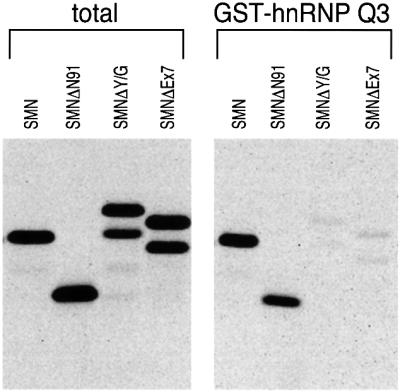
Fig. 5. The C-terminal domain of SMN is required for binding to hnRNP Q1, and SMNΔEx7, the most prevalent mutant found in SMA patients, shows defective interaction with hnRNP Q1. In vitro translated and [35S]methionine-labeled wild-type SMN and myc-tagged, N-terminal (SMNΔN91) or C-terminal (SMNΔY/G and SMNΔEx7) SMN deletions were incubated with recombinant GST–hnRNP Q1. Bound proteins were resolved by SDS–PAGE and detected by fluorography. The slower migration of SMNΔY/G is due to its anomalous electrophoretic behavior on SDS–PAGE gels. The doublets seen in the C-terminal myc-tagged SMN deletion proteins are due to the utilization of two translation initiation sites, one contributed from the myc epitope and the other from the first codon of SMN.
