Abstract
Activated lymphocytes must increase in size and duplicate their contents (cell growth) before they can divide. The molecular events that control cell growth in proliferating lymphocytes and other metazoan cells are still unclear. Here, we utilized transgenesis to provide evidence suggesting that the basic helix–loop– helix–zipper (bHLHZ) transcriptional repressor Mad1, considered to be an antagonist of Myc function, inhibits lymphocyte expansion, maturation and growth following pre-T-cell receptor (pre-TCR) and TCR stimulation. Furthermore, we utilized cDNA microarray technology to determine that, of the genes repressed by Mad1, the majority (77%) are involved in cell growth, which correlates with a decrease in size of Mad1 transgenic thymocytes. Over 80% of the genes repressed by Mad1 have previously been found to be induced by Myc. These results suggest that a balance between Myc and Mad levels may normally modulate lymphocyte proliferation and development in part by controlling expression of growth-regulating genes.
Keywords: cell growth/lymphocyte development/Mad/microarray/Myc
Introduction
Binding of the T-cell antigen receptor (TCR) with peptide and MHC molecules on the surface of antigen-presenting cells provokes a sequence of biochemical events culminating in lymphokine production, acquisition of an effector phenotype and expansion of antigen-specific lymphocytes. Central to the expansion phase is the coordinated process whereby cells must duplicate their contents and double their size (termed cell growth) prior to progressing through the cell cycle and dividing into equal-sized daughter cells (reviewed by Neufeld et al., 1998; Polymenis and