H-cholesterol in DMEM/2% BSA for 40 h at 37°C. Cholesterol efflux was studied by incubation of the cells with DMEM/2% BSA supplemented with 100 μg/ml of HDL. Cell protein was measured according to Lowry et al. (22). Specific HDL-mediated efflux is defined as the difference between the efflux in the presence of HDL and 2% BSA minus the efflux in the presence of 2% BSA only.
Serum Lipid and Apolipoprotein Analyses.
After an overnight fasting period, approximately 100 μl of blood was drawn from each individual mouse by tail bleeding. The concentrations of total cholesterol, triglycerides, phospholipids, and the distribution of cholesterol and triglycerides over the different lipoproteins in serum were determined as described (21). The serum apoAI concentration was determined by ELISA, by using polyclonal goat-anti-mouse apoAI (Harlan Sera-lab, Loughborough, U.K.) as coating antibody and biotinylated goat-anti-mouse apoAI as sandwich antibody. Finally, biotinylated horseradish peroxidase-conjugated streptavidin was applied, and plates were incubated with 3,3′,5,5′-tetramethylbenzidine. Pooled serum of C57BL/6 mice was used as standard. ApoE was measured by using a sandwich ELISA specific for mouse apoE, as described (21).
Hepatic Triglyceride Production and Postheparin Plasma Lipolytic Activity.
Hepatic triglyceride production was determined at 8 weeks posttransplant by i.v. injection of 500 mg/kg of Triton WR1339 (Sigma) after an overnight fast (23). Blood samples were taken at 0, 1, 2, 3, and 4 h after Triton WR1339 injection, and plasma triglyceride levels were measured enzymatically. The hepatic triglyceride production rate was calculated from the slope of the curve. Postheparin plasma for determination of lipolytic activity was obtained after i.v. injection of 1,000 units/kg of heparin (Leo Pharmaceutical Products, Ballerup, Denmark) 20 min before blood collection. Plasma samples were subsequently assayed for lipolytic activity by using a substrate-based assay with a radiolabeled triolein emulsion as described by Zechner (24). The lipolytic activity was calculated from the amount of free fatty acids released per ml/h.
Histological Analysis of the Aortic Root.
To analyze the development of atherosclerosis at the aortic root, transplanted mice were killed at 5 months posttransplant after 12 weeks of feeding the high-cholesterol Western-type diet. The arterial tree was perfusion-fixed (Zinc Formal Fixx, Shandon, U.K.), and atherosclerosis was analyzed as described (21, 25). Mean lesion area (in μm2) was calculated from 10 oil red O/hematoxylin-stained sections, starting at the appearance of the tricuspid valves. Sections were immunolabeled against MOMA-2 (a generous gift of G. Kraal, Vrije Universiteit, Amsterdam; dilution 1:50) or CD68 (a generous gift of S. Gordon, Oxford University, Oxford, U.K.; FA-11 dilution 1:500) for detection of macrophages as well as freshly infiltrated monocytes (26–28). Collagen content of the lesions was visualized with aniline blue by using Masson's Trichrome accustain according to the manufacturer's instructions. The MOMA-2 or CD68 positive lesion area and collagen content of the lesions were subsequently quantified in five consecutive sections by computer-aided morphometric analysis using the Leica image analysis system. Furthermore, a histological classification of atherosclerotic lesions in the aortic root was performed on oil red O/hematoxylin and Masson's Trichrome (Sigma) stained sections according to the recommendations of the American Heart Association (29–31). Ranking was performed blinded on four sections of nine mice from each group by two independent investigators.
Macrophage Quantification in Liver and Spleen.
Paraffin sections (5 μm) of liver and spleen were stained for the F4/80 antigen, which specifically recognizes mature tissue macrophages (28), by subsequent incubation with rat-anti-mouse Cl:A3–1 (Bachem; dilution 1:50) and goat-anti-rat IgG conjugated to alkaline phosphatase (Sigma; dilution 1:200). The F4/80-positive area in liver and spleen was quantified by using the Leica (Deerfield, IL) image analysis system.
Morphological Analysis and Immunophenotypic Characterization of Hematopoietic Cells.
Total cell counts were analyzed from EDTA blood by using an ADVIA 120 hematology analyzer (Bayer Diagnostics, Tarrytown, NY). The microscopic white blood cell and bone marrow differential was obtained from May–Grünwald–Giemsa-stained smears. For further characterization, whole blood or bone marrow samples were preincubated with the antibody 2.4G2 directed against the Fc-binding region of Fc γ-receptor II and III followed by staining with antibodies directed against CD3, CD4, CD8a, CD11c, CD13, CD34, CD41, CD45, CD117, and Ly-6A/E. All antibodies were obtained from PharMingen. Cells were analyzed on a dual laser FACSCalibur (Becton Dickinson) followed by data analysis by using predefined clusters (attractors software). Peritoneal leukocytes were analyzed on May–Grünwald–Giemsa-stained cytospin preparations and by flow cytometry based on four-color analysis of CD13, CD11c, CD45, and CD4.
Statistical Analyses.
