Abstract
Mouse chimeras from embryonic stem cells in which the X-linked glucose 6-phosphate dehydrogenase (G6PD) gene had been targeted were crossed with normal females. First-generation (F1) G6PD(+/–) heterozygotes born from this cross were essentially normal; analysis of their tissues demonstrated strong selection for cells with the targeted G6PD allele on the inactive X chromosome. When these F1 G6PD(+/–) females were bred to normal males, only normal G6PD mice were born, because: (i) hemizygous G6PD(–) male embryos died by E10.5 and their development was arrested from E7.5, the time of onset of blood circulation; (ii) heterozygous G6PD(+/–) females showed abnormalities from E8.5, and died by E11.5; and (iii) severe pathological changes were present in the placenta of both G6PD(–) and G6PD(+/–) embryos. Thus, G6PD is not indispensable for early embryo development; however, severe G6PD deficiency in the extraembryonic tissues (consequent on selective inactivation of the normal paternal G6PD allele) impairs the development of the placenta and causes death of the embryo. Most importantly, G6PD is indispensable for survival when the embryo is exposed to oxygen through its blood supply.
Keywords: G6PD deficiency/hemizygotes/heterozygotes/lethality/oxidative damage
Introduction
Genetic inactivation of an individual gene is a powerful tool for investigating a gene even when its function is totally unknown. On the other hand, when the gene encodes a known enzyme, genetic inactivation is still crucial for obtaining definitive evidence as to the physiological role of the respective metabolic pathway in a cell, in a tissue or in the entire organism. The glucose 6-phosphate dehydrogenase (G6PD) gene is a prototype housekeeping gene (Vulliamy et al., 1992; Kletzien et al., 1994) encod ing the first enzyme of the pentose phosphate pathway, an NADP-linked dehydrogenase (Luzzatto and Testa, 1978; Stryer, 1995). Therefore, G6PD helps to provide (i) pentose for nucleic acid synthesis and (ii) reductive potential in the form of NADPH (Stryer, 1995). G6PD deficiency is a common genetic abnormality in humans: it affects millions of people in many populations (Beutler, 1978; Luzzatto, 1998), as a result of Darwinian selection in areas of the world where malaria has been or still is endemic (Luzzatto and Notaro, 2001). The distinctive phenotype of subjects with G6PD deficiency consists of hemolytic anemia. In the majority of cases, this is triggered by exposure to oxidative agents, suggesting that the redox role of G6PD is paramount. Recent databases contain >130 different human G6PD mutants (Beutler and Vulliamy, 2002) displaying a spectrum of clinical severity, which can be correlated in some measure with the structural– functional consequences of the respective amino acid changes (Notaro et al., 2000). It is important to note that no large deletions or frameshift mutations have ever been observed: in other words, there is no known human null mutant, and some residual enzyme activity is found in all cases.
We have shown previously that targeted inactivation of the X-linked G6PD gene produces viable mouse embryonic stem (ES) cells, but these are exquisitely sensitive to oxidative stress (Pandolfi et al., 1995). We now show that severely G6PD-deficient male embryos [G6PD(–)] stop growing at E7.5, concomitantly with the development of blood circulation, supporting the notion that G6PD is incompatible with aerobic life: indeed, the embryos die at E10.5. In contrast, the fate of heterozygous female embryos depends on the source of the X chromosome with the G6PD(–) allele. If this X is maternal, the embryos die because of damage to the G6PD(–) placenta, in which the normal paternal X is selectively inactivated (Lyon and Rastan, 1984; Tan et al., 1993). Instead, if the G6PD(–) X chromosome in heterozygotes is paternal, the placenta is normal thanks to the normal maternal X, and the embryos survive to yield adult animals. In these animals, we report an interesting epigenetic phenomenon: namely, the phenotype is essentially normal, with normal G6PD activity in many tissues, as a result of somatic cell selection favoring the survival and growth of G6PD normal cells after X chromosome inactivation.
Results
Characterization of residual enzyme activity in G6PD ‘knock-out’ CJ7 ES cells
We have reported previously that ES cells in which G6PD was inactivated by homologous recombination were extremely sensitive to oxidative stress, but they were viable (Pandolfi et al., 1995). Before injecting these targeted ES cells, we carried out a karyotype analysis because we have shown that euploidy is predictive of germline transmission (Longo et al., 1997). As most of these ES cells had an abnormal karyotype, we decided to repeat the targeting procedure.
We have now used the same targeting vector to transfect the 129 Sv-derived CJ7 ES cells (Swiatek and Gridley, 1993) that are isogenic with the previously used AB1 ES cells (McMahon and Bradley, 1990). After double selection with G418 and gancyclovir, we have isolated several homologous recombinant ES cell clones: three of those with a normal 40, XY karyotype were characterized further (Table I).
Table I. Formation of embryoid bodies from G6PD(–) ES cells.
| Atmospheric O2 (∼20%) |
5% O2 |
|||||
|---|---|---|---|---|---|---|
| G6PD + | G6PD(–) | G6PD(–) | G6PD(–) | G6PD + | G6PD(–) | |
| ES cell clone (no. of experiments) | CJ7 (n = 4) | 110a (n = 1) | 135a (n = 1) | 302b (n = 3) | CJ7 (n = 4) | 302b (n = 4) |
| Embryoid bodies, n ± SD | 167 ± 67 | 3 | 0 | 4 ± 3 | 115 ± 13 | 33 ± 12 |
| EBs with hematopoietic differentiation, n (%) | 57 (34) | 1 (33) | 2 (50) | 45 (39) | 6 (18) | |
aG6PD(–) ES cell clones previously reported in Pandolfi et al. (1995).
bG6PD(–) ES cell clone produced in this study.
