Abstract
AP-1 family transcription factors have been implicated in the control of proliferation, apoptosis and malignant transformation. However, their role in oncogenesis is unclear and no recurrent alterations of AP-1 activities have been described in human cancers. Here, we show that constitutively activated AP-1 with robust c-Jun and JunB overexpression is found in all tumor cells of patients with classical Hodgkin’s disease. A similar AP-1 activation is present in anaplastic large cell lymphoma (ALCL), but is absent in other lymphoma types. Whereas c-Jun is up-regulated by an autoregulatory process, JunB is under control of NF-κB. Activated AP-1 supports proliferation of Hodgkin cells, while it suppresses apoptosis of ALCL cells. Furthermore, AP-1 cooperates with NF-κB and stimulates expression of the cell-cycle regulator cyclin D2, proto-oncogene c-met and the lymphocyte homing receptor CCR7, which are all strongly expressed in primary HRS cells. Together, these data suggest an important role of AP-1 in lymphoma pathogenesis.
Keywords: lymphoma/MAPK/metastasis/oncogenesis/target genes
Introduction
In the multi-stage process of oncogenesis, tumor cells acquire a series of genetic alterations of regulatory genes that result in their escape from normal proliferation and cell-death control (Hanahan and Weinberg, 2000). An oncogenic potential is therefore inherent to components of mitogenic and apoptotic signaling pathways, including transcription factors such as activator protein-1 (AP-1) and nuclear factor κB (NF-κB). AP-1 is composed of homo- or heterodimers formed by related Jun (c-Jun, JunB, JunD), Fos (c-Fos, FosB, Fra-1, Fra-2) and ATF family proteins (Karin et al., 1997). Transcription of c-Jun and other AP-1 family members is rapidly and transiently stimulated by a number of extracellular signals, which trigger activation of the JNK, ERK1/2 or p38 MAP kinase pathways (Chang and Karin, 2001). AP-1 activity is regulated at the level of expression and post-translational modification of the different subunits (Karin et al., 1997; Leppä and Bohmann, 1999). This regulation requires a tight balance, as heterodimers composed of different Jun family members differ functionally and may even act antagonistically, as has been shown for c-Jun and JunB (Bakiri et al., 2000; Szabowski et al., 2000). AP-1 proteins are usually activated in response to stress signals, such as UV irradiation, but also by growth factor pathways, and promote mitogen-induced cell-cycle progression or regulate apoptosis by modulating cyclin D1 and p53 expression (Schreiber et al., 1999; Wisdom et al., 1999). The role of Jun/AP-1 in programmed cell death is complex and, depending on the cellular context and cell type, pro- or anti-apoptotic functions have been reported (Leppä and Bohmann, 1999). In line with the transcriptional activation in response to mitogenic signals, Jun proteins, alone or in cooperation with oncogenic proteins, can induce transformation, which is best characterized for c-Jun (Mechta-Grigoriou et al., 2001); however, JunB might also act as a tumor suppressor in certain cell types (Passegué et al., 2001). Although an oncogenic potential of Jun proteins was indicated in mouse models and mammalian cell lines, no recurrent involvement of these proteins, including chromosomal translocation or alterations of protein expression, have yet been identified in human diseases. Thus, their role for malignant transformation of human cells is not clear.
A common lymphoma in humans is classical Hodgkin’s disease (cHD). Characteristically, the malignant mononuclear Hodgkin-/multinuclear Reed–Sternberg (HRS) cells, which are considered to be derived from clonally expanded germinal center B cells, represent only a minor cellular fraction of the affected lymph nodes (Staudt, 2000). They are surrounded by reactive cells attracted by cytokines and chemokines, abundantly produced by HRS cells (Staudt, 2000). Despite great effort, the pathogenesis of HD is not understood. Molecules like CD30 are frequently expressed by HRS cells, but there is no hint of a link for these molecules to disease development (Dürkop et al., 1992). Furthermore, the pathogenic role of clonal copies of Epstein–Barr virus (EBV), present in cHD, has not yet been clarified (Chapman and Rickinson, 1998). As a characteristic for HRS cells, a constitutive activation of transcription factor NF-κB has been recognized (Bargou et al., 1996; Krappmann et al., 1999). In most normal cell types, NF-κB is only transiently activated, mainly by stress signals and in immune and inflammatory signaling events (Karin and Ben Neriah, 2000). As a major cause of constitutive NF-κB activation in HRS cells, the IκB kinase complex (IKK) is persistently activated, inducing high turnover of IκB proteins (Krappmann et al., 1999). Constitutive NF-κB accounts for the elevated expression of several genes typically associated with HRS cells, including cell cycle-regulating and anti-apoptotic genes, and contributes to proliferation, apoptosis resistance and tumorigenicity of HRS cells (Bargou et al., 1997; Hinz et al., 2001). Many physiological signaling pathways that activate IKK and NF-κB also stimulate MAP kinase signaling cascades and AP-1. The simultaneous activation of both groups of transcription factors allows a functional synergism, and relevant target genes, such as cyclin D1, GM-CSF and others, are regulated by both NF-κB and AP-1 (Stein et al., 1993; Herber et al., 1994; Thomas et al., 1997; Hinz et al., 1999). We therefore investigated the activity of AP-1 and MAP kinases in Hodgkin and non-Hodgkin lymphoma. We identified a constitutive, aberrant expression of the AP-1 complex, containing c-Jun and JunB, as a pathogenetic hallmark of Hodgkin lymphoma. With a specificity rarely documented for other oncogenes, this activity is found in the entire tumor cell population of all cHD patients tested. Surprisingly, in cultured cHD cells, AP-1 is up-regulated in a cell autonomous manner, independent of MAP kinases. AP-1 is required for cHD cell proliferation and stimulates expression of cyclin D2, proto-oncogene c-met and the CCR7 lymphocyte homing receptor.