Statistical analyses were performed utilizing the unpaired Student's t test (instat, GraphPad Software, San Diego).
Results and Discussion
Generation of LDLr Knockout Mice Deficient in Leukocyte ABCA1.
To assess the biological role of macrophage ABCA1 in lipoprotein metabolism and atherogenesis, we used the technique of bone marrow transplantation to selectively disrupt ABCA1 in hematopoietic cells. Bone marrow from previously generated mice, lacking functional ABCA1 in all tissues (17), was transplanted into LDLr knockout mice, which represent an established model for the development of atherosclerosis (18, 19) (Fig. 1a). Successful reconstitution of recipients with cells of donor origin after bone marrow transplantation was established at 20 weeks posttransplant by PCR-assisted amplification of the wild-type and the ABCA1 null mutant gene, respectively. Genomic DNA isolated from bone marrow of ABCA1+/+ → LDLr−/− mice displayed only the 1.3-kb wild-type-specific band. In contrast, in bone marrow of ABCA1−/− → LDLr−/− chimeras a prominent 1.0-kb band diagnostic of the disrupted ABCA1 gene was visible, whereas the wild-type ABCA1 gene was only faintly detectable (Fig. 1b). Semiquantitative PCR analysis revealed that ≈90% of the bone marrow cells from ABCA1−/− → LDLr−/− chimeras were of donor origin indicating that the bone marrow transfer was nearly complete. Successful bone marrow transfer was also confirmed in a functional assay in 3H-cholesterol-labeled peritoneal macrophages at 20 weeks posttransplant, which showed, as expected, a reduced cholesterol efflux by the macrophages isolated from ABCA1−/− → LDLr−/− chimeras, as compared with ABCA1+/+ → LDLr−/− mice (Fig. 1c).
Figure 1.
Design of bone marrow transplantation experiment and verification of success of bone marrow transplantation. (a) One day before transplantation, mice were exposed to total body irradiation and subsequently engrafted with 0.5 × 107 bone marrow cells from ABCA1-deficient mice or wild-type littermates. During the first 8 weeks, mice were maintained on a chow diet containing 5.7% fat and no cholesterol. At 8 weeks after bone marrow transplantation, the diet was switched to a high-cholesterol Western-type diet (WTD) containing 15% fat and 0.25% cholesterol. Serum lipid levels were carefully monitored throughout the experiment. After 12 weeks feeding WTD, the chimeras were killed for lesion assessment in the aortic root. (b) Verification of successful reconstitution with donor hematopoietic cells by PCR at 20 weeks posttransplant using genomic DNA isolated from bone marrow. Amplification of the wild-type ABCA1 gene resulted in a 1.3-kb PCR band, whereas the ABCA1 null mutant gene yielded a 1.0-kb band. (c) HDL mediated cellular cholesterol efflux using 3H-cholesterol-labeled peritoneal macrophages isolated from mice transplanted with either ABCA1+/+ (○, n = 3) or ABCA1−/− (●, n = 3) bone marrow at 20 weeks posttransplant.
Effect of Disruption of Leukocyte ABCA1 Function on Plasma Lipid Levels.
During the course of the experiment, the effects of the absence of ABCA1 from hematologic cells on serum lipid levels were carefully monitored. In contrast to patients with TD and ABCA1 knockout mice, both displaying only low plasma levels of HDL precursor particles (6–8, 13, 17, 32, 33), no significant effect of leukocyte ABCA1 deficiency on HDL cholesterol levels (Fig. 2a) or on HDL lipid composition (data not shown) was observed. Serum apoAI levels, however, were reduced by 24% (n = 11, P < 0.05; Table 1), whereas apoB100 levels remained unchanged (data not shown). In TD patients and in ABCA1 knockout mice, newly synthesized lipid-poor apoAI cannot acquire phospholipid and cholesterol from tissues because of the absence of ABCA1, which leads to a dramatic reduction of apoAI in plasma (13) caused by hypercatabolism of preβ-HDL precursors (33). Interestingly, the moderate reduction of plasma apoAI levels in ABCA1−/− → LDLr−/− chimeras indicates that the absence of ABCA1 in hematologic cells alone can already affect plasma apoAI catabolism.
Figure 2.
Effect of leukocyte ABCA1 deficiency on the plasma cholesterol and triglycerides distribution. Blood samples were drawn after an overnight fast at 8 weeks posttransplant, while feeding regular chow diet (a and b) and at 16 weeks after bone marrow transplantation after 8 weeks of feeding a high-cholesterol Western-type diet (c and d). Sera from individual mice were loaded onto a Superose 6 column, and fractions were collected. Fractions 3–7 represent VLDL; fractions 8–14, LDL; and fractions 15–19, HDL, respectively. The distribution of cholesterol (a and c) and triglycerides (b and d) over the different lipoproteins in ABCA1+/+ → LDLr−/− (○) and ABCA1+/+ → LDLr−/− (●) chimeras is shown. Values represent the mean of 10–11 mice. SEM are shown only for fractions containing the top of the VLDL, LDL, and HDL peaks.
Table 1.