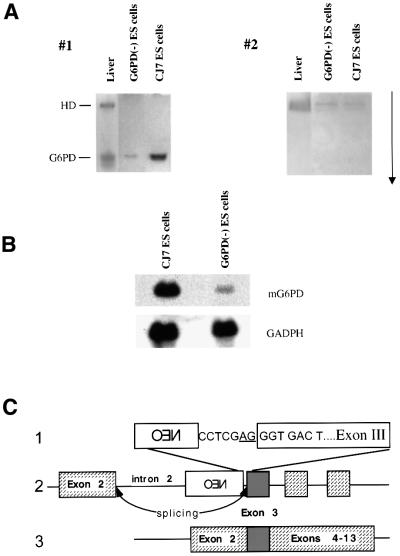
Spectrophotometric measurement of NADPH production (in the presence of glucose 6-phosphate; G6P) in three independent ES cell clones, even after 12 passages in the absence of feeder layer, revealed a level of ∼14% of normal (data not shown). To exclude the possibility of contamination by wild-type cells, we re-cloned the G6PD(–) ES cells by limiting dilution. In 37 clones in which the G6PD(–) allele was confirmed by PCR analysis, the G6PD activity ranged between 2 and 30% of normal. The two clones with the highest activity (nos 302.12 and 302.25) and the two with the lowest activity (nos 302.7 and 302.32) were subcloned. In 10 subclones, the activity varied within 5% of the respective parental clones; and over 3 weeks the activity fluctuated by <4% (data not shown). Thus, the residual G6PD activity was stable, and it did not arise from contamination by wild-type cells. We suspected that this ‘residual’ activity might be contributed by the autosomal enzyme, hexose dehydrogenase (HD): unlike G6PD, which is highly specific for G6P, HD has a broad substrate specificity. Spectrophotometric determinations of dehydrogenase activity with three sugars (glucose, galactose 6-phosphate and mannose 6-phosphate) other than G6P (data not shown) and acrylamide gel electrophoresis (which resolves HD from G6PD; Figure 1A) have indeed shown that some but not all of the ‘residual’ enzyme activity was due to HD; but some was definitely due to G6PD. Northern blot analysis, using as probe full-length human G6PD cDNA, revealed a 2.5 kb band, the size of normal G6PD mRNA. By normalization to a signal obtained with a GAPDH probe, we estimate that this mRNA was ∼5% of that in normal ES cells (Figure 1B). These data indicate that the G6PD(–) ES cells are still able to produce a low amount of G6PD. These results were in apparent contrast to the almost undetectable residual G6PD activity we found in our previous targeting study (Pandolfi et al., 1995). In order to explain this surprising result, we carried out DNA sequence analysis of the targeting vector, and we found a normal exon 3 at the 3′ end of the NEO cassette. The same result was obtained on a PCR-amplified fragment from G6PD(–) ES cells. This can be explained by the fact that after insertion of XhoI linkers, a splicing acceptor site has been artificially recreated 5′ of G6PD exon 3, immediately following the NEO cassette (Figure 1C). The same result was obtained on a PCR-amplified fragment from G6PD(–) ES cells. From this analysis, we have to conclude that although the NEO cassette is cloned within exon 3 as previously reported (Pandolfi et al., 1995), it is in fact positioned more proximally as a result of the insertion of the XhoI linkers (Figure 1C). We presume that the NEO cassette can be spliced out as a rare event during processing of the primary transcript. It is possible that the difference in G6PD expression between the AB1 G6PD-targeted ES cells and the CJ7 G6PD-targeted ES cells is due to chromosomal abnormalities present in the former, that could affect the splicing machinery. However, we cannot rule out that these differences are due to some unrelated difference in biology between AB1 and CJ7.
Fig. 1. Analysis of residual dehydrogenase activity in G6PD(–) ES cells. (A) Lysates from mouse liver, CJ7 and G6PD(–) ES cells were applied to a polyacrylamide gel and electrophoresis was carried out at 4°C and 40 W for 24 h. The arrow indicates the direction of migration. Gel 1 was stained with glucose 6-phosphate as substrate; gel 2 was stained with galactose 6-phosphate as substrate. The HD band is visible in gel 2 in both the wild-type and G6PD(–) ES cells. (B) Northern blot analysis of G6PD mRNA. By densitometry, the band in the G6PD(–) ES cells is estimated to be ∼15% of normal. (C) Diagram of the genomic murine G6PD locus after integration of the targeting vector (not drawn to scale). This structure of the targeting vector is revised from that shown in Figure 1 of the original description (Pandolfi et al., 1995). Exon 3 is highlighted in gray. (1) Junction between the NEO cassette and G6PD exon 3: an artificial splicing acceptor site is underlined upstream of exon 3. (2) Diagram of the primary transcript from the targeted G6PD. (3) Normal G6PD mRNA is produced at a low level because a splicing acceptor site has been recreated artificially by the insertion of an XhoI linker between the NEO cassette and exon 3.
Since this means that the ‘knock-out’ ES cells have a small but measurable G6PD activity, we will refer to them as severely G6PD-deficient ES cells, or G6PD(–), rather than G6PD null.
Generation of embryoid bodies from G6PD(–) ES cells
In order to assess the impact of the almost complete loss of G6PD activity on the developmental competence of ES cells, we tested their ability to form embryoid bodies (EBs) in vitro. G6PD(–) ES cells form very few EBs when compared with normal controls (Table I); however, the ability of those few EBs to undergo haematopoietic differentiation was unimpaired. In an environment in which the oxygen tension was lowered deliberately, the number of G6PD(–) EBs is markedly increased, indicating that the impaired EB formation results from oxidative damage (Table I).
Generation and characterization of G6PD-heterozygous mutant mice
Three euploid G6PD(–) ES cell clones (with 15, 3 and 2% residual enzyme activity) were injected into C57BL/6J mouse blastocysts. Chimeric mice were born, and germline transmission was obtained upon mating with normal females (Figure 2). Females heterozygous for the G6PD(–) allele were produced, as confirmed by Southern blot analysis (Table II and Figure 2B) and by PCR analysis (data not shown).

Fig. 2. Generation of G6PD-deficient mice from G6PD(–) ES cells. (A) Design of the genetic crosses. *X indicates the X chromosome bearing the knock-out allele. Note that in the F1 heterozygotes, this is paternal (*XP); whereas in the F2 heterozygotes it is maternal (*XM). (B) Southern blot analysis (after DNA digestion with HpaI; Pandolfi et al., 1995) is diagnostic of the heterozygotes genotype [G6PD(+/–)] in the right lane by comparison in the other two lanes with normal CJ7 ES cells and with G6PD(–) ES cells, respectively.
Table II. Germline transmission of the G6PD(–) allele.
| F × M controls | F control × M chimeric (F1) | F F1 G6PD (+/–) × M control (F2) | |
|---|---|---|---|
| Litters analyzed, n | 77 | 45 | 32 |
| Litter size, mean ± SD | 7.6 ± 1.5 | 7.3 ± 1.3 | 4 ± 1.4 |
| Sex ratio (F/M) | 1.06 | 1.21 | 1.15 |
| F G6PD (+/+), n | 300 | 76 | 69 |
| M G6PD (+), n | 283 | 148 | 60 |
| F G6PD (+/–), n | NE | 103 | 0 |
| M G6PD (–), n | NE | NE | 0 |
F, female; M, male.
NE, not expected.
Since the G6PD gene is X linked, the heterozygous mice are expected to exhibit somatic cell mosaicism with respect to G6PD expression as a result of X chromosome inactivation. To investigate this phenomenon, we applied a G6PD-specific histochemical staining method to frozen sections of intestinal epithelium. Since each intestinal crypt grows from a single stem cell (Griffiths et al., 1988; Campbell et al., 1994), we found indeed that in the heterozygous mice individual crypts either were severely deficient in G6PD activity or they were just as intensely stained as in control animals (Figure 3A). We next analyzed individual peripheral red blood cells by a similar cytochemical test. Each cell with sufficient G6PD activity produces one or more discrete granules of formazan within 5 min of staining; accordingly, individual cells can be scored as positive or negative for G6PD activity. In 10 normal animals, negative red blood cells ranged between 0 and 0.7%. In 12 G6PD(+/–) heterozygotes, the majority of red cells were still positive, but some had no detectable formazan granules; negative cells ranged between 0.9 and 4.2% (Figure 3B and C). Although this is a significant number of G6PD-deficient blood red cells, their proportion is too low to cause any significant decrease in the overall G6PD activity as measured in a standard assay on a red blood cell lysate of these G6PD(+/–) heterozygotes, which is therefore similar to that of normal female mice (Figure 4, left panel). Thus, mosaicism in heterozygous mice can also be demonstrated in blood; but it is clear that somatic cell selection against G6PD(–) cells operates strongly in the hematopoietic tissue.