Results
Strongly elevated AP-1 DNA-binding activity and Jun protein expression in HRS and ALCL cells, but not in other non-Hodgkin lymphoma cells
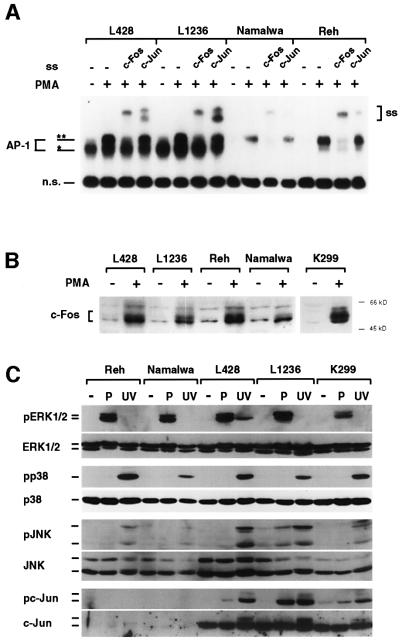
A number of unstimulated Hodgkin and non-Hodgkin lymphoma cell lines were tested for AP-1 DNA-binding activity. A dramatically elevated constitutive activity was detected in all seven HRS (Figure 1A, lanes 1–7) and anaplastic large cell lymphoma (ALCL) cell lines (lanes 15–17), whereas all other non-Hodgkin cell lines lacked a comparable DNA-binding activity (lanes 8–14). Supershift analysis with c-Jun, JunB, JunD, c-Fos, Fra-1, Fra-2 or ATF-2 antibodies indicated that the AP-1 complex in all HRS cells predominantly contained c-Jun (Figure 1A). In addition, JunB was detectable in some HRS cell lines. In ALCL cells, c-Jun and JunB were also detectable, but Fra-2 was the main component (Figure 1A; data not shown). JunD was weakly present in all cell lines. In agreement with these results, highly elevated c-Jun mRNA and protein expression was seen in all seven HRS cell lines and, though weaker, in ALCL cells (Figure 1B). JunB mRNA and protein up-regulation was found in the majority of HRS and ALCL cell lines (Figure 1B). In contrast, JunD mRNA was equally present in all cell lines tested, although JunD protein expression appeared to be elevated in some HRS cell lines (Figure 1B). Thus, all HRS cell lines reveal a striking accumulation and enhanced DNA-binding activity of c-Jun, and in addition JunB is selectively overexpressed in most cases.
Fig. 1. Abundant constitutive Jun/AP-1 DNA-binding activity in unstimulated HRS cell lines. (A) Top panel, nuclear extracts of Hodgkin cell lines, as indicated, pro-B lymphoblastic leukemia (Reh), Burkitt’s lymphoma (Namalwa, Daudi, BL60), myeloma (L363, INA-6), T lymphocytic leukemia (Molt-4) or ALCL [K299, SU-DHL-1 (DHL-1), DEL] cells were assayed for AP-1 DNA-binding activity by EMSA using the TRE site of the human collagenase promoter. Free DNA is not shown. n.s., non-specific. Bottom panel, supershift analysis (ss) of AP-1 components with nuclear extracts of lymphoma cell lines, as indicated. (B) Top panel, expression of c-Jun, JunB and JunD mRNA in various lymphoma cell lines, as indicated. GAPDH expression is shown as a control. NB, northern blot. Bottom panel, protein expression of c-Jun, JunB and JunD in various lymphoma cell lines, as indicated. As a control, expression of α-tubulin is shown. WB, western blot.
Patients with cHD reveal high level c-Jun and JunB expression in the entire tumor cell population
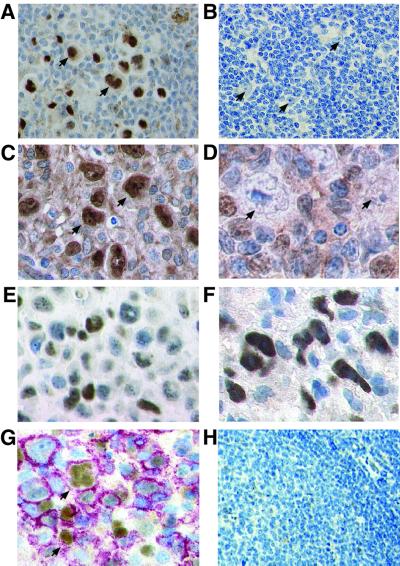
The expression pattern of c-Jun and JunB in cell lines suggests that the strong c-Jun and JunB expression could serve as a marker to discriminate various lymphoma subtypes. Indeed, when lymph node sections of patients with cHD were analyzed by immunohistochemistry, all HRS cells in all cases examined revealed strong and selective nuclear staining for c-Jun and JunB using various poly- or monoclonal antibodies (Figure 2A and C; Table I; data not shown). No differences were detectable between the histological subtypes or EBV-positive and -negative cases of cHD (data not shown). In marked contrast to cHD, neither c-Jun nor JunB expression was detectable in the tumor cells of patients with lymphocyte predominance Hodgkin’s disease (LPHD), a rare subtype distinct from cHD (Figure 2B and D; Table I). Among a number of precursor and peripheral B- and T-cell non-Hodgkin lymphomas, only t(2;5)-positive ALCL stained positive for c-Jun and JunB, although inconsistently and less intensely, when compared with cHD (Figure 2E and F; Table I). The characteristic pattern of c-Jun and JunB overexpression among lymphoid malignancies thus establishes these proteins as unique markers for cHD. Interestingly, c-Jun-positive, activated extrafollicular B cells with an expression level similar to HRS cells were found in tonsils from patients with acute EBV infec tion (EBV latency III). Some of these cells revealed multi nuclear Reed–Sternberg cell morphology (Figure 2G). The further analysis of 12 cases of lymphoproliferations associated with an EBV infection (latency I, Burkitt lymphoma; latency II and III, post-transplantation lymphoproliferative disorders) revealed only one case, which strongly labeled for c-Jun, whereas in the other cases, no staining or only weak staining of the minority of tumor cells was observed (data not shown). In order to identify a possible normal counterpart of c-Jun- and JunB-positive malignant cells, we further analyzed sections from activated tonsillar lymphoid tissue from an additional 20 patients. Apart from rare, weakly stained lymphoid cells in the germinal center of some tonsils, this lymphoid tissue was negative for both proteins (Figure 2H; data not shown).
Fig. 2. High level c-Jun and JunB expression is a hallmark of malignant cells in cHD. Immunohistochemistry of representative biopsy specimen. (A) All HRS cells (arrows) in cHD, but not surrounding benign cells, reveal strong nuclear c-Jun staining. (B) Malignant cells (arrows) in LPHD do not express c-Jun in detectable amounts. (C) In all HRS cells in cHD, JunB is abundantly expressed, with mostly nuclear localization (arrows). (D) Malignant cells (arrows) in LPHD do not show JunB expression. (E) c-Jun and (F) JunB expression in ALCL. In contrast to cHD, all cells shown are tumor cells. (G) Tonsil of a patient with infectious mononucleosis analyzed by double staining for c-Jun and CD20, indicating B-cell origin. The membrane staining of CD20 appears red, nuclear staining of c-Jun, brown. B cells with HRS cell-like morphology stain positive for c-Jun (arrows). (H) Normal tonsil. No c-Jun-positive cells are detectable.