Effect of leukocyte ABCA1-deficiency on plasma lipids and apolipoprotein concentrations
| Donor bone marrow | Time, weeks | Diet | n | Total cholesterol, mg/dl | HDL cholesterol, mg/dl | Triglycerides, mg/dl | Phospholipids, mg/dl | ApoAI, %‡ | ApoE, mg/dl |
|---|---|---|---|---|---|---|---|---|---|
| ABCA1+/+ | 0 | Chow | 11 | 337 ± 12 | ND | 101 ± 7 | 394 ± 16 | ND | ND |
| 8 | Chow | 11 | 366 ± 22 | 118 ± 6 | 88 ± 6 | 435 ± 19 | 255 ± 11 | 23 ± 2 | |
| 16 | WTD | 11 | 1,465 ± 69 | 269 ± 11 | 173 ± 23 | 815 ± 43 | 158 ± 21 | 110 ± 10 | |
| ABCA1−/− | 0 | Chow | 11 | 325 ± 12 | ND | 74 ± 5 | 388 ± 8 | ND | ND |
| 8 | Chow | 11 | 314 ± 91* | 103 ± 6 | 153 ± 15† | 451 ± 12 | 193 ± 24* | 13 ± 1 | |
| 16 | WTD | 10 | 1,068 ± 63† | 238 ± 14 | 149 ± 15 | 679 ± 34* | 110 ± 20 | 33 ± 2 |
Plasma lipids and apolipoprotein concentrations in LDLr−/− mice transplanted with ABCA1+/+ or ABCA1−/− bone marrow and maintained on a chow diet or a high-cholesterol Western-type diet (WTD) for 8 weeks. Data represent means ± SEM. Statistically significant difference of
P < 0.05 and
P < 0.001 compared to ABCA1+/+ → LDLr−/− mice.
The concentration apoAI is expressed as percentage of the apoAI level found in pooled serum of C57bl/6 mice. ND, not determined.
Total serum cholesterol levels on a chow diet were moderately decreased by 14% (n = 11, P < 0.05) in ABCA1−/− → LDLr−/− chimeras, as compared with controls, whereas triglyceride levels were 74% (n = 11, P < 0.001) increased (Table 1). As indicated in Fig. 2 a and b, changes in these lipid levels are caused by a slight decrease in cholesterol and an increase in triglycerides in VLDL and LDL. Moreover, by analysis of the percentile lipid composition of isolated VLDL and LDL fractions from pooled sera, it was established that the decrease in serum cholesterol and the increase in triglycerides were largely accounted for by changes in VLDL and LDL lipid composition. Both VLDL and LDL were enriched in triglycerides at the expense of cholesteryl esters. Triglycerides in VLDL were increased in the ABCA1−/− → LDLr−/− chimeras as compared with the controls from 30 to 58%, whereas cholesteryl esters were lowered from 50 to 23%. For LDL the triglyceride content was increased in ABCA1−/− → LDLr−/− chimeras (18 versus 10% in controls), whereas cholesteryl esters were lowered (52 versus 60% in controls). Mild hypertriglyceridemia because of enrichment of LDL with triglycerides has also been reported for patients with TD (34–36). However, data available on the postheparin lipolytic activity in these patients are inconclusive (36). In our studies, no effect of leukocyte ABCA1-deficiency on postheparin lipolytic activity was observed (14.2 ± 1.24 μmol of free fatty acids (FFA) per ml/h for ABCA1−/− → LDLr−/− mice, n = 6 and 14.8 ± 1.42 μmol of FFA per ml/h for controls, n = 6) indicating that the increased triglyceride contents of VLDL and LDL are not caused by impaired lipolysis. Lipolysis of circulating lipoproteins can also be inhibited by high levels of apoE because of inhibition of LPL by displacement or masking of its activator apoCII (37, 38). Interestingly, analysis of serum apoE levels by ELISA showed a reduction in serum apoE levels from 23 ± 2 mg/dl (n = 11) in ABCA1+/+ → LDLr−/− mice to 13 ± 1 mg/dl (n = 11; P < 0.0001) in the ABCA1−/− → LDLr−/− chimeras, indicating that leukocyte ABCA1 has a significant effect on apoE metabolism. We then tested whether leukocyte ABCA1 deficiency affects triglyceride synthesis by in vivo inactivation of lipolysis by using Triton WR1339. The triglyceride production rates did not differ significantly between the two chimera groups [5,045 ± 478 μg/ml/h for mice transplanted with ABCA1−/− bone marrow (n = 3) and 4,821 ± 283 μg/ml/h for ABCA1+/+ → LDLr−/− mice (n = 3)]. Neither lipolysis nor the VLDL triglyceride production by the liver could explain these effects. It is therefore possible that the observed moderate combined hypertriglyceridemia and hypocholesterolemia in ABCA1−/− → LDLr−/− chimeras are a direct consequence of compromised lipid transport in hematologic cells lacking ABCA1.