Fig. 3. Histochemical staining for G6PD activity in cells from F1 heterozygotes. (A) Large intestine from first-generation heterozygotes and wild-type female littermates. The two top sections of this panel are negative controls stained without the substrate G6P. Mosaicism in G6PD expression is evident in heterozygotes. Similar results have been obtained in the small intestine. (B) Cytochemical analysis of peripheral blood red cells in a heterozygous mouse shows a small but significant proportion of G6PD-deficient red cells. (C) Scattergram of quantitative cytochemical analysis carried out on peripheral blood red cells: there is a statistically significant difference between heterozygotes and wild-type mice (Fisher’s exact test, P < 0.00001).
Fig. 4. Quantitative analysis of G6PD expression in F1 heterozygotes in various tissues. Adr, adrenal glands.
In order to estimate the relative contribution of normal cells and of G6PD(–) cells to the formation of tissues other than blood in heterozygotes, we carried out quantitative assays of G6PD activity in seven different tissues. Although these animals have only one functional X chromosome, the activity levels in each tissue were in the same range as in control female mice (Figure 4, right panel). The most likely explanation of this result is that, due to strong selection against the mutant cells early in organogenesis and in hematopoiesis, it is G6PD normal cells that contribute most to the formation of most tissues.
Characterization of the offspring of G6PD heterozygous mutant mice
When the first-generation heterozygous female mice, F1 G6PD(+/–), were crossed with wild-type males (Figure 2A), the average litter size at birth was about half that of controls. The sex ratio was normal. Genotyping of the newborn mice revealed only G6PD(+) males and G6PD(+/+) females (Table II). These data suggested that both males hemizygous for G6PD deficiency, G6PD(–), and F2 females heterozygous for G6PD deficiency, F2 G6PD(+/–), had died in utero. Indeed, DNA analysis of the embryos at days E6.5 and E7.5 demonstrated the presence of wild-type embryos, heterozygous G6PD(+/–) female embryos and hemizygous G6PD(–) male embryos in Mendelian ratios (Table III).
Table III. Identification of living G6PD(+/–) and G6PD(–) embryos during development.
| d.p.c. | Litter (n) | Litter size | Sex ratio (F/M) | G6PD (+/–) (%) | G6PD (–) (%) |
|---|---|---|---|---|---|
| 6.5 | 4 | 7.3 ± 1.8 | 12/17 | 24 | 17 |
| 7.5 | 7 | 8.2 ± 1.2 | 30/28 | 17 | 22 |
| 8.5 | 6 | 7.8 ± 1.4 | 20/27 | 19 | 23 |
| 9.5 | 5 | 8.8 ± 1.7 | 21/23 | 23 | 18 |
| 10.5 | 5 | 7.4 ± 1.3 | 17/20 | 19 | 27 |
| 11.5 | 4 | 8.5 ± 1.2 | 18/16 | 26 | 26 |
| 12.5 | 3 | 5 ± 1.7 | 9/6 | – | – |
d.p.c., days post-coitus.
G6PD(–) hemizygous embryos. Until E6.5, the G6PD(–) embryos are undistinguishable in appearance from their littermates. At E7.5, some G6PD(–) embryos were smaller but they continued to develop, producing mesoderm and head folds. By PECAM (CD31) protein expression, we determined that the G6PD(–) embryos undergo vasculogenesis; and timely erythropoiesis was detected by benzidine staining (data not shown). There was no significant difference in the histochemical expression of the cell proliferation marker Ki-67; on the other hand, cell death was somewhat accelerated in the G6PD(–) embryos, as assessed by the TUNEL reaction (data not shown). By E8.5, the wild-type embryos differentiate somites (Figure 5A); organogenesis is initiated and the heart and the central nervous system develop (Figures 5A and 6A). The embryos undergo major spatial rearrangements in a precisely controlled manner, such that the body shape changes from S-like to C-like (described by the term ‘embryo turning’). In strong contrast, the G6PD(–) embryos fail to turn and to acquire fetal position, and essentially stop developing [compare G6PD(–) with G6PD(+/+) in Figure 5]. These embryos do initiate organogenesis but they form an aberrant head and distended heart, with onset of heartbeat (Figures 5E and 6I). Typical somites could not be identified. In histological sections, the neuroepithelium was thin and wavy, and embryonic tissues generally contained sparse cells (Figures 6H and I, and 7H and I). Large regions within the embryo were occupied by dilated blood vessels and there was evidence of hemorrhage (Figure 7I). Widespread cell death was detected by TUNEL staining (Figure 6I and J). As the lesions in the G6PD(–) embryos become increasingly severe, by E10.5 most embryos are dead; they gradually disintegrate thereafter (Figure 5H and K).
Fig. 5. Different deadly impacts of the G6PD(–) allele on the development of G6PD(–) and G6PD(+/–) embryos in the F2 generation. Embryos from mating of F1 G6PD(+/–) females with wild-type males (see Figure 2) were dissected, and examples of all genotypes (indicated at the top) are shown. The d.p.c. are shown on the left. It is seen that G6PD(–) embryos (C, E, H and K) arrest at around E8, fail to turn and then die. The asterisk in (E) indicates the heart. On the other hand, G6PD(+/–) embryos exhibit abnormalities as early as E8.5, but they continue to grow until E11.5 (compare B, G and J with A, F and I, respectively). Magnification: (A–C) 5×; (D and E) 2.5×; (F–K) 1.25×.
Fig. 6. Histological analysis of E8.5 mutants demonstrates major damage in the central nervous sytem and heart. All sections are from F2 embryos at E8.5; asterisks in (A), (B), (C), (F) and (I) indicate the heart; long arrows in (A), (C), (D), (E), (H) and (I) point to the neuroepithelium. Short arrows in (F) and (I) indicate the areas magnified in the corresponding insets. Sections from G6PD(–) hemizygous embryos confirm a major defect in the neuroepithelium (compare H with A), which is associated with extensive apoptosis (J). Apoptosis is also seen in the heart (compare I with B). Similar but less severe changes are seen in heterozygous G6PD(+/–) embryos (C–G). (A), (C), (D), (E) and (H) are stained with H&E. (B), (F), (G), (I) and (J) show TUNEL staining. Magnification: (A), (C), (D) and (E) 10×; (B), (F), (H) and (I) 20×; (G) and (J) and insets in (B), (F) and (I) 100×.
