Abstract
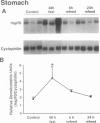
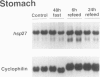
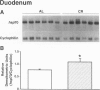
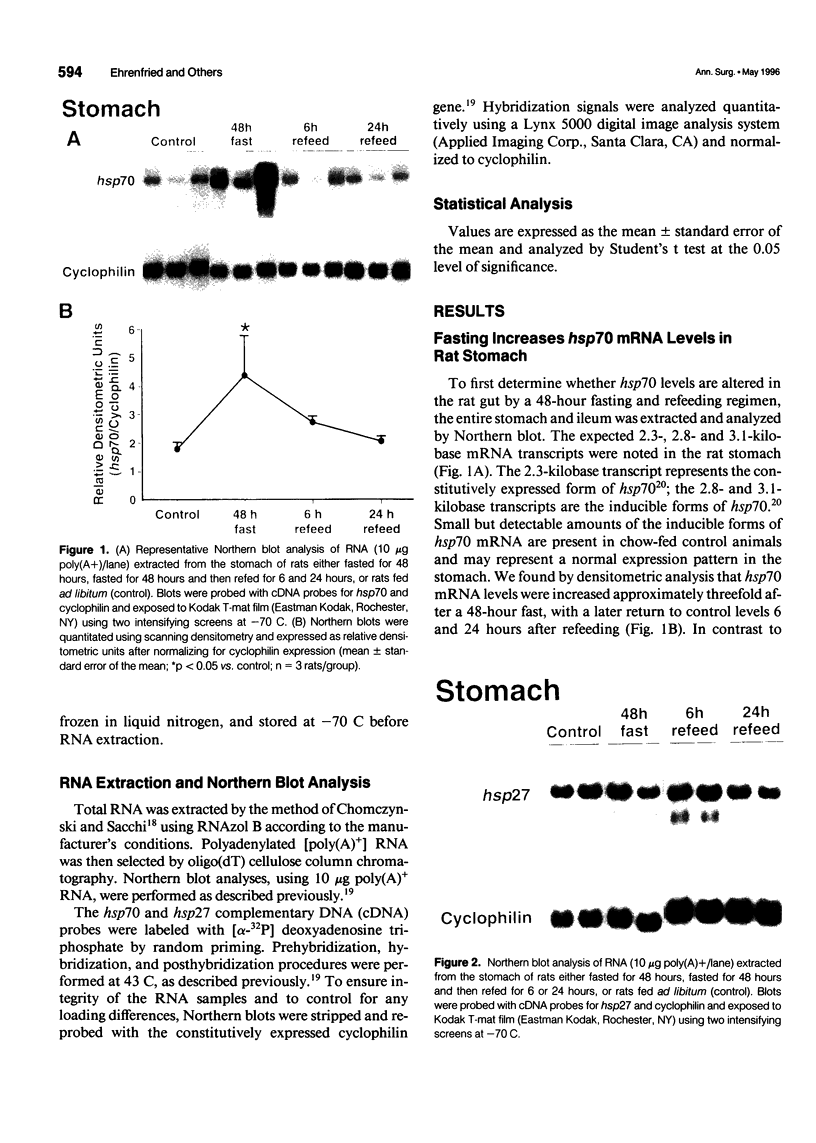
OBJECTIVE: The authors determined whether caloric restriction (CR) either acutely or chronically, alters heat shock protein 70 (hsp70) gene expression in the gut. SUMMARY BACKGROUND DATA: Caloric restriction prolongs the life span and delays age-related disease (e.g., cancer) in mammals; the mechanisms responsible for these effects are not known. Heat shock proteins are a group of stress-responsive genes of which the most prominent member is hsp70. METHODS: In the first experiment, adult (4-month-old) rats (n = 3/group) were killed after a 48-hour fast or 6 and 24 hours after refeeding. In addition, three rats (controls) were killed without fasting or refeeding. The stomach was removed and RNA was extracted for hsp70 gene expression. In the second experiment, aged (22- to 26-month-old) rats were fed ad libitum (AL) or a CR diet (60% caloric intake of AL diet). Rats were killed, the stomach and duodenum were removed, and RNA was extracted for determination of hsp70 gene expression. RESULTS: In the first experiment, hsp70 mRNA levels were increased approximately threefold in the stomach of rats fasted for 48 hours; levels decreased to control values by 6 and 24 hours after refeeding. In the second experiment, hsp70 mRNA levels were increased significantly in both the stomach and duodenum of aged CR rats compared with AL controls. CONCLUSIONS: The authors have demonstrated that hsp70 mRNA levels are increased in the proximal gut of young and old rats, either acutely (with fasting) or with CR. Increased expression of the cytoprotective hsp70 gene in the gut may provide a possible cellular mechanism for the beneficial effects noted with CR.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G. Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat. 1972 Apr;133(4):391–400. doi: 10.1002/aja.1001330403. [DOI] [PubMed] [Google Scholar]
- Aly K. B., Pipkin J. L., Hinson W. G., Feuers R. J., Duffy P. H., Hart R. W. Temporal and substrate-dependent patterns of stress protein expression in the hypothalamus of caloric restricted rats. Mech Ageing Dev. 1994 Oct 1;76(1):1–10. doi: 10.1016/0047-6374(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Aly K. B., Pipkin J. L., Hinson W. G., Feuers R. J., Duffy P. H., Lyn-Cook L., Hart R. W. Chronic caloric restriction induces stress proteins in the hypothalamus of rats. Mech Ageing Dev. 1994 Oct 1;76(1):11–23. doi: 10.1016/0047-6374(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Blake M. J., Udelsman R., Feulner G. J., Norton D. D., Holbrook N. J. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9873–9877. doi: 10.1073/pnas.88.21.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin D. E., Buchman T. G. Molecular biology of circulatory shock. Part III. Human hepatoblastoma (HepG2) cells demonstrate two patterns of shock-induced gene expression that are independent, exclusive, and prioritized. Surgery. 1990 Nov;108(5):902–912. [PubMed] [Google Scholar]
- Cai J. W., Hughes C. S., Shen J. W., Subjeck J. R. Induction of heat-shock proteins by glutamine. The 'feeding effect'. FEBS Lett. 1991 Aug 19;288(1-2):229–232. doi: 10.1016/0014-5793(91)81041-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Weissman J. S., Horwich A. L. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994 Aug 12;78(3):365–372. doi: 10.1016/0092-8674(94)90416-2. [DOI] [PubMed] [Google Scholar]
- Duffy P. H., Feuers R. J., Leakey J. A., Nakamura K., Turturro A., Hart R. W. Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev. 1989 May;48(2):117–133. doi: 10.1016/0047-6374(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Duffy P. H., Feuers R., Nakamura K. D., Leakey J., Hart R. W. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int. 1990;7(2):113–124. doi: 10.3109/07420529009056963. [DOI] [PubMed] [Google Scholar]
- Ehrenfried J. A., Chen J., Li J., Evers B. M. Glutamine-mediated regulation of heat shock protein expression in intestinal cells. Surgery. 1995 Aug;118(2):352–357. doi: 10.1016/s0039-6060(05)80344-7. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Ishizuka J., Chung D. H., Townsend C. M., Jr, Thompson J. C. Neurotensin expression and release in human colon cancers. Ann Surg. 1992 Oct;216(4):423–431. doi: 10.1097/00000658-199210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Harman D. Free-radical theory of aging. Increasing the functional life span. Ann N Y Acad Sci. 1994 Jun 30;717:1–15. doi: 10.1111/j.1749-6632.1994.tb12069.x. [DOI] [PubMed] [Google Scholar]
- Heydari A. R., Wu B., Takahashi R., Strong R., Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993 May;13(5):2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Dowling R. H. Speed of onset of adaptive mucosal hypoplasia and hypofunction in the intestine of parenterally fed rats. Clin Sci (Lond) 1980 Nov;59(5):317–327. doi: 10.1042/cs0590317. [DOI] [PubMed] [Google Scholar]
- Johnson L. R., Lichtenberger L. M., Copeland E. M., Dudrick S. J., Castro G. A. Action of gastrin on gastrointestinal structure and function. Gastroenterology. 1975 May;68(5 Pt 1):1184–1192. [PubMed] [Google Scholar]
- Johnston R. N., Kucey B. L. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science. 1988 Dec 16;242(4885):1551–1554. doi: 10.1126/science.3201244. [DOI] [PubMed] [Google Scholar]
- Kochevar D. T., Aucoin M. M., Cooper J. Mammalian heat shock proteins: an overview with a systems perspective. Toxicol Lett. 1991 May;56(3):243–242. doi: 10.1016/0378-4274(91)90154-x. [DOI] [PubMed] [Google Scholar]
- MCCAY C. M., POPE F., LUNSFORD W. Experimental prolongation of the life span. Bull N Y Acad Med. 1956 Feb;32(2):91–101. [PMC free article] [PubMed] [Google Scholar]
- Masoro E. J. Food restriction in rodents: an evaluation of its role in the study of aging. J Gerontol. 1988 May;43(3):B59–B64. doi: 10.1093/geronj/43.3.b59. [DOI] [PubMed] [Google Scholar]
- Morin C. L., Ling V., Bourassa D. Small intestinal and colonic changes induced by a chemically defined diet. Dig Dis Sci. 1980 Feb;25(2):123–128. doi: 10.1007/BF01308310. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Mizzen L. A., Welch W. J. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988 Oct 21;242(4877):433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Udelsman R., Blake M. J., Holbrook N. J. Molecular response to surgical stress: specific and simultaneous heat shock protein induction in the adrenal cortex, aorta, and vena cava. Surgery. 1991 Dec;110(6):1125–1131. [PubMed] [Google Scholar]
- Udelsman R., Blake M. J., Stagg C. A., Holbrook N. J. Endocrine control of stress-induced heat shock protein 70 expression in vivo. Surgery. 1994 May;115(5):611–616. [PubMed] [Google Scholar]