On challenging the mice with a high-cholesterol Western-type diet containing 0.25% cholesterol and 15% fat, serum cholesterol levels increased from 314 ± 91 mg/dl to 1,068 ± 63 mg/dl (3.4-fold, n = 10) in ABCA1−/− → LDLr−/− chimeras and from 366 ± 22 mg/dl to 1,465 ± 69 (4-fold, n = 11) mg/dl in ABCA1+/+ → LDLr−/− mice (Table 1). Under these conditions, phospholipids were slightly reduced (17%, P < 0.05) in mice transplanted with ABCA1−/− bone marrow as compared with controls, whereas triglycerides and apoAI did not differ significantly. Furthermore, ABCA1−/− → LDLr−/− chimeras displayed a less pronounced increase in apoB100 in response to the high-cholesterol Western-type diet relative to ABCA1+/+ → LDLr−/− mice (data not shown). Fractionation of serum lipoproteins after 8 weeks feeding the Western-type diet indicated that the increase in cholesterol levels in both groups of mice was mainly caused by an increase in cholesterol associated with VLDL and LDL (Fig. 2c). The ABCA1−/− → LDLr−/− chimeras, however, showed an attenuated response to the diet as compared with control ABCA1+/+ → LDLr−/− mice in the form of lower VLDL and LDL cholesterol levels (Table 1, Fig. 2c). No significant difference in VLDL and LDL triglycerides was observed (Fig. 2d). Lipid composition analysis of isolated VLDL and LDL fractions indicated that VLDL and LDL are enriched in cholesteryl esters (≈60% of the total lipid content), whereas triglycerides are largely reduced in both groups of mice as compared with chow diet. Nevertheless, VLDL and LDL from ABCA1−/− → LDLr−/− mice still contain slightly more triglycerides (8.7 and 1.7%, respectively) as compared with ABCA1+/+ → LDLr−/− mice (6.3 and 0.8%, respectively). The minor effect of leukocyte ABCA1 deficiency on the lipid composition of VLDL and LDL indicates that the differences in cholesterol levels between the two groups under these dietary conditions are mainly caused by reduced numbers of circulating VLDL and LDL particles and only to a lesser degree by changes in particle composition. This notion is supported by our observation that ABCA1−/− → LDLr−/− chimeras display a less pronounced increase in apoB100 levels (a direct measure for the number of circulating VLDL and LDL particles (39) in response to the high-cholesterol Western-type diet relative to ABCA1+/+ → LDLr−/− mice (data not shown). Feeding the high-cholesterol diet increased serum apoE levels to 110 ± 10 mg/dl (n = 11) in ABCA1+/+ → LDLr−/− mice and to 33 ± 2 mg/dl (n = 10; P < 0.0001) in ABCA1−/− → LDLr−/− chimeras. Recently, we have shown that macrophage apoE deficiency does not affect apoE concentration in the circulation, indicating that apoE production by macrophages does not significantly influence serum apoE levels (40). Thus, the effects of leukocyte ABCA1 deficiency on serum apoE levels are probably caused not by direct effects on macrophage apoE synthesis but rather by indirect effects on hepatic apoE production or apoE catabolism in the periphery.
Disruption of Leukocyte ABCA1 Function Induces Increased Susceptibility to Atherosclerosis.
The phenotype of familial HDL-deficiency syndromes such as TD is characterized by excessive accumulation of cholesteryl esters in macrophages (12, 13). Premature development of atherosclerosis, however, occurs in only half of the individuals with TD (41). One subgroup of individuals develops premature atherosclerosis, whereas another presents predominantly splenomegaly (42), indicating differences in macrophage targeting to tissues. In patients with systemic ABCA1-deficiency cholesterol efflux from macrophages is affected not only by the absence of functional ABCA1 from the macrophage itself but also by the inability to convert preβ-HDL to mature HDL, which leads to failure of critical steps in the process of reverse cholesterol transport.