Fig. 7. Progression of pathological alterations eventually leads to necrosis in all mutant embryos. H&E-stained sections of E10.5 (A, D, E, H and I) and E11.5 (B, C, F and G) embryos. The G6PD(–) hemizygous embryo in (H) is nearly reabsorbed; at higher magnification (I), one sees markedly dilated blood vessels and hemorrhages in the mesenchyme of the head. (D) and (E) (compared with A) demonstrate the variable extent of tissue damage in heterozygous embryos (more severe in E than in D). At E11.5, the damage is already substantial (compare F with B), with extensive lysis of tissue (compare G with C). Magnification: (A), (B), (D), (E), (F) and (H) 2.5×; (I) 10×; (C) and (G) 40×.
F2 G6PD(+/–) embryos. Since the first-generation heterozygous mice, F1 G6PD(+/–), were healthy and fertile, it was not expected that no heterozygous mice would be born in the second generation [F2 G6PD(+/–)]. By E8.5, some of these embryos were still undistinguishable from controls in whole-mount preparations (Figure 5B); others, however, were already delayed in development. All heterozygote embryos generate somites and undergo turning, unlike their G6PD(–) littermates (Figure 5). Histological studies (including TUNEL staining) revealed at this time various degrees of deviation from normal development (Figures 6 and 7); and even embryos that looked normal in whole mounts (e.g. Figure 5B) showed abnormalities on sections (Figure 6C and D). The neuroepithelium and the mesenchyme have sparse cells and dilated blood vessels, and cell death was more pronounced than in controls (compare Figure 6F and G with B). Specifically, in sharp contrast to normal littermates (Figure 6B), F2 G6PD(+/–) embryos had apoptotic cells in the heart (Figure 6F), as also seen in the G6PD(–) male embryos (Figure 6I). Tissue damage becomes more pronounced as development progresses (Figure 7D–G). Nevertheless, unlike the G6PD(–) embryos, the F2 G6PG(+/–) embryos increase in size (Figures 5 and 7, for comparison), and in most cases they remain alive until E11.5. By that time, there are many necrotic cells in the neuroepithelium of the brain (Figure 7F and G) and in the spinal cord. Lysis of the tissue is also significant in the head and trunk mesenchyme. There are widespread hemorrhages in all organs. The F2 G6PD(+/–) embryos died between E11.5 and E12.5.
Development of the placenta in G6PD(–) and G6PD(+/–) embryos
By conventional histology, no significant alterations were observed up to E7.5, and by E8.5, mutant embryos showed timely fusion of the allantois with the chorion, just as in wild-type littermates (data not shown). However, at day E8.5, significant numbers of apoptotic cells were seen by TUNEL staining in the allantois of all mutant embryos, G6PD(–) as well as F2 G6PD(+/–) (Figure 8). These apoptotic cells were found predominantly in the region of fusion of the allantois that is in close proximity to the chorion (Figure 8C–F). Since this cell death pattern was in sharp contrast to the healthy allantois of the F1 G6PD(+/–) embryos (Figure 8A), we suspected that the G6PD(–) trophoblast fails to supply factors that are required for survival and differentation of the allantois: cell death within the allantois perturbs its function in the differentiation of the placenta.
Fig. 8. Severe abnormalities of the placenta are similar in F2 G6PD(–) and G6PD(+/–) embryos. At day 8.5, TUNEL staining demonstrates widespread apoptosis in allantoic cells (arrows) in (C) and (E) (compare with A). Magnification: (A), (C) and (E) 20×; (B), (D) and (F) 100×. At day 9.5, the development of the placenta is obviously markedly impaired (see H and I compared with G; magnification 10×). The sections were stained with an anti-CD31 antibody.
Normally, around E9, a connection between fetal and maternal blood circulation is established through the vitelline blood vessels. At this stage, allantoic blood vessels abundantly and deeply invade the chorion in wild-type embryos (Figure 8G), as well as in F1 G6PD(+/–) (data not shown). Thus, the labyrinthine layer of the placenta is formed, with the spongiotrophoblast overlying it (Tanaka et al., 1998). In contrast, at E8.5, the chorion of the G6PD(–) and F2 G6PD(+/–) embryos is organized as a chorionic plate and lacks fetal blood vessels (Figure 8C and E). By E9.5, although some blood vessels have invaded the chorion, both the labyrinthine and the spongiotrophoblast layers remain significantly underdeveloped. By analysis of serially sectioned embryos (compare Figure 8G with H and I), we determined that by E9.5 the placenta, in both G6PD(–) and F2 G6PD(+/–) embryos, is less than half the normal size. These pathological changes in the placenta are severe enough to explain the death of the F2 G6PD(+/–) embryos.
Discussion
Human G6PD deficiency is usually asymptomatic or has a conditional phenotype such as fava bean-induced hemolytic anemia (Beutler, 1995). However, a subset of patients have chronic hemolytic anemia (Mason et al., 1995). Although it has been claimed in the past that some of these patients have ‘complete’ absence of this enzyme (Escobar et al., 1964), residual G6PD activity is always detectable in these patients by sensitive assays (Luzzatto, 1975). Currently, from a database of >130 human mutants, it is apparent that all of them are either missense or small in-frame deletions (Beutler and Vulliamy, 2002), although a single instance of a downstream nonsense mutation has been reported (Xu et al., 1995). Moreover, two-thirds of these mutations are in amino acid residues that are highly and moderately conserved in the course of evolution, whereas relatively few are in fully conserved amino acid residues (Notaro et al., 2000). Thus, nature’s experiments point clearly to the notion that complete G6PD deficiency is lethal for a mammalian organism. We show now that G6PD inactivation is still compatible with embryo formation; however, this biochemical defect becomes lethal at a precise stage in embryonic development.
G6PD inactivation in ES cells
We have confirmed that our targeting construct causes inactivation of the G6PD gene. However, the G6PD(–) ES cells are capable of some G6P-dependent NADPH production. This activity is accounted for in part by HD, and in part by true G6PD activity, resulting from alternative splicing of the G6PD transcript. Since we obtained ES cell clones with as little as 2% G6PD (including HD) activity, it is clear that alternative splicing produces only a minute amount of G6PD protein. This leakiness in our knock-out did not prevent the severe phenotypic changes that we have described in both ES cells and embryos.
In control ES cells, we estimate that >95% of the total G6P-dependent NADPH production can be attributed to G6PD, and only <5% to HD (data not shown). Therefore, we can surmise that shortage of G6PD activity will become critical for survival or growth in any kind of cell for either or both of the following reasons: (i) the cell does not express HD; or (ii) the cell is exposed to conditions in which the HD activity is insufficient. In the case of ES cells, it is clear that the residual G6PD activity, together with the existing HD activity, is sufficient for survival and growth; but it becomes limiting when the cells are exposed to an oxidative stress, or when they are diluted to the low density required for cloning, whether in their original undifferentiated state or in EBs. We presume that high dilution entails greater exposure to oxygen, because decreasing oxygen tension has a protective effect (Table II).