Table I. c-Jun and JunB expression analysis in sections of B- and T-cell lymphoma.
| Differentiation stage | Lymphoma entity | No. of cases | Immunohistochemistry staining intensity |
||
|---|---|---|---|---|---|
| ABSENTa |
WEAK Proportion of tumor cellsb |
STRONG Majority of tumor cellsc |
|||
| c-Jun expression | |||||
| Pre-GC B cells | Mantle cell lymphoma | 12 | 12 | ||
| GC B cells (early) | Burkitt lymphoma | 13 | 11 | 2 | |
| Follicular lymphoma | 10 | 10 | |||
| LPHD | 16 | 16 | |||
| GC B cells (late) | cHD | 26 | 26 | ||
| Post-GC B cells | Diffuse large B-cell lymphoma | 11 | 7 | 4 | |
| Extranodal marginal zone lymphoma | 10 | 8 | 2 | ||
| Plasmacytoma | 13 | 6d | 7 | ||
| Precursor T cells | T lymphoblastic | 5 | 5 | ||
| Peripheral T cells | Not otherwise specified | 5 | 2 | 3 | |
| ALCL t(2;5) | 7 | |
3 |
4 |
|
| |
JunB expression |
|
|||
| Classical HD | 18 | 18 | |||
| LPHD | 10 | 10 | |||
| ALCL t(2;5) | 16 | 2 | 14 | ||
Results of immunohistochemical analysis of diverse lymphoma entities, ordered for the cellular differentiation stages, are indicated.
aIn some cases, occasional neoplastic cells exhibited weak immunostaining (<5% of the neoplastic cell population).
b10–70% of neoplastic cells exhibited weak immunostaining.
c70–100% of neoplastic cells were mostly labeled intensively, with nearly 100% of HRS cells labeled strongly in all cases of cHD.
dIn four plasmacytoma cases, only cytoplasmic staining was observed.
GC, germinal center.
The constitutive AP-1 complex is distinct from the mitogen-induced complex and is formed in the absence of MAP kinase activity
Since c-Jun and JunB complexes are usually activated by extracellular stimuli, we analyzed the effect of phorbolester treatment on AP-1 DNA-binding activity in HRS cells compared with non-Hodgkin cell lines (Figure 3A). AP-1 complexes with distinct electrophoretic migration compared with the constitutive complexes in HRS cells were rapidly induced by PMA in all cell types. These complexes were predominantly composed of c-Fos. Induction of expression and phosphorylation of c-Fos protein was confirmed by western blot analysis (Figure 3B). These data indicate that constitutive and induced AP-1 complexes in HRS cells are distinct. As MAP kinases are strong AP-1 inducers, we next analyzed MAPK expression and activity in the same cell lines (Figure 3C). No significant differences of JNK1, ERK1/2 or p38 protein expression were observed. All three groups of MAPKs were activated by either PMA or UV irradiation, as detected with phosphospecific antibodies, but HRS cells revealed no increased basal MAPK activity. Stimulation of JNK activity was verified by JNK1 in in vitro kinase assays (data not shown). UV light strongly induced c-Jun Ser63 phosphorylation in HRS and ALCL cells, leading to reduced protein mobility. However, c-Jun in unstimulated HRS cells was not Ser63 phosphorylated, confirming the absence of MAPK activity. Compared with the PMA or UV induction of c-Jun in Reh and Namalwa cells, its abundancy in unstimulated HRS cells was remarkably high. Furthermore, MAPK independence of the AP-1 activity in HRS cells was supported by treatment with the ERK1/2 inhibitor PD98059 and the p38 inhibitor SB203580, which did not alter the constitutive AP-1 activity (data not shown).
Fig. 3. Modulation of AP-1 and MAPK activity in Hodgkin and non-Hodgkin cell lines. (A) Phorbolester-induction of a distinct AP-1 DNA-binding activity in HRS and non-Hodgkin cells. Cells were left untreated or stimulated with PMA, as indicated. Extracts were assayed by EMSA with or without preincubation with antibodies against c-Fos or c-Jun. Supershifted protein–DNA complexes are indicated (ss). The PMA-induced AP-1 complex (**) migrates slower than the constitutive complex in HRS cells (*). n.s., non-specific. (B) Extracts of unstimulated or stimulated cells were analyzed in a western blot for c-Fos induction. (C) MAPK pathways are not constitutively activated in HRS cells, as revealed by mitogen and UV-light induction. The HRS cell lines L428 and L1236 or control cell lines were stimulated with PMA (P), UV-irradiated (UV) or left untreated. Whole-cell extracts were analyzed in western blots for MAPK activation and c-Jun N-terminal phosphorylation with phosphospecifc antibodies (pERK1/2, pp38, pJNK, pc-Jun), and for expression of the kinases and c-Jun with the respective antibodies, as indicated.
In HRS cells, c-Jun is largely activated by an autoregulatory mechanism at the transcriptional level, while JunB is under NF-κB control
One reason for highly elevated c-Jun protein levels in HRS cells in the absence of MAPK activity, which usually precedes induction of c-Jun, might be enhanced protein or RNA stability. We therefore determined c-Jun protein stability by pulse–chase analysis (Figure 4A). The half-life of c-Jun protein in L428, L1236 and Namalwa cells was between 120 and 170 min; in KMH-2 cells, it was slightly longer. This is in good agreement with the determined c-Jun half-life in other cell types (Lamph et al., 1988); thus prolonged protein stability cannot explain the drastic c-Jun accumulation in HRS cells. Furthermore, the c-Jun mRNA half-life in HRS cells was ∼40 min, similar to the value reported for other cell types (Figure 4B; Blattner et al., 2000). Consequently, elevated expression must be primarily due to increased transcription.

Fig. 4. High level c-Jun/AP-1 expression is not caused by increased protein or mRNA half-life. (A) For pulse–chase analysis, cells were labeled with 35[S]methionine and chased with medium containing unlabeled amino acids for the times indicated. Immunoprecipitated c-Jun was separated by SDS–PAGE and quantitated with a phosphoimager. Note that exposition time for KMH-2, L1236 and L428 samples was overnight and for Namalwa, 10 days. (B) c-Jun transcript stability was assessed by actinomycin D treatment of L1236 cells for the times indicated, followed by northern analysis (bottom panel). A quantitation of the c-Jun signal, corrected for GAPDH, is shown for a triplicate experiment (top panel). Error bars denote SDs.
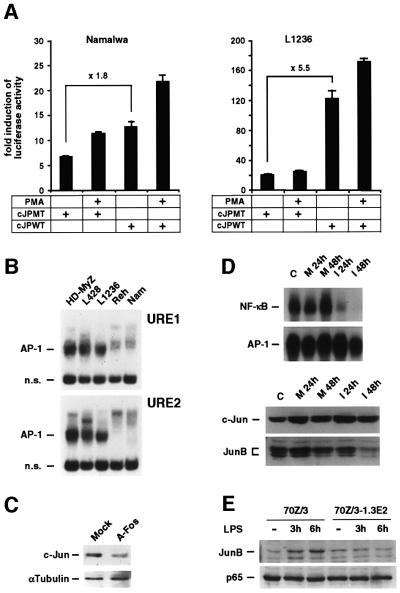
The c-Jun promoter activity is autoregulated by c-Jun/AP-1 (Angel et al., 1988). When comparing the activities of the wild-type promoter (WT) with a mutant (MT) lacking AP-1 sites, the latter conferred a several-fold activation in HRS but not in Namalwa cells (Figure 5A; data not shown). This indicates that AP-1 in HRS cells is transcriptionally active and that the c-Jun promoter is autoregulated. The finding of an autoregulation of c-Jun by Jun/AP-1 was supported by two further experiments. First, the AP-1 DNA-binding activity to both AP-1-like sites of the c-Jun promoter (URE1, –72 to –63; URE2, –191 to –182; Stein et al., 1992) was strongly enhanced in HRS cell lines compared with non-Hodgkin cell lines, as observed for the TRE site (Figures 5B and 1A). Supershift analysis revealed, especially at the URE2 site, a similar subunit composition as found for the TRE site (data not shown). Secondly, down-regulation of the AP-1 activity in L428 HRS cells by A-Fos, a dominant repressor of AP-1 (Olive et al., 1997), reduced the c-Jun protein expression level (Figure 5C).