We assessed whether and to what degree the selective disruption of ABCA1 in circulating cells, in particular macrophages, affects lesion formation in the arterial wall by using our murine chimera model—i.e., under conditions of nearly normal apoAI and HDL plasma levels. Lesion development was analyzed in the aortic root of ABCA1−/− → LDLr−/− and ABCA1+/+ → LDLr−/− mice, respectively, 20 weeks after transplantation—i.e., after 12 weeks of feeding a high-cholesterol Western-type diet (see Fig. 1). As shown in Fig. 3 a and b, absence of ABCA1 from leukocytes leads to a 60% (P < 0.0001) increase in the mean atherosclerotic lesion area from 104 ± 10 × 103 μm2 (n = 11) in ABCA1+/+ → LDLr−/− mice to 164± × 103 μm2 (n = 10) in ABCA1−/− → LDLr−/− chimeras. It is of particular interest that this increase in lesion size is observed despite the 27% reduction in non-HDL cholesterol levels (Fig. 2c). Quantitative morphological analysis of the atherosclerotic lesions demonstrated that the total area of lesional macrophages was significantly reduced in ABCA1−/− → LDLr−/− mice compared with ABCA1+/+→ LDLr−/− mice (31.9 ± 2.9%, n = 10 versus 51.3 ± 4.5%, n = 11, respectively; P < 0.005; Fig. 3 c and d, Table 2). Strikingly, as determined by Masson's Trichrome staining, collagen deposition was 6-fold increased in lesions of ABCA1−/− → LDLr−/− chimeras (Table 2). Classification of lesions in the aortic root indicated that progression of the disease was more advanced in ABCA1−/− → LDLr−/− chimeras as compared with ABCA1+/+ → LDLr−/− mice. In control transplanted mice, 13% of the lesions were classified as type II fatty streaks, 38% as type III intermediate lesions, 26% as type IV atheroma lesions, and 21% as type V advanced fibroatheroma lesions with calcification, cholesterol clefts, and/or necrosis. In ABCA1−/− → LDLr−/− chimeras, however, only 2% of the lesions were type II fatty streaks, 24% type III intermediate lesions, 42% type IV atheroma lesions, and 32% type V advanced fibroatheroma lesions. The decreased macrophage content and increased collagen deposition in atherosclerotic lesions in the absence of leukocyte ABCA1 are consistent with the presence of more advanced lesions, which characteristically display a higher degree of fibroatheromatous tissue components (43). Because macrophages are by far the predominant leukocyte cell type within atherosclerotic lesions (43) and lymphocytes, which occur in lesions only in limited numbers, express only minute amounts of ABCA1 mRNA, if any (unpublished observation), our results strongly suggest that the observed increased susceptibility to atherosclerosis is a direct consequence of the absence of ABCA1 from lesional macrophages. Recently, Joyce et al. reported that overexpression of human ABCA1 in both liver and macrophages protects against the development of diet-induced atherosclerosis in C57bl/6 mice (44). However, drawing conclusions on direct effects of ABCA1 overexpression on atherosclerotic lesion development was not possible, as the reduction in atherosclerosis coincided with a marked reduction in the serum concentration of the highly atherogenic lipoprotein VLDL and an increase in HDL cholesterol. In contrast, using our bone marrow transplantation model, we now provide direct evidence that locally in the arterial wall macrophage ABCA1 plays a pivotal role in the prevention of atherosclerotic lesion development.
Figure 3.
Leukocyte ABCA1 controls susceptibility to atherosclerosis. Formation of atherosclerotic lesions was determined at 20 weeks posttransplant at the aortic root of ABCA1+/+ → LDLr−/− and ABCA1−/− → LDLr−/− chimeras that were fed a high-cholesterol Western-type diet for 12 weeks. (a and b) The mean lesion area was calculated from oil red O-stained cross sections of the aortic root at the level of the tricuspid valves. Values represent the mean of 10–11 mice. Original magnification, ×50. (c and d) Immunohistochemical quantification of lesion macrophages by using the MOMA-2 antibody that identifies macrophages as well as freshly infiltrated monocytes. The macrophage lesion area is indicated as % of the total lesion area. Original magnification, ×200. Statistically significant difference of ***, P < 0.0001, and **, P < 0.005 compared with ABCA1+/+ → LDLr−/− mice.
Table 2.
Characteristics of atherosclerotic lesions in LDL receptor knockout mice reconstituted with ABCA1+/+ or ABCA1−/− bone marrow
| ABCA1+/+ → LDLr−/− | ABCA1−/− → LDLr−/− | P value | |
|---|---|---|---|
| Total lesion area, 104⋅μm2 | 10.4 ± 0.1 | 16.4 ± 0.1 | <0.0001 |
| Lesion macrophage area, 104⋅μm2 | 5.6 ± 0.1 | 5.1 ± 0.1 | 0.0020 |
| MOMA-2 positive area, % of lesion area | 51.3 ± 4.5 | 31.9 ± 2.9 | 0.4560 |
| CD68-positive area, % of lesion area | 54.0 ± 6.9 | 28.1 ± 1.8 | 0.4600 |
| Macrophage number per 104 lesion area | 23.2 ± 3.5 | 9.3 ± 1.1 | 0.0020 |
| Lesion collagen area, 104⋅μm2 | 1.1 ± 0.3 | 6.6 ± 0.5 | <0.0001 |
Data represent means ± SEM. P value < 0.05 indicates statistically significant difference compared to ABCA1+/+ → LDLr−/− mice.
Enhanced Recruitment of Leukocytes into the Circulation and Peripheral Tissues.