Highly skewed X-inactivation in viable G6PD(–) heterozygotes
It is probably the rule rather than the exception for ‘knock-out’ heterozygotes to be viable, even with indispensable genes. The fact that F1 G6PD(+/–) heterozygotes are viable has helped us in two ways: (i) these animals can be used to produce hemizygous G6PD(–) embryos; and (ii) we can assess for the first time the impact of G6PD inactivation on the fate of different types of somatic cells. The most important finding that emerges from this analysis is that, once X-inactivation has produced mosaicism for the G6PD cellular phenotype, there is a strong selection against the G6PD(–) cells. In fact, somatic cell selection in these animals is highly reminiscent of what happens in women who are heterozygous for certain severe human G6PD mutations (Filosa et al., 1996). However, in these women, drastic selection is essentially confined to blood cells, consistent with the notion that, since the main mechanism of G6PD deficiency in humans is instability of the protein, it never becomes extreme in cells that are competent for protein synthesis (Mason, 1996). In contrast, our targeting vector has produced cells in which G6PD deficiency is caused by impaired synthesis of functional G6PD, and therefore cell selection is much more widespread (Figure 4). We have not studied systematically at what stage cell selection takes place. However, since hemizygous G6PD(–) embryos are already quite abnormal by E8.5, we presume that selection takes place quite early, either before or with the onset of organogenesis. Nevertheless, some G6PD(–) stem cells persist in the G6PD(+/–) mice, as we have shown in the blood and the intestine (Figure 3).
G6PD(–) hematopoiesis
Apart from clarifying the physiological role of G6PD, one of the motives for producing a G6PD(–) animal was to develop a model of human G6PD deficiency, a condition in which hemolytic anemia is the main clinical manifestation. Therefore, obtaining G6PD(–) red cells is especially relevant. We have attained this goal in three ways. (i) EBs from G6PD(–) ES cells can undergo hematopoietic differentiation, and the red blood cells they produce are G6PD(–). (ii) G6PD(–) hemizygous embryos have reached the stage of yolk sac hematopoiesis, and the red blood cells circulating in these embryos are also G6PD(–). (iii) First-generation heterozygous adults have a small but measurable proportion of G6PD(–) red cells circulating in their peripheral blood. Thus, G6PD(–) hematopoietic stem cells are viable.
The G6PD(–) allele is an embryonic lethal
It has been shown previously that the litter size is reduced in mice slightly deficient in G6PD activity (Nicol et al., 2000). In keeping with this, when the first-generation heterozygous female mice, F1 G6PD(+/–), were crossed with wild-type males (Figure 2A), the average litter size at birth was about half that in controls (Table II). However, it was surprising that in our model the sex ratio was normal and that all newborn mice were G6PD normal (Table II). These data indicated that in the F2 generation, severe G6PD deficiency causes the demise of both male embryos hemizygous for G6PD deficiency and female embryos heterozygous for G6PD deficiency. During early gestation, the genotypes of the offspring of G6PD heterozygotes were in Mendelian ratios in both sexes (Table III), indicating that the initial development of all embryos was unimpaired. In fact, the embryos appear essentially normal until E7.5. Since the early development of the G6PD(–) embryos is normal, G6PD is not required for cell viability in vivo [just as in unchallenged G6PD(–) ES cells in vitro]. At E8.5, however, the G6PD(–) hemizygous embryos rather abruptly stop growing and show severe abnormalities. Glycolysis is the major metabolic pathway in the embryo before the placenta develops and blood circulation is established (Cox and Gunberg, 1972; Clough and Whittingham, 1983; Ellington, 1987). Embryos homozygous for a null allele of glucose phosphate isomerase (GPI) (Kelly and West, 1996), which have a block in glycolysis, arrest at E7.5, presumably having survived until then thanks to the pentose phosphate pathway. In contrast, the metabolic defect in embryos null for G6PD strikes somewhat later. Indeed, these embryos initiate erythropoiesis at the appropriate time, and they progress to angiogenesis; however, with the establishment of blood circulation, they arrest and begin to deteriorate, presumably because, with the onset of aerobic metabolism, oxygen radicals cause widespread tissue damage. Interestingly, rat embryos cultured in excess glucose fail to turn (Ellington, 1997), just like G6PD(–) embryos.
A maternal G6PD(+) allele is required in the trophoblast for placenta development
Since the first-generation heterozygous mice, F1 G6PD(+/–), were healthy and fertile, it was not immediately obvious why no heterozygous mice (that have the same genotype as their mothers) were born in the second generation. An important difference between the F1 and F2 G6PD(+/–) mice is that in the former the G6PD(–) allele is on the paternal X chromosome [we can therefore designate them as F1 G6PD(+/–)*XP]; whereas in the latter the G6PD(–) allele is on the maternal X chromosome [we can therefore designate them as F2 G6PD(+/–)*XM] (Figure 2). Epigenetic imprinting at the G6PD locus might explain why the first-generation heterozygotes were completely normal, whereas the second-generation heterozygotes died in utero. Indeed, it is well known that in the mouse tropho-ectoderm and in the primitive endoderm, X chromosome inactivation is non-random, whereby the paternal X chromosome is inactivated selectively (Frels and Chapman, 1980; Lyon and Rastan, 1984; Krumlauf et al., 1986; Tan et al., 1993; Goto and Monk, 1998). The XM chromosome has a normal G6PD allele in the first generation of heterozygous embryos; the trophoblast will have normal G6PD activity. In contrast, in the second generation, the XM chromosome has a G6PD(–) allele, and as a result the trophoblast will have no G6PD activity. Trophoblast derivatives give rise to the chorion and to the ectoplacental cone, which gradually develop into the labyrinthine layer and part of the spongiotrophoblast layer of the fetal placenta (Guillemot et al., 1994, 1995; Tanaka et al., 1998; Kaufman et al., 1999).
The fact that the fetal placenta of the F2 G6PD(+/–)*XM and the G6PD(–) embryos have similar defects provides novel direct evidence that the consequences of a genetic and of an epigenetic defect are similar. Although the allantois has fused with the chorion, both the labyrinthine and the spongiotrophoblast layers of the fetal placenta in the mutants were significantly underdeveloped. At E9.5, the placenta in both F2 G6PD(+/–)*XM and G6PD(–) embryos, compared with that of the G6PD wild-type embryos, is less than half the thickness. Therefore, it is not surprising that the F2 G6PD(+/–)*XM embryos will not progress in development, once the function of the placenta is required. The defective placenta will not allow the establishment of a normal connection of the embryos with the maternal circulation; thus, nutrient supply and waste disposal will be impaired. From this point of view, the heterozygous female embryo finds itself just as handicapped as the hemizygous male embryo. However, the development of the heterozygous embryos progresses somewhat further, presumably because, unlike in hemizygotes, the cells of the embryo itself have one normal G6PD allele.