Fig. 5. Constitutive c-Jun is positively autoregulated, whereas JunB is a target gene of NF-κB. (A) Autostimulatory regulation of the c-Jun promoter by Jun/AP-1. c-Jun promoter–luciferase constructs with wild-type (c-JunPWT) or mutated (c-JunPMT) AP-1 sites were transfected into Namalwa (left panel) or L1236 HRS (right panel) cells. As a control, both constructs were activated by PMA, which acts through AP-1 and non-AP-1 sites (Marinissen et al., 1999). Luciferase activity was determined for triplicate experiments. Error bars denote SDs. (B) Nuclear extracts of the HRS cell lines HD-MyZ, L428 and L1236, as well as the non-Hodgkin cell lines Reh and Namalwa, were analyzed by EMSA for AP-1 DNA-binding activity to the URE sites 1 and 2 of the c-Jun promoter. Free DNA is not shown. n.s., non specific. (C) c-Jun protein expression is down-regulated by inhibition of AP-1. Forty hours after transfection of L428 cells with A-Fos, cells were FACS-sorted and whole-cell extracts were analyzed for c-Jun and α-tubulin expression in western blots. (D) JunB but not c-Jun is activated by constitutive NF-κB in HRS cells. L428 cells were infected with an adenovirus expressing IκBαΔN (I) or with a control adenovirus (M). Whole-cell extracts of uninfected cells (C) and cells infected for 24 and 48 h with Adv–IκBαΔN (I 24h and I 48h, respectively) and control virus (M 24h and M 48h, respectively) were analyzed by EMSA for NF-κB and AP-1 DNA-binding activity (top panel) and western blot analysis for expression of c-Jun and JunB (bottom panel). (E) 70Z/3 and IKKγ-deficient 70Z/3–1.3E2 cells were stimulated with LPS. Whole-cell extracts were analyzed for JunB and, as a control, p65 protein expression by western blot analysis.
To examine whether AP-1 activity was connected to the constitutive NF-κB activation in HRS cells, we infected L428 cells with an adenovirus encoding the NF-κB repressor IκBαΔN. NF-κB DNA-binding activity was strongly reduced after infection, as described previously (Figure 5D; Hinz et al., 2001). AP-1 activity decreased only moderately at 48 h. While c-Jun protein levels were not affected, JunB amounts dropped strongly at the latest time point. Thus, our data indicate that, in contrast to c-Jun, JunB is a cellular target gene of constitutive NF-κB in HRS cells. To confirm regulation of JunB by the IKK/NF-κB pathway, 70Z/3 pre-B cells and an IKKγ-deficient variant, 70Z/3–1.3E2 (Yamaoka et al., 1998), were stimulated with lipopolysaccharide (LPS; Figure 5E). In fact, JunB was induced only in IKKγ-expressing cells. To analyze a possible modulation of AP-1 transcriptional activity by NF-κB, we determined the effect of IκBαΔN on the pRTU14 AP-1 reporter in L428 cells. No alteration of AP-1 transcriptional activity was detectable (data not shown), indicating that c-Jun, not JunB, is the major transactivating component of the constitutive AP-1 complex in HRS cells.
Constitutive c-Jun/AP-1 promotes proliferation and activates expression of cyclin D2, c-Met and CCR7 in HRS cells
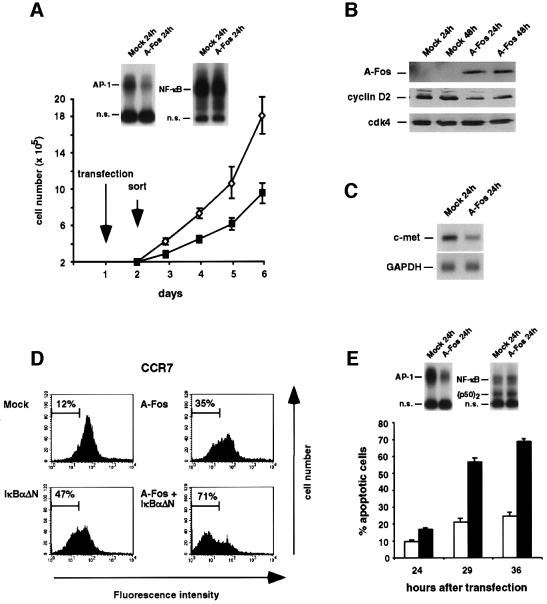
To investigate the functional consequences of constitutive AP-1 activity, we transiently transfected HRS cells with A-Fos. Due to low transfection efficiencies, green fluorescent protein (GFP)-cotransfected cells were sorted by FACS. As expected, AP-1 DNA-binding activity was diminished upon A-Fos expression, whereas NF-κB DNA binding and transcriptional activities remained unchanged (Figure 6A; data not shown). Down-modulation of AP-1 activity resulted in a marked reduction of cell growth, compared with mock-transfected cells (Figure 6A). No growth inhibition was observed after transfection of the Burkitt lymphoma cell line BL60, which lacks constitutive AP-1 activity (data not shown; see Figure 1A). G1–S-phase transition in HRS cells could be under the control of cyclin D2, which is expressed at elevated levels in HRS cells, is stimulated by constitutive NF-κB, and contains AP-1 sites in the promoter region (Brooks et al., 1996; Hinz et al., 2001). Indeed, A-Fos caused a noticeable decline of cyclin D2 expression (Figure 6B), indicating that constitutively activated AP-1 and NF-κB cooperate to induce cyclin D2 expression in HRS cells.
Fig. 6. Functional analysis of AP-1 activity in Hodgkin and ALCL cell lines. (A) Down-regulation of constitutive AP-1 activity by the c-Jun/AP-1 repressor A-Fos reduces proliferation in L428 HRS cells. Whole-cell lysates of FACS-sorted cells were analyzed by EMSA for AP-1 and NF-κB DNA-binding activity. Mock- (open diamonds) or A-Fos- (filled squares) transfected cells (2 × 105) were seeded after sorting and cell numbers were determined in the following 4 days. Results are the means of triplicate measurements. Error bars denote SDs. (B) Western blot analysis for A-Fos protein expression with an anti-FLAG tag antibody and for cyclin D2 and, as a control, cdk4 expression with specific antibodies. (C) Forty hours after transfection, L428 cells were sorted and mRNA expression of c-met was analyzed by northern blot analysis. Thereafter, the blot was reprobed with GAPDH. (D) NF-κB and AP-1 co-stimulate expression of CCR7 in HRS cells. L428 cells were transfected with control plasmid (Mock), A-Fos, IκBαΔN or A-Fos and IκBαΔN expression plasmids along with a GFP expression construct. GFP-positive cells were analyzed by flow cytometric analysis for expression of CCR7. The percentage of cells with weak or absent CCR7 expression is shown. (E) AP-1 suppression induces apoptosis in K299 ALCL cells. Transfection, cell sorting and EMSA analysis was as in (A). The fraction of apoptotic cells determined at the indicated times is shown as means of triplicate measurements for mock- (open bars) and A-Fos- (filled bars) transfected cells, respectively. Error bars denote SDs.