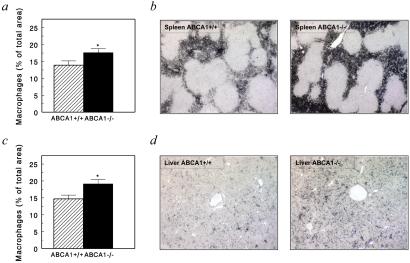
Recent studies in patients with TD have suggested a dual function for macrophage ABCA1 in both lipid metabolism and inflammation (42). To assess potential morphological changes associated with leukocyte ABCA1 deficiency outside the vasculature, a complete necropsy of the transplanted mice was performed. No significant effects of leukocyte ABCA1 deficiency on body weight were observed at the time of death. Immunohistological quantification of macrophages in the spleens of the transplanted mice revealed that the area of macrophages was 21% increased (P < 0.05) from 13.9 ± 1.3% (n = 11) in spleens of ABCA1+/+ → LDLr−/− mice to 17.6 ± 1.2% (n = 10) for spleens of ABCA1−/− → LDLr−/− chimeras (Fig. 4). Macroscopically, in 3 of 10 ABCA1−/− → LDLr−/− mice, splenomegaly with focal expansion of the white pulp was observed, whereas the spleens of all control mice appeared normal. In the liver, the area of Kupffer cells, which constitute the largest population of tissue macrophages in the body (45), was increased by 30% (n = 10, P < 0.05) in ABCA1−/− → LDLr−/− chimeras. Furthermore, the absolute amount of resident leukocytes in the peritoneal cavity was increased 3.4-fold (P < 0.01) from 1.77 ± 0.4 × 106 cells (n = 8) to 6.04 ± 1.27 × 106 cells (n = 7) (Fig. 5a). Although lymphocytes form the largest resident cell population of the peritoneal cavity under these conditions (ABCA1−/− → LDLr−/− mice: 61.7 ± 7.9%, n = 4; ABCA1+/+ → LDLr−/− mice: 54.8 ± 1.9%, n = 6), we also found a 2.5-fold increase in macrophage numbers in this compartment. Total peripheral blood leukocyte counts were increased in ABCA1−/− → LDLr−/− mice (5.6 ± 0.6 × 109 cells/liter, n = 14; P < 0.001), compared with ABCA1+/+ → LDLr−/− chimeras (3.5 ± 0.4 × 109 cells/liter, n = 16). As indicated in Fig. 5b, this was mainly caused by an increase in granulocyte (0.9 ± 0.2 × 109 cells/liter versus 0.6 ± 0.08 × 109 cells/liter, P < 0.01) and lymphocyte counts (4.2 ± 0.4 × 109 cells/liter versus 2.7 ± 0.3 × 109 cells/liter, P < 0.01). Analysis of the lymphocyte subsets indicated that the increase in lymphocyte counts was caused by an expansion of non-T helper (CD4 − cells (32.9 ± 2.2% of total leukocytes in ABCA1−/− → LDLr−/− chimeras versus 19.1 ± 2.4% in ABCA1+/+ → LDLr−/− mice, P < 0.001) (Fig. 5b). Importantly, monocyte (Fig. 5b), as well as platelet and red blood cell counts (data not shown), did not differ between ABCA1−/− → LDLr−/− mice and controls. No significant effect of leukocyte ABCA1 deficiency on the relative number of progenitors was found in the bone marrow (Fig. 5c), indicating that the observed increased peripheral granulocyte and lymphocyte counts in ABCA1−/− → LDLr−/− mice are thus rather the consequence of sustained leukocyte recruitment from the bone marrow into the circulation than of enhanced hematopoiesis. Taken together, the elevated leukocyte counts in the circulation and the increased presence of macrophages in tissues such as liver and spleen demonstrate that leukocyte ABCA1, in addition to its role in cholesterol efflux, exerts a regulatory function in the recruitment of inflammatory cells to the periphery.
Figure 4.
Increased macrophage content in spleen and liver of mice lacking functional leukocyte ABCA1. At 20 weeks after bone marrow transplantation, the macrophage contents of spleen and liver were determined by immunohistochemistry by using F4/80, a specific marker for mature tissue macrophages. Values represent the mean of 10–11 mice. Original magnification, ×50. Statistically significant difference of *, P < 0.05, compared with ABCA1+/+ → LDLr−/− mice.
Figure 5.
Effect of leukocyte ABCA1 deficiency on peripheral leukocyte and bone marrow progenitor counts. (a) Peritoneal leukocyte numbers of ABCA1+/+ → LDLr−/− mice (hatched bars, n = 8) and ABCA1−/− → LDLr−/− mice (closed bars, n = 7) bone marrow were determined by flushing the peritoneal cavity with sterilized PBS and subsequent cell counting. (b and c) Counts of peripheral leukocytes and bone marrow progenitors were assessed on May–Grünwald–Giemsa-stained smears and by flow cytometry of blood and bone marrow from ABCA1+/+ → LDLr−/− (hatched bars, n = 16) and ABCA1−/− → LDLr−/− (closed bars, n = 14) mice. Statistically significant difference of **, P < 0.01, ***, P < 0.001 compared with ABCA1+/+ → LDLr−/− mice.
An important mediator in the growth and survival of mononuclear phagocytes is macrophage colony-stimulating factor (M-CSF) (46). Interestingly, M-CSF deficiency increases hypercholesterolemia in several murine models of atherosclerosis (47, 48), whereas injection of M-CSF reduces plasma cholesterol levels by enhancing the clearance of non-HDL lipoproteins through both LDL receptor-dependent and independent pathways (49). Thus, M-CSF might provide an important link between the observed effects of leukocyte ABCA1 on both non-HDL lipoproteins and Kupffer cells and other tissue macrophages.