In conclusion, we have defined the consequences of genetic inactivation of an X-linked housekeeping gene that has a major role in redox metabolism. It is interesting that naturally occurring G6PD(–) mutants, extensively characterized in human patients, give a phenotype almost exclusively confined to erythrocytes, which undergo premature destruction (hemolysis) (Dern et al., 1954; Luzzatto et al., 2001). It seems now clear that this results from the fact that even the most severe of these human mutants have enzyme deficiency because of instability of the G6PD protein (Morelli et al., 1978; Naylor et al., 1996; Roos et al., 1999); and there is enough residual enzyme activity to satisfy the requirements of cells other than erythrocytes. In contrast, we have now shown that when severe G6PD deficiency is produced by targeting the G6PD gene in a way that drastically reduces G6PD biosynthesis, the effects are devastating for the embryo, from the time that cells are exposed to oxygen from circulating blood. In hemizygous G6PD(–) males, G6PD deficiency directly damages the embryo, effectively arresting its development. In heterozygous F2 G6PD(+/–) females, the damage to the embryo is indirect, i.e. secondary to an impaired development of the placenta. These findings indicate that the role of G6PD is quite basic in mammalian development.
Materials and methods
ES culture and transfection
The 129 Sv-derived CJ7 ES cell line (Swiatek and Gridley, 1993) was cultured on a monolayer of mitomycin-treated primary embryonic fibroblasts in the presence of 1000 U/ml of leukemia inhibitory factor (LIF; Gibco-BRL, Gaithersburg, MD) as previously described (Rosti et al., 1997). ES cells have been transfected by electroporation with the targeting vector we have described previously (Pandolfi et al., 1995). After 6 days of double selection with 400 µg/ml G418 (Geneticin, Sigma, St Louis, MO) and 2 µM gancyclovir (Cytovene®, Syntex Inc., Palo Alto, CA), several individual ES cell clones were isolated and expanded. Homologous recombination in the g6pd locus was assessed by Southern blot analysis as we have described previously (Pandolfi et al., 1995).
Assay for G6PD activity
ES cells were harvested from tissue culture dishes by trypsinization and washed in phosphate-buffered saline (PBS). Cell extracts were prepared by four cycles of flash-freezing–thawing cell pellets in G6PD lysis buffer (10 mM Tris–HCl pH 7.4, 1 mM EDTA, 1 µM EACA, 10 mM NaCl, 3 mM MgCl2, 20 µM NADP). Cell extracts were cleared by centrifugation and were assayed spectrophotometrically for G6PD activity as previously described (De Angioletti et al., 2001). The measured rate was converted into IU, and divided by the amount of protein measured with the Bradford method (Bio-Rad Protein Assay; Bio-Rad Laboratories, Hercules, CA).
In vitro differentation of ES cells; primary culture for embryoid body formation and hematopoietic differentiation
EB formation was obtained using the methylcellulose assay (Rosti et al., 1997). A single-cell suspension was obtained from subconfluent and undifferentiated ES cells. ES cells were plated at a density of 6 × 103 cells/ml in 1 ml of 0.9% (w/v) methylcellulose in IMDM medium (Stem Cell Techonologies Inc.) supplemented with 15% fetal bovine serum (HyClone), 450 mM monothioglycerolin (Gibco-BRL) and 3 IU of recombinant human erythropoeitin (Ortho Diagnostic Systems Inc., Raritan, NJ). Each assay was performed in duplicate. After 7–10 days incubation at 37°C in a fully humidified atmosphere supplemented with 5% CO2, the number of EBs formed was scored using an inverted microscope. The same experiments have been repeated in the presence of low oxygen tension (5% O2, 5% CO2, 90% N2). Hematopoietic differentiation within the EBs was assessed according to the presence of hemoglobinized cells and/or the appearance of invading macrophages. The identity of these cells was confirmed by light microscopy of May–Grünwald–Giemsa-stained cytospin preparations obtained from individual EBs.
Generation of G6PD(–) chimeric and heterozygous mice
Homologous recombined ES cell clones showing normal 40, XY karyotype (Longo et al., 1997) were used for the production of chimeric mice. Five to ten G6PD(–) ES cells were injected into C57BL/6J blastocysts obtained from females 3.5 days post-coitus (d.p.c.). After injection, blastocysts were transferred into pseudo-pregnant (CBA × C57BL/6J) F1 mice. Coat chimerism (percentage of agouti coat color) was estimated at the age of 2 weeks. All animal procedures were in compliance with the NIH Guide for the Care and Use of Laboratory Animals. G6PD heterozygous mutants have been obtained by crossing germline transmitter chimeric males with C57BL/6J females.
Histochemical localization of G6PD activity on fresh frozen sections
Heterozygous mice were killed by CO2 inhalation. The bowels or the embryos in decidua were removed and frozen in isopentane cooled to –70°C with dry ice. G6PD activity was demonstrated in 6 µm sections by incubation in medium containing 5 mM nitroblue tetrazolium, 2 mM NADP, 5 mM G6P, 4 mM MgCl2, 5 mM NaN3, 0.32 mM phenazine methosulfate in 0.05 M Tris–HCl buffer at pH 7.4 for 30 min to 1 h at 37°C. After post-fixation in 4% formol saline and washing in PBS, sections were mounted in Permount gel. The specificity of the reaction was confirmed by the use of control sections incubated in medium devoid either of G6P alone or of both G6P and NADP (Griffiths et al., 1988).
Cytochemical test for G6PD deficiency in red blood cells
Blood from heterozygous mice was collected by eye puncture under anesthesia. Blood was centrifuged at 4°C for 20 min at 1200–1500 g. The supernatant was discarded and 50 µl of the packed red cells were added to 900 µl of 9 g/l NaCl and 50 µl of sodium nitrite solution, and incubated at 37°C for 20 min. The suspensions was centrifuged at 4°C for 15 min at 500 g, and the supernatant fluid was discarded without disturbing the buffy coat and uppermost layer of red cells. Cells were washed three times in cold saline. After the last wash, the buffy coat was removed, the packed cells were mixed well and 50 µl were transferred to a tube containing 1 ml of the incubation medium (4 ml of 9 g/l NaCl, 1 ml of 50 g/l glucose, 2 ml of 0.3 mol/l phosphate buffer pH 7.0, 1 ml of 0.11 g/l Nile Blue sulfate, 2 ml of water). The suspension was incubated undisturbed at 37°C for 30 min. After that, 0.2 ml of MTT tetrazolium solution [5 g/l of 3-(4,5-dimethyl-thiazolyl-1-2)-2,5-diphenyltetrazolium bromide in 9 g/l NaCl] was added, gently mixed and incubated at 37°C for 1 h. Cells were resuspended thoroughly and one drop was placed on a glass slide adjacent to one drop of hypotonic saline (6 g/l NaCl) and covered with a cover glass. Red cells were examined with an oil immersion objective noting the presence of formazan granules (Van Noorden and Vogels, 1985).