Furthermore, we identified the proto-oncogene c-met and the lymphocyte homing receptor CCR7 as AP-1 target genes in HRS cells. c-met is strongly expressed in primary HRS cells and its expression is suggested to be regulated by AP-1 (Seol et al., 2000; Teofili et al., 2001). In HRS cells, inhibition of AP-1 by A-Fos down-regulated c-met expression (Figure 6C). By screening high density cDNA microarrays with mRNAs of Ad-IκBαΔN-infected HRS cells, we identified the chemokine receptor CCR7 as an NF-κB-regulated gene, which is strongly expressed by HRS cell lines (data not shown). CCR7 protein expression in L428 cells was in fact significantly reduced by IκBαΔN (Figure 6D). Inspection of the promoter sequence revealed the presence of several AP-1 sites and similarities to NF-κB sites (Schweickart et al., 1994). Indeed, A-Fos expression reduced CCR7 expression, while simultaneous inhibition of NF-κB and AP-1 resulted in a much stronger CCR7 reduction (Figure 6D). Thus, in HRS cells CCR7 is a cellular target gene co-regulated by AP-1 and NF-κB.
Constitutive AP-1 prevents apoptosis of ALCL cells
A-Fos expression was also used to assess AP-1 function in K299 ALCL cells. Compared with mock-transfected cells, a pronounced induction of apoptosis was caused by AP-1 inhibition (Figure 6E). Induction of apoptosis was confirmed by analysis of caspase-3 activation (data not shown). Again, we excluded A-Fos-mediated alterations of constitutive NF-κB activity, which is weak in comparison to HRS cells and contains a larger fraction of p50 homodimers (Figure 6E; data not shown). Due to the strong induction of apoptosis, it is difficult to investigate any further effect of AP-1 on proliferation. An anti-apoptotic function of AP-1 was not seen in HRS cells (data not shown), which might be due to the anti-apoptotic function of the constitutive NF-κB activity in these cells (Bargou et al., 1997; Hinz et al., 2001).
Discussion
In this study, we report that the c-Jun and JunB transcription factors are constitutively expressed at a high level in primary and cultivated tumor cells of cHD and, to a lesser extent, in ALCL. As a selective marker, this striking expression pattern discriminates cHD and ALCL from all the other hematopoietic neoplasias investigated. c-Jun and JunB overexpression is found in the entire tumor cell population of all cHD patients tested, indicating a fundamental pathogenetic significance.
Normally, c-Jun is expressed broadly at a low level in many cell types and its expression is transiently amplified by a number of different stimuli, classically involving MAPK pathways (Karin et al., 1997; Leppä and Bohmann, 1999). Therefore, a striking observation is the MAPK-independence of constitutive AP-1 activation in HRS cells. This also rules out that CD30 or CD40, both highly expressed by HRS cells, autocrine mechanisms via secreted cytokines, or EBV–LMP1 expression, all of which should stimulate JNK, activate AP-1. We have also shown that c-Jun expression is independent of constitutive NF-κB in HRS cells. In contrast, JunB is activated by NF-κB. Thus, NF-κB does have an effect on the composition of constitutive AP-1 in HRS cells, but not on its activation. c-Jun mRNA abundance is ascribed to increased transcription, and the c-Jun promoter is highly activated in HRS cells through its AP-1 sites. In other cell types, these sites confer autoregulation via c-Jun–ATF2 heterodimers, and transiently activated p38 and JNK kinases stimulate the transcriptional activity of the bound dimers (Angel et al., 1988; van Dam et al., 1995). Activation of the c-Jun promoter without MAPK activity likely involves as yet unknown signaling pathways in HRS cells, perhaps acting synergistically with c-Jun, or increased activity of transcriptional co-factors.
In theory, long-lasting AP-1 activation could be a consequence of viral infection, since viral proteins, like HTLV-1 Tax or EBV-LMP1 are efficient inducing agents (Xu et al., 1996; Kieser et al., 1997). We found strong c-Jun up-regulation in activated B cells, some revealing Reed–Sternberg cell morphology, in tonsils from patients with acute EBV infection. Although c-Jun up-regulation in cHD is independent of the present EBV status, clonal copies of EBV are present in 40–60% of patients with cHD (Chapman and Rickinson, 1998). Whether EBV-induced AP-1 up-regulation can persist after loss of the virus must await future analysis in model infection systems. Interestingly, activated AP-1, containing JunD, is present in HTLV-1-associated adult T-cell leukemia (ATL) cells, and was also detected in cells carrying the provirus, but not expressing the viral tax protein (Mori et al., 2000). Taken together, these observations point to a connection between constitutive Jun/AP-1 activity and a history of viral infection of lymphoid cells.
The combined constitutive activation of AP-1 and NF-κB mimics a state of chronic inflammation, and the synergism between the two transcription factors may explain the high production of cytokines by HRS cells. As an important target gene cooperatively activated by AP-1 and NF-κB, we identified the lymphocyte homing receptor CCR7, which bears AP-1- and NF-κB-binding sites in its promoter region (Schweickart et al., 1994). In line with this, CCR7 has been found as an IKK-dependent, LPS-regulated target gene in a pre-B cell line (Li et al., 2001). CCR7 is expressed on B, T and activated dendritic cells and controls migration and localization of lymphocytes to secondary lymphoid organs (Förster et al., 1999). Compared with B cell lines, HRS cell lines produce high amounts of functionally active CCR7 receptors (Höpken et al., 2002). Importantly, an analysis of lymph node sections has revealed up-regulation of CCR7 by the great majority of HRS cells in cHD, but not by L & H cells in LPHD, perfectly matching the c-Jun/JunB expression shown in this study. This different expression pattern of CCR7 between cHD and LPHD might contribute to the distinct dissemination pattern of cHD and LPHD (Höpken et al., 2002).