In summary, our results demonstrate that more advanced and larger lesions of atherosclerosis develop when leukocyte ABCA1 is absent, providing direct evidence that leukocyte ABCA1 exerts a pronounced antiatherosclerotic activity. Several factors may account for this effect, which operates independently of plasma HDL. First, because ABCA1 facilitates cellular cholesterol and phospholipid export (4, 50), it is likely that excessive cholesterol uptake by macrophages in the vessel wall is compensated to a significant degree by ABCA1-mediated cholesterol efflux. Second, ABCA1 has been implicated in the engulfment of apoptotic cells by macrophages (51, 52). It is thus conceivable that a compromised phagocytic activity of lesion macrophages because of defective ABCA1 may lead to the enhanced accumulation of apoptotic material that in return may stimulate the inflammatory response within the vascular wall. Finally, it is also possible that, on the basis of as-yet-unknown molecular mechanisms, the recruitment of ABCA1-deficient monocytes to specific target sites such as the arterial wall is enhanced, as evidenced for the liver and the spleen. In such a scenario, it can be expected that facilitated influx of monocytes into the vascular subendothelium would also significantly contribute to lesion progression. Whatever its molecular mechanism, the clear-cut antiatherosclerotic activity of ABCA1 reported in this study renders this ABC transporter an attractive target for the development of antiatherosclerotic drugs.
Acknowledgments
This work was supported by The Netherlands Heart Foundation (Grant 98.192), the Deutsche Forschungsgemeinschaft (Grants AN 111/6-1 and KA 1078/2-1), and the Alexander von Humboldt Foundation.
Abbreviations
- ABC
ATP-binding cassette
- ABCA1
ABC transporter 1
- LDL
low-density lipoprotein
- LDLr
LDL receptor
- VLDL
very LDL
- HDL
high-density lipoprotein
- TD
Tangier disease
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Higgins C F. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 2.Broccardo C, Luciani M-F, Chimini G. Biochim Biophys Acta. 1999;1461:395–404. doi: 10.1016/s0005-2736(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 3.Borst P, Zelcer N, Van Helvoort A. Biochim Biophys Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz G, Kaminski W E, Orsó E. Curr Opin Lipidol. 2000;11:493–501. doi: 10.1097/00041433-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Oram J F, Vaughan A M. Curr Opin Lipidol. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, et al. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 7.Brooks-Wilson A, Marcil M, Clee S M, Zhang L H, Roomp K, van Dam M, Yu L, Brewer C, Collins J A, Molhuizen H O, et al. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 8.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette J C, Deleuze J F, Brewer H B, Duverger N, Denefle P, Assmann G. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 9.Glomset J A. Adv Intern Med. 1980;25:91–116. [PubMed] [Google Scholar]
- 10.Frederickson D S. J Clin Invest. 1964;43:228–236. doi: 10.1172/JCI104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assman G, Schmitz G, Brewer H B., Jr . In: The Metabolic Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1989. pp. 1267–1282. [Google Scholar]
- 12.Langmann T, Klucken J, Reil M, Liebisch G, Luciani M F, Chimini G, Kaminski W E, Schmitz G. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 13.Orsó E, Broccardo C, Kaminski W E, Bottcher A, Liebisch G, Drobnik W, Gotz A, Chambenoit O, Diederich W, Langmann T, et al. Nat Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 14.Von Eckardstein A, Nofer J R, Assmann G. Arterioscler Thromb Vasc Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 15.Oram J F, Lawn R M, Garvin M R, Wade D P. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 16.Chinetti G, Lestavel S, Bocher V, Remaley A T, Neve B, Torra I P, Teissier E, Minnich A, Jaye M, Duverger N, et al. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen-Weber T A, Voland J R, Wu Y, Ngo K, Roland B L, Nguyen S, Peterson P A, Fung-Leung W P. Am J Pathol. 2000;157:1017–1029. doi: 10.1016/S0002-9440(10)64614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishibashi S, Brown M S, Goldstein J L, Gerard R D, Hammer R E, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishibashi S, Goldstein J L, Brown M S, Herz J, Burns D K. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishina P M, Verstuyft J, Paigen B. J Lipid Res. 1990;31:859–869. [PubMed] [Google Scholar]
- 21.Van Eck M, Herijgers N, Yates J, Pearce N J, Hoogerbrugge P M, Groot P H E, Van Berkel Th J C. Arterioscler Thromb Vasc Biol. 1997;17:3117–3126. doi: 10.1161/01.atv.17.11.3117. [DOI] [PubMed] [Google Scholar]