Analysis of the embryos at successive stages
Pregnant heterozygotes were sacrificed at successive stages from E6.5 to E12.5. Embryonic stage was determined according to Downs and Davies (1993). Embryos were dissected in Hank’s balanced salt solution (HBSS) and fixed overnight in 4% paraformaldehyde. After fixation, embryos were dehydrated and embedded in paraffin, and 8 µm serial sections were prepared according to the standard procedure and stained with hematoxylin and eosin (H&E). For immunohistochemistry, slides were deparaffinized, quenched in 1% H2O2 and washed in PBS. Tissue antigens were unmasked and sections were incubated with either monoclonal anti-mouse Ki67 antibody (NCL-Ki67-MM1; Novocastra Laboratories, Newcastle, UK) or monoclonal anti-mouse PECAM-1 (CD31) antibody (MEC 13.3; PharMingen), followed by incubation with appropriate biotinylated secondary antibodies (Lyden et al., 1999). For detection of apoptotic cells, tissues fixed in 4% paraformaldehyde were processed for the TUNEL reaction where biotinylated dUTP is added to DNA double-strand breaks using terminal transferase as previously described (Kissel et al., 2000).
Genotyping by PCR
Genomic DNA was extracted from individual yolk sacs. Genotyping was also carried out on a few paraffin sections from each embryo set aside before or after staining. PCR was performed using different sets of primers. Sex was established using a pair of primers amplifying the SRY gene located on the Y chromosome (SRY1, GAGAGCATGGAGG GCCAT; SRY2, CCACTCCTCTGTGACACT). Mutant embryos were established using one primer inside the genomic region of the G6PD gene and the other primer inside the NEO cassette (G6PD-6, ATCTT GAACCCAGCAGTGTCA; NEO 3′-end, CCTTCTTGACGAGTTCT TCTG). Control primers were used to double-check all DNAs and particularly those not amplified by the other two sets of primers (MZF-7, CTGCGCACGAAGCCCTGGCCA; MZF-13, TGCCTCACCAACCAG TTCCAA). For the three sets of primers, the conditions of PCR were identical: 35 cycles, 94°C for 1 min, 56°C for 1.5 min and 72°C for 1.5 min.
Acknowledgments
Acknowledgements
We are extremely grateful to D.Araten, M.De Angioletti, C.Fein-Levy, A.Karadimitris, K.Nafa, A.Rovira and D.Tabarini for much support and advice. This work was supported by MSKCC and by grants HL 59312, HL 56678 and HL57612 from the National Institutes of Health.
References
- Beutler E. (1978) Glucose 6-phosphate dehydrogenase deficiency. In Beutler,E. (ed.), Hemolytic Anemia in Disorders of Red Cell Metabolism. Plenum Medical Book Co., New York, NY, pp. 23–167.
- Beutler E. (1991) Glucose 6-phosphate dehydrogenase deficiency. N. Engl. J. Med., 324, 169–174. [DOI] [PubMed] [Google Scholar]
- Beutler E. (1995) Glucose 6-Phosphate Dehydrogenase Deficiency. McGraw-Hill, New York, NY.
- Beutler E. and Vulliamy,T. (2002) Hematologically important mutations: glucose 6-phosphate dehydrogenase. Blood Cells Mol. Dis., 28, 93–103. [DOI] [PubMed] [Google Scholar]
- Campbell F., Fuller,C.E., Williams,G.T. and Williams,E.D. (1994) Human colonic stem cell mutation frequency with and without irradiation. J. Pathol., 174, 175–182. [DOI] [PubMed] [Google Scholar]
- Clough J.R. and Whittingham,D.G. (1983) Metabolism of [14C]glucose by postimplantation mouse embryos in vitro. J. Embryol. Exp. Morphol., 74, 133–142. [PubMed] [Google Scholar]
- Cox S.J. and Gunberg,D.L. (1972) Metabolite utilization by isolated embryonic rat hearts in vitro. J. Embryol. Exp. Morphol., 28, 235–245. [PubMed] [Google Scholar]
- De Angioletti M., Rovira,A., Notaro,R., Camacho Vanegas,O., Sadelain,M. and Luzzatto,L. (2001) Glucose 6-phosphate dehydrogenase expression is less prone to variegation when driven by its own promoter. Gene, 267, 221–231. [DOI] [PubMed] [Google Scholar]
- Dern R.J., Beutler,E. and Alving,A.S. (1954) The hemolytic effect of primaquine II. The natural course of the hemolytic anemia and the mechanism of its self-limiting character. J. Lab. Clin. Med., 44, 171–175. [PubMed] [Google Scholar]
- Downs K.M. and Davies,T. (1993) Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development, 118, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Ellington S.K. (1987) In vitro analysis of glucose metabolism and embryonic growth in postimplantation rat embryos. Development, 100, 431–439. [DOI] [PubMed] [Google Scholar]
- Ellington S.K. (1997) Effects of excess glucose on mammalian post-implantation embryos. Int. J. Dev. Biol., 41, 299–306. [PubMed] [Google Scholar]
- Escobar M.A., Heller,P. and Trobaugh,F.E. (1964) ‘Complete’ erythrocyte glucose-6-phosphate dehydrogenase deficiency. Arch. Intern. Med., 113, 428–434. [DOI] [PubMed] [Google Scholar]
- Filosa S., Giacometti,N., Wangwei,C., De Mattia,D., Pagnini,D., Alfinito,F., Schettini,F., Luzzatto,L. and Martini,G. (1996) Somatic cell selection is a major determinant of the blood cell phenotype in heterozygotes for glucose 6-phosphate dehydrogenase mutations causing severe enzyme deficiency. Am. J. Hum. Genet., 59, 887–895. [PMC free article] [PubMed] [Google Scholar]
- Frels W.I. and Chapman,V.M. (1980) Expression of the maternally derived X chromosome in the mural trophoblast of the mouse. J. Embryol. Exp. Morphol., 56, 179–190. [PubMed] [Google Scholar]
- Goto T. and Monk,M. (1998) Regulation of X-chromosome inactivation in development in mice and humans. Microbiol. Mol. Biol. Rev., 62, 362–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D.F., Davies,S.J., Williams,D., Williams,G.T. and Williams,E.D. (1988) Demonstration of somatic mutation and colonic crypt clonality by X-linked enzyme histochemistry. Nature, 333, 461–463. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Nagy,A., Auerbach,A., Rossant,J. and Joyner,A.L. (1994) Essential role of Mash-2 in extraembryonic development. Nature, 371, 333–336. [DOI] [PubMed] [Google Scholar]
- Guillemot F. et al. (1995) Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat. Genet., 9, 235–242. [DOI] [PubMed] [Google Scholar]
- Kaufman K.A., Bowen,J.A., Tsai,A.F., Bluestone,J.A., Hunt,J.S. and Ober,C. (1999) The CTLA-4 gene is expressed in placental fibroblasts. Mol. Hum. Reprod., 5, 84–87. [DOI] [PubMed] [Google Scholar]
- Kelly A. and West,J.D. (1996) Genetic evidence that glycolysis is necessary for gastrulation in the mouse. Dev. Dyn., 207, 300–308. [DOI] [PubMed] [Google Scholar]
- Kissel H. et al. (2000) Point mutation in Kit receptor tyrosine kinase reveals essential roles for Kit signaling in spermatogenesis and oogenesis without affecting other Kit responses. EMBO J., 19, 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R.F., Harris,P.K. and Foellmi,L.A. (1994) Glucose-6-phosphate dehydrogenase: a ‘housekeeping’ enzyme subject to tissue-specific regulation by hormones, nutrients and oxidant stress. FASEB J., 8, 174–181. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Chapman,V.M., Hammer,R.E., Brinster,R. and Tilghman,S.M. (1986) Differential expression of α-fetoprotein genes on the inactive X chromosome in extraembryonic and somatic tissues of a transgenic mouse line. Nature, 319, 224–226. [DOI] [PubMed] [Google Scholar]
- Longo L., Bygrave,A., Grosveld,F.G. and Pandolfi,P.P. (1997) The chromosome make-up of mouse embryonic stem cells is predictive of somatic and germ cell chimaerism. Transgenic Res., 6, 321–328. [DOI] [PubMed] [Google Scholar]
- Luzzatto L. (1975) Inherited haemolytic states: glucose-6-phosphate dehydrogenase deficiency. Clin. Hematol., 4, 83–108. [PubMed] [Google Scholar]
- Luzzatto L. (1998) Glucose 6-phosphate dehydrogenase deficiency and hemolytic anemia. In Nathan,D.G. and Orkin,S.H. (eds), Nathan and Oski’s Hematology of Infancy and Childhood, 5th edn. W.B.Saunders Company, Philadelphia, PA, Vol. 2, pp. 704–726.