The second important gene product activated by AP-1 in HRS cells is cyclin D2. Cyclin D2 is strongly expressed in primary and cultivated HRS cells and its expression also depends on NF-κB (Hinz et al., 1999; Teramoto et al., 1999). In fibroblasts and epithelial cells, NF-κB and AP-1 both stimulate expression of cyclin D1, and NF-κB activity is required for growth factor-induced, cyclin D1-dependent G1–S-phase transition (Herber et al., 1994; Hinz et al., 1999). Similarly, the cyclin D2 promotor contains NF-κB and AP-1 sites (Brooks et al., 1996). It is therefore likely that NF-κB and AP-1, in concert, support HRS cell proliferation by activating cyclin D2. This combined action of c-Jun/AP-1 and NF-κB might explain the high fraction of proliferating cells of ∼80–90% in most cases of cHD (Gerdes et al., 1987; data not shown). A third identified AP-1-dependent target gene in HRS cells is the proto-oncogene c-met. As shown for the other target genes, c-met is also strongly expressed in HRS cells of cHD (Teofili et al., 2001). Furthermore, the c-Met/hepatocyte growth factor pathway has been suggested as important for the survival and the interaction of HRS cells with the surrounding reactive cells (Teofili et al., 2001).
At this point we cannot discriminate the relative contributions of c-Jun and JunB in HRS cell proliferation and target gene activation, since both are repressed by A-Fos (Olive et al., 1997). In other cell types, c-Jun and JunB were described to have antagonistic functions in the regulation of cell-cycle progression and AP-1 target gene activation. JunB was even reported to act as a tumor repressor in mice (Passegué et al., 2001). However, besides its potential repressor function, JunB is a transcriptional activator of genes such as IL-4 (Li et al., 1999). The exact functional contribution of the single components of constitutive AP-1, which differ between HRS and ALCL cells, has to await further analysis.
Based on its ancestral relationship to the avian viral oncogene v-Jun and its transforming capacities in model systems, the c-Jun proto-oncogene has long been implicated in tumor pathogenesis, but no recurrent involvement in human cancers could yet be found. Our data demonstrate for the first time that aberrantly expressed c-Jun and JunB are a hallmark of a common human lymphoma. Apart from serving as a diagnostic tool, the impact on proliferation, apoptosis regulation and expression of essential genes identifies constitutive Jun/AP-1, especially in combination with constitutive NF-κB, as an important target for the development of therapeutic approaches.
Materials and methods
Cell lines and culture conditions
HRS [L428, L1236, KMH-2, HDLM-2, L540, L591 (EBV+), HD-MyZ], ALCL [K299, SU-DHL-1, DEL, all t(2;5)+], Reh, Namalwa, Daudi, BL-60, L363, INA-6, Molt-4, 70Z/3 and 70Z/3–1.3E2 cells were cultured as described previously (Mathas et al., 2000). Cells were treated with 200 ng/ml 4β-phorbol-12-myristate-13-acetate (PMA; Sigma, Deisenhofen, Germany), 10 µg/ml LPS (Sigma), 20 J/m2 UV or 5 µg/ml actinomycin D, where indicated. Electroporation was performed using a Gene-Pulser II (Bio-Rad, München, Germany) with 960 µF and 0.18 (L428, HD-MyZ, L1236, K299) or 0.25 kV (Namalwa). Transfection efficiency was determined by pEGFP-N3 (Clontech Laboratories, Heidelberg, Germany) cotransfection and subsequent FACS analysis. Enrichment of transfected L428 and K299 cells was performed by FACS sorting of GFP-positive cells 24 h after transfection using FACS Vantage and CELLQuest software (Becton Dickinson, Heidelberg, Germany). Adenoviral infection of L428 cells was described previously (Hinz et al., 2001).
DNA constructs
pCMVA-Fos was kindly provided by C.Vinson (NCI, Bethesda, MD). c-Jun –1600/+170 full-length promoter wild-type (WT) and mutant (MT) (–1600/+170Δ2.UREΔAP1) constructs were kindly provided by P.Angel (DKFZ, Heidelberg, Germany). The PCR-amplified promoter sequence was cloned into pGL2 (Promega, Mannheim, Germany) as a KpnI/HindIII fragment. The pcDNA3-IκBαΔN expression construct was described previously (Krappmann et al., 1999).
Metabolic labeling and immunoprecipitation
For metabolic labeling, cells were washed with methionine-free EMEM, supplemented with 10% dialyzed fetal calf serum (FCS; Gibco) and 2 mM glutamine, and kept in this medium for 30 min before adding [35S]methionine (100 µCi/ml; 5 × 106 cells/ml for each time point). After 60 min incubation the medium was replaced by RPMI (10% FCS). Extracts were prepared at the indicated times. The cells were washed twice with phosphate-buffered saline and lysed in RIPA buffer. After 20 min at 4°C, extracts were centrifuged for 5 min at 15 800 g. The supernatant was used for immunoprecipitation. Extracts were precleared with 20 µl of protein A–Sepharose for 1 h at 4°C, thereafter c-Jun antibodies (5 µl) and 10 µl of protein A–Sepharose were added and incubated at 4°C for 3–4 h. Protein A–Sepharose was washed four times with ice-cold RIPA buffer and boiled for 5 min in SDS loading buffer. The supernatant was separated by SDS–PAGE followed by autoradiography.
Immunofluorescence and flow cytometry
CCR7 expression was detected indirectly by an anti-CCR7 antibody (clone 3D12; kindly provided by M.Lipp, MDC, Berlin, Germany) and a PE-conjugated anti-rat antibody (Dianova, Hamburg, Germany). An irrelevant isotype-matched antibody was used as control. CCR7 expression after transfection experiments was determined on viable, GFP-positive cells. Immunofluorescence was analyzed using a FACS Calibur flow cytometer and CELLQuest software (Becton Dickinson).
Analysis of apoptosis
Cells were stained with Acridine orange (5 µg/ml; Sigma) and analyzed by fluorescence microscopy. The number of cells with fragmented or condensed nuclei were counted, and the percentages determined. All assays were performed in triplicate.
Luciferase assays
For reporter assays, HD-MyZ, L428, L1236 and Namalwa cells were transfected by electroporation with a total plasmid DNA amount of 15 µg. c-Jun promotor WT or MT luciferase constructs (10 µg) were transfected with 5 µg of pRL-TKLuc as an internal control. Eighteen hours after transfection, cells were stimulated with PMA for 6 h, 24 h after transfection cells were lysed and the ratio of the two luciferases was determined with the dual luciferase kit (Promega).