- 22.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Borensztajn J, Rone M S, Kotlar T J. Biochem J. 1976;156:539–543. doi: 10.1042/bj1560539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zechner R. Biochim Biophys Acta. 1990;1044:20–25. doi: 10.1016/0005-2760(90)90213-h. [DOI] [PubMed] [Google Scholar]
- 25.Groot P H E, van Vlijmen B J M, Benson G M, Hofker M H, Schiffelers R, Vidgeon-Hart M, Havekes L M. Arterioscler Thromb Vasc Biol. 1996;16:926–933. doi: 10.1161/01.atv.16.8.926. [DOI] [PubMed] [Google Scholar]
- 26.Van Eck M, Zimmermann R, Groot P H E, Zechner R, Van Berkel Th J C. Arterioscler Thromb Vasc Biol. 2000;20:E53–E62. doi: 10.1161/01.atv.20.9.e53. [DOI] [PubMed] [Google Scholar]
- 27.Kraal G, Rep M, Janse M. Scand J Immunol. 1987;26:653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 28.Leenen P J, de Bruijn M F, Voerman J S, Campbell P A, van Ewijk W. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 29.Stary H C, Chandler A B, Glagov S, Guyton J R, Insull W, Jr, Rosenfeld M E, Schaffer S A, Schwartz C J, Wagner W D, Wissler R W. Arterioscler Thromb. 1994;14:840–856. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- 30.Stary H C, Chandler A B, Dinsmore R E, Fuster V, Glagov S, Insull W, Jr, Rosenfeld M E, Schwartz C J, Wagner W D, Wissler R W. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 31.Stary H C. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 32.McNeish J, Aiello R J, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe K L, Roach M L, Royer L J, de Wet J, et al. Proc Natl Acad Sci USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kay L L, Ronan R, Schaefer E J, Brewer H B. Proc Natl Acad Sci USA. 1982;79:2485–2489. doi: 10.1073/pnas.79.8.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C-S, Alaupovic P, Gregg R E, Brewer H B., Jr Biochim Biophys Acta. 1987;920:9–19. doi: 10.1016/0005-2760(87)90305-5. [DOI] [PubMed] [Google Scholar]
- 35.Kunitake S T, Young S G, Chen G C, Pullinger C R, Zhu S, Pease R J, Scott J, Hass P, Schilling J, Kane J P. J Biol Chem. 1990;265:20739–20746. [PubMed] [Google Scholar]
- 36.Takizawa A, Komoda T, Hokari S, Sakagishi Y, Tanaka A. Clin Chim Acta. 1991;203:97–100. doi: 10.1016/0009-8981(91)90161-5. [DOI] [PubMed] [Google Scholar]
- 37.Rensen P C N, Van Berkel Th J C. J Biol Chem. 1996;271:14791–14799. doi: 10.1074/jbc.271.25.14791. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y. J Biol Chem. 1998;273:26388–26393. doi: 10.1074/jbc.273.41.26388. [DOI] [PubMed] [Google Scholar]
- 39.Schumaker V N, Phillips M L, Chatterton J E. Adv Protein Chem. 1994;45:205–248. doi: 10.1016/s0065-3233(08)60641-5. [DOI] [PubMed] [Google Scholar]
- 40.Van Eck M, Herijgers N, Vidgeon-Hart M, Pearce N J, Hoogerbrugge P M, Groot P H E, Van Berkel Th J C. Atherosclerosis. 2000;150:71–80. doi: 10.1016/s0021-9150(99)00372-x. [DOI] [PubMed] [Google Scholar]
- 41.Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus E D, Smith M P, Jr, Pritchard P H, Frohlich J, Lees R S, et al. Atherosclerosis. 1994;107:85–98. doi: 10.1016/0021-9150(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz G, Kaminski W E, Porsch-Ozcurumez M, Klucken J, Orsó E, Bodzioch M, Buchler C, Drobnik W. Pathobiology. 1999;67:236–240. doi: 10.1159/000028100. [DOI] [PubMed] [Google Scholar]
- 43.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 44.Joyce C W, Amar M J A, Lamber G, Vaisman B L, Paigen B, Najib-Fruchart J, Hoyt R F, Neufeld E D, Remaley A T, et al. Proc Natl Acad Sci USA. 2002;99:407–412. doi: 10.1073/pnas.012587699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S-H, Starkey P M, Gordon S. J Exp Med. 1995;161:475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tushinski R J, Olive I T, Guilbert L J, Tynan P W, Warner J L, Stanley E R. Cell. 1982;28:71–77. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- 47.De Villiers W J S, Smith J D, Miyata M, Dansky H M, Darley E, Gordon S. Arterioscler Thromb Vasc Biol. 1998;18:631–640. doi: 10.1161/01.atv.18.4.631. [DOI] [PubMed] [Google Scholar]
- 48.Rajavashisth T, Qiao J-H, Tripathi S, Tripathi J, Mishra N, Hua M, Wang X-P, Loussararian A, Clinton S, Libby P, Lusis A. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoudemire J B, Garnick M B. Blood. 1989;77:750–755. [PubMed] [Google Scholar]
- 50.Lawn R M, Wade D P, Garvin M R, Wang X, Schwartz K, Porter J G, Seilhamer J J, Vaughan A M, Oram J F. J Clin Invest. 1999;104:R25–R31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luciani M-F, Chimini G. EMBO J. 1996;15:296–235. [PMC free article] [PubMed] [Google Scholar]
- 52.Hamon Y, Broccardo C, Chambenoit O, Luciani M F, Toti F, Chaslin S, Freyssinet J M, Devaux P F, McNeish J, Marguet D, et al. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]