- Luzzatto L. and Notaro,R. (2001) Malaria. Protecting against bad air. Science, 293, 442–443. [DOI] [PubMed] [Google Scholar]
- Luzzatto L. and Testa,U. (1978) Human erythrocyte G6PD: structure and function in normal and mutant subjects. Curr. Top. Hematol., 1, 1–70. [PubMed] [Google Scholar]
- Luzzatto L., Mehta,A. and Vulliamy,T. (2001) Glucose 6-phosphate dehydrogenase deficiency. In Scriver,C.R., Beaudet,A.L., Sly,W.S. and Valle,D. (eds), The Metabolic and Molecular Bases of Inherited Disease. Vol. 3. McGraw-Hill, New York, NY, pp. 4517–4553.
- Lyden D. et al. (1999) Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature, 401, 670–677. [DOI] [PubMed] [Google Scholar]
- Lyon M.F. and Rastan,S. (1984) Parental source of chromosome imprinting and its relevance for X chromosome inactivation. Differentiation, 26, 63–67. [DOI] [PubMed] [Google Scholar]
- Mason P.J. (1996) New insights into G6PD deficiency. Br. J. Haematol., 94, 585–591. [PubMed] [Google Scholar]
- Mason P.J. et al. (1995) New glucose 6-phosphate dehydrogenase mutations associated with chronic anemia. Blood, 85, 1377–1380. [PubMed] [Google Scholar]
- McMahon A.P. and Bradley,A. (1990) The Wnt-1 (int-1) proto-oncogene is required for development of a large region of mouse brain. Cell, 62, 1073–1085. [DOI] [PubMed] [Google Scholar]
- Morelli A., Benatti,U., Gaetani,G.F. and De Flora,A. (1978) Biochemical mechanisms of glucose-6-phosphate dehydrogenase deficiency. Proc. Natl Acad. Sci. USA, 75, 1979–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor C.E., Rowland,P., Basak,A.K., Gover,S., Mason,P.J., Bautista,J.M., Vulliamy,T.J., Luzzatto,L. and Adams,M.J. (1996) Glucose 6-phosphate dehydrogenase mutations causing enzyme deficiency in a model of the tertiary structure of the human enzyme. Blood, 87, 2974–2982. [PubMed] [Google Scholar]
- Nicol C.J., Zielenski,J., Tsui,L.C. and Wells,P.G. (2000) An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. FASEB J., 14, 111–127. [DOI] [PubMed] [Google Scholar]
- Notaro R., Afolayan,A. and Luzzatto,L. (2000) Human mutations in glucose 6-phosphate dehydrogenase reflect evolutionary history. FASEB J., 14, 485–494. [DOI] [PubMed] [Google Scholar]
- Pandolfi P.P., Sonati,F., Rivi,R., Mason,P., Grosveld,F. and Luzzatto,L. (1995) Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J., 14, 5209–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D. et al. (1999) Molecular basis and enzymatic properties of glucose 6-phosphate dehydrogenase volendam, leading to chronic nonspherocytic anemia, granulocyte dysfunction and increased susceptibility to infections. Blood, 94, 2955–2962. [PubMed] [Google Scholar]
- Rosti V., Tremml,G., Soares,V., Pandolfi,P.P., Luzzatto,L. and Bessler,M. (1997) Murine embryonic stem cells without pig-a gene activity are competent for hematopoiesis with the PNH phenotype but not for clonal expansion. J. Clin. Invest., 100, 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. (1995) Biochemistry. W.H.Freeman, New York, NY.
- Swiatek P.J. and Gridley,T. (1993) Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev., 7, 2071–2084. [DOI] [PubMed] [Google Scholar]
- Tan S.S., Williams,E.A. and Tam,P.P.L. (1993) X-chromosome inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nat. Genet., 3, 170–174. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Kunath,T., Hadjantonakis,A.K., Nagy,A. and Rossant,J. (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science, 282, 2072–2075. [DOI] [PubMed] [Google Scholar]
- Van Noorden C.J.F. and Vogels,I.M.C. (1985) A sensitive cytochemical staining method for glucose-6-phosphate dehydrogenase activity in individual erythrocytes. II. Further improvements of the staining procedure and some observations with glucose-6-phosphate dehydrogenase deficiency. Br. J. Haematol., 60, 57–63. [DOI] [PubMed] [Google Scholar]
- Vulliamy T., Mason,P.J. and Luzzatto,L. (1992) The molecular basis of glucose 6-phosphate dehydrogenase deficiency. Trends Genet., 8, 138–143. [DOI] [PubMed] [Google Scholar]
- Xu W., Westwood,B., Bartsocas,C.S., Malcorra-Azpiazu,J.J., Indrak,K. and Beutler,E. (1995) Glucose 6-phosphate dehydrogenase mutations and haplotypes in various ethnic groups. Blood, 85, 257–263. [PubMed] [Google Scholar]