Electrophoretic mobility shift assay (EMSA) and western blotting
EMSA, whole-cell and nuclear extract preparation were described previously (Krappmann et al., 1999). The following double-stranded oligonucleotides were used: AP-1–TRE, 5′-AGCTAGCATGAGTCAGACAC-3′ and 5′-AGCTGTGTCTGACTCATGCT-3′; c-Jun promoter, jun 1 URE (URE1) –72 to –63, 5′-AGCTTGTGACAT CATCCG-3′and 5′-GATCCGGATGATGTCACA-3′; c-Jun promoter, jun 2 URE (URE2) –191 to –182, 5′-AGCTTGATTACCTCATCCG-3′ and 5′-GATCCGGATGAGGTAATCA-3′ (Stein et al., 1992). A double-stranded oligonucleotide with a mutated AP-1 consensus binding site served as a control. The antibodies used for supershift analysis were from Santa Cruz (Heidelberg, Germany). For western blot analysis, the following primary antibodies were used: polyclonal and monoclonal c-Jun (sc-7481, sc-1694), polyclonal JunD (sc-74), c-Fos (sc-52), p38 (sc-535), cdk-4 (sc-601), IκBα (sc-371; all from Santa Cruz), polyclonal anti-JunB, monoclonal anti-phospho-Ser63 c-Jun (both as described in Lallemand et al., 1998), monoclonal anti-phospho-ERK1/2 (Thr202/Tyr204; Ab9106), polyclonal anti-phospho-p38 MAPK (Thr180/Tyr182; 9211S), polyclonal anti-phospho-JNK (Thr183/Tyr185; 9251; all from New England Biolabs, Beverly, MA), polyclonal ERK1/2 (442704; Calbiochem, Bad Soden, Germany), polyclonal anti-caspase-3 (556425), monoclonal anti-JNK1 (554286), monoclonal anti-cyclin D2 (14821A; all PharMingen, Hamburg, Germany), polyclonal anti-p65 (SA-171; Biomol, Hamburg, Germany), monoclonal anti-tubulinα (MCA78A; Serotec, München, Germany), anti-Flag M5 (F-4042; Sigma). Filters were incubated with horseradish peroxidase-conjugated secondary antibodies. Bands were visualized using the enhanced chemiluminescence system (Amersham).
Northern blot analysis
Total RNA was prepared using RNeasy kit (Qiagen, Hilden, Germany). Total RNA (10 µg) were subjected to gel electrophoresis and transferred to a nylon membrane (Appligene, Heidelberg, Germany). The membrane was hybridized (ExpressHyb solution; Clontech) with [α-32P]dCTP-labeled random prime-labeled DNA probes [c-Jun, JunB, JunD, c-met, glyceraldehyde-3-phosphate dehydrogenase (GAPDH)].
Immunohistology
All cases were drawn from the files of the Consultation and Reference Center for Haematopathology at the Institute of Pathology, Benjamin Franklin Clinic, Free University of Berlin, Germany. The final diagnosis was established after extensive immunohistological analysis according to the criteria of the REAL classification (Harris et al., 1994). All cases of ALCL were ALK and t(2;5) positive. For EBV-associated lymphoproliferations, the EBV infection type was established by in situ hybridization analysis of EBV-encoded nuclear RNAs (EBER1 and EBER2) and immunohistological analysis of EBV protein expression (LMP-1, EBNA2 and ZEBRA). Immunostains were performed on paraffin-embedded tissue specimens. Prior to the incubation of antibodies against c-Jun (sc-1694, sc-7481), JunB (sc-46) and JunD (sc-74; all from Santa Cruz), a heat-induced antigen retrieval was performed. Immunodetection was performed with biotinylated anti-mouse and anti-rabbit immunoglobulins, followed by peroxidase or alkaline phosphatase-labeled streptavidine (DAKO, Glostrup, Denmark). In the case of peroxidase, diaminobenzidin was applied as a substrate, while alkaline phosphatase was developed using Fast Red. Cross-reactivity of the antibodies with the various Jun proteins in the immunostains was excluded by analysis of paraffin-embedded HD-MyZ, L428, L1236 and Namalwa cells.
Acknowledgments
Acknowledgements
We are indebted to Peter Angel (Heidelberg), Dirk Bohmann (Heidelberg) and Charles Vinson (Bethesda) for the gift of c-Jun promoter and AP-1 reporter constructs, c-Jun cDNA and A-Fos cDNA, respectively. Ad5-IκBαΔN was provided by Peter Löser (Berlin) and c-met cDNA by Walter Birchmeier (Berlin). We thank Peter Grasshoff (Berlin) for cell sorting, Moshe Yaniv (Paris) and Achim Leutz (Berlin) for critical reading of the manuscript.
References
- Angel P., Hattori,K., Smeal,T. and Karin,M. (1988) The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell, 55, 875–885. [DOI] [PubMed] [Google Scholar]
- Bakiri L., Lallemand,D., Bossy Wetzel,E. and Yaniv,M. (2000) Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J., 19, 2056–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargou R.C., Leng,C., Krappmann,D., Emmerich,F., Mapara,M.Y., Bommert,K., Royer,H.D., Scheidereit,C. and Dörken,B. (1996) High-level nuclear NF-κB and Oct-2 is a common feature of cultured Hodgkin/Reed–Sternberg cells. Blood, 87, 4340–4347. [PubMed] [Google Scholar]
- Bargou R.C. et al. (1997) Constitutive nuclear factor-κB–RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J. Clin. Invest., 100, 2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner C., Kannouche,P., Litfin,M., Bender,K., Rahmsdorf,H.J., Angulo,J.F. and Herrlich,P. (2000) UV-induced stabilization of c-fos and other short-lived mRNAs. Mol. Cell. Biol., 20, 3616–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.R., Shiffman,D., Chan,C.S., Brooks,E.E. and Milner,P.G. (1996) Functional analysis of the human cyclin D2 and cyclin D3 promoters. J. Biol. Chem., 271, 9090–9099. [DOI] [PubMed] [Google Scholar]
- Chang L. and Karin,M. (2001) Mammalian MAP kinase signalling cascades. Nature, 410, 37–40. [DOI] [PubMed] [Google Scholar]
- Chapman A.L. and Rickinson,A.B. (1998) Epstein–Barr virus in Hodgkin’s disease. Ann. Oncol., 9, S5–S16. [DOI] [PubMed] [Google Scholar]
- Dürkop H., Latza,U., Hummel,M., Eitelbach,F., Seed,B. and Stein,H. (1992) Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell, 68, 421–427. [DOI] [PubMed] [Google Scholar]
- Förster R., Schubel,A., Breitfeld,D., Kremmer,E., Renner Müller,I., Wolf,E. and Lipp,M. (1999) CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell, 99, 23–33. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Van Baarlen,J., Pileri,S., Schwarting,R., Van Unnik,J.A. and Stein,H. (1987) Tumor cell growth fraction in Hodgkin’s disease. Am. J. Pathol., 128, 390–393. [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. and Weinberg,R.A. (2000) The hallmarks of cancer. Cell, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- Harris N.L. et al. (1994) A revised European–American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood, 84, 1361–1392. [PubMed] [Google Scholar]
- Herber B., Truss,M., Beato,M. and Müller,R. (1994) Inducible regulatory elements in the human cyclin D1 promoter. Oncogene, 9, 1295–1304. [PubMed] [Google Scholar]
- Hinz M., Krappmann,D., Eichten,A., Heder,A., Scheidereit,C. and Strauss,M. (1999) NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol. Cell. Biol., 19, 2690–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M., Löser,P., Mathas,S., Krappmann,D., Dörken,B. and Scheidereit,C. (2001) Constitutive NF-κB maintains high expression of a characteristic gene network, including CD40, CD86 and a set of anti-apoptotic genes in Hodgkin/Reed–Sternberg cells. Blood, 97, 2798–2807. [DOI] [PubMed] [Google Scholar]
- Höpken U.E., Foss,H.-D., Meyer,D., Hinz,M., Leder,K., Stein,H. and Lipp,M. (2002) Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte predominant Hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood, 99, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Karin M. and Ben Neriah,Y. (2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol., 18, 621–663. [DOI] [PubMed] [Google Scholar]
- Karin M., Liu,Z.G. and Zandi,E. (1997) AP-1 function and regulation. Curr. Opin. Cell Biol., 9, 240–246. [DOI] [PubMed] [Google Scholar]
- Kieser A., Kilger,E., Gires,O., Ueffing,M., Kolch,W. and Hammerschmidt,W. (1997) Epstein–Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J., 16, 6478–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappmann D., Emmerich,F., Kordes,U., Scharschmidt,E., Dörken,B. and Scheidereit,C. (1999) Molecular mechanisms of constitutive NF-κB/Rel activation in Hodgkin/Reed–Sternberg cells. Oncogene, 18, 943–953. [DOI] [PubMed] [Google Scholar]
- Lallemand D., Ham,J., Garbay,S., Bakiri,L., Traincard,F., Jeannequin,O., Pfarr,C.M. and Yaniv,M. (1998) Stress-activated protein kinases are negatively regulated by cell density. EMBO J., 17, 5615–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamph W.W., Wamsley,P., Sassone Corsi,P. and Verma,I.M. (1988) Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature, 334, 629–631. [DOI] [PubMed] [Google Scholar]
- Leppä S. and Bohmann,D. (1999) Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene, 18, 6158–6162. [DOI] [PubMed] [Google Scholar]
- Li B., Tournier,C., Davis,R.J. and Flavell,R.A. (1999) Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J., 18, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Peet,G.W., Balzarano,D., Li,X., Massa,P., Barton,R.W. and Marcu,K.B. (2001) Novel NEMO/IκB kinase and NF-κB target genes at the pre-B to immature B cell transition. J. Biol. Chem., 276, 18579–18590. [DOI] [PubMed] [Google Scholar]
- Marinissen M.J., Chiariello,M., Pallante,M. and Gutkind,J.S. (1999) A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-Jun promoter: a role for c-Jun NH2-terminal kinase, p38s and extracellular signal-regulated kinase 5. Mol. Cell. Biol., 19, 4289–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathas S., Rickers,A., Bommert,K., Dörken,B. and Mapara,M.Y. (2000) Anti-CD20- and B-cell receptor-mediated apoptosis: evidence for shared intracellular signaling pathways. Cancer Res., 60, 7170–7176. [PubMed] [Google Scholar]
- Mechta-Grigoriou F., Gerald,D. and Yaniv,M. (2001) The mammalian Jun proteins: redundancy and specificity. Oncogene, 20, 2378–2389. [DOI] [PubMed] [Google Scholar]
- Mori N. et al. (2000) Constitutive activation of transcription factor AP-1 in primary adult T-cell leukemia cells. Blood, 95, 3915–3921. [PubMed] [Google Scholar]
- Olive M., Krylov,D., Echlin,D.R., Gardner,K., Taparowsky,E. and Vinson,C. (1997) A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J. Biol. Chem., 272, 18586–18594. [DOI] [PubMed] [Google Scholar]
- Passegué E., Jochum,W., Schorpp-Kistner,M., Möhle-Steinlein,U. and Wagner,E.F. (2001) Chronic myeloid leukemia with increased granulocyte progenitors in mice lacking JunB expression in the myeloid lineage. Cell, 104, 21–32. [DOI] [PubMed] [Google Scholar]
- Schreiber M., Kolbus,A., Piu,F., Szabowski,A., Möhle-Steinlein,U., Tian,J., Karin,M., Angel,P. and Wagner,E.F. (1999) Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev., 13, 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickart V.L., Raport,C.J., Godiska,R., Byers,M.G., Eddy,R.L., Shows,T.B. and Gray,P.W. (1994) Cloning of human and mouse EBI1, a lymphoid-specific G-protein-coupled receptor encoded on human chromosome 17q12-q21.2. Genomics, 23, 643–650. [DOI] [PubMed] [Google Scholar]
- Seol D.W., Chen,Q. and Zarnegar,R. (2000) Transcriptional activation of the hepatocyte growth factor receptor (c-met) gene by its ligand (hepatocyte growth factor) is mediated through AP-1. Oncogene, 19, 1132–1137. [DOI] [PubMed] [Google Scholar]
- Staudt L.M. (2000) The molecular and cellular origins of Hodgkin’s disease. J. Exp. Med., 191, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Angel,P., van Dam,H., Ponta,H., Herrlich,P., van der Eb,A. and Rahmsdorf,H.J. (1992) Ultraviolet-radiation induced c-Jun gene transcription: two AP-1 like binding sites mediate the response. Photochem. Photobiol., 55, 409–415. [DOI] [PubMed] [Google Scholar]
- Stein B., Baldwin,A.S.,Jr, Ballard,D.W., Greene,W.C., Angel,P. and Herrlich,P. (1993) Cross-coupling of the NF-κB p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J., 12, 3879–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabowski A., Maas-Szabowski,N., Andrecht,S., Kolbus,A., Schorpp-Kistner,M., Fusenig,N.E. and Angel,P. (2000) c-Jun and JunB antagonistically control cytokine-regulated mesenchymal–epidermal interaction in skin. Cell, 103, 745–755. [DOI] [PubMed] [Google Scholar]
- Teofili L. et al. (2001) Expression of the c-met proto-oncogene and its ligand, hepatocyte growth factor, in Hodgkin disease. Blood, 97, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Teramoto N., Pokrovskaja,K., Szekely,L., Polack,A., Yoshino,T., Akagi,T. and Klein,G. (1999) Expression of cyclin D2 and D3 in lymphoid lesions. Int. J. Cancer, 81, 543–550. [DOI] [PubMed] [Google Scholar]
- Thomas R.S., Tymms,M.J., McKinlay,L.H., Shannon,M.F., Seth,A. and Kola,I. (1997) ETS1, NFκB and AP1 synergistically transactivate the human GM-CSF promoter. Oncogene, 14, 2845–2855. [DOI] [PubMed] [Google Scholar]
- van Dam H., Wilhelm,D., Herr,I., Steffen,A., Herrlich,P. and Angel,P. (1995) ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-Jun induction in response to genotoxic agents. EMBO J., 14, 1798–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom R., Johnson,R.S. and Moore,C. (1999) c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J., 18, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Heidenreich,O., Kitajima,I., McGuire,K., Li,Q., Su,B. and Nerenberg,M. (1996) Constitutively activated JNK is associated with HTLV-1 mediated tumorigenesis. Oncogene, 13, 135–142. [PubMed] [Google Scholar]
- Yamaoka S., Courtois,G., Bessia,C., Whiteside,S.T., Weil,R., Agou,F., Kirk,H.E., Kay,R.J. and Israel,A. (1998) Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell, 93, 1231–1240. [DOI] [PubMed] [Google Scholar]