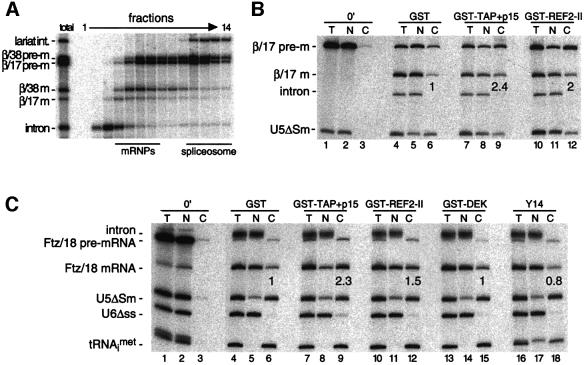
Fig. 3. (A) Glycerol gradient fractionation of splicing substrates and products present in Xenopus oocyte nuclei 30 min after injection of β/38 and β/17 pre-mRNAs shows that both spliced mRNAs are released from spliceosomes in vivo. RNAs were extracted and analyzed as in Figure 1B. Bars indicate positions of mRNPs and spliceosomes. (B and C) Stimulation of spliced β/17 and Ftz/18 mRNA export by co-injection of recombinant proteins. Xenopus oocyte nuclei were injected with indicated control RNAs (at left) and either β/17 pre-mRNA (B) or Ftz/18 pre-mRNA (C). Where indicated, purified recombinant proteins (25 nl of 1 mg/ml stocks) were co-injected with RNAs (lanes ≥7) and co-injection of purified GST served as a control (lanes 4–6). RNA samples from total oocytes (T), nuclear (N) and cytoplasmic (C) fractions were collected immediately after injection (0 min; lanes 1–3) or 3 h after injection (lanes 4–18) and analyzed as in Figure 2. Export efficiencies of β/17 and Ftz/18 mRNAs in the presence of test proteins are indicated relative to their export efficiencies with GST alone.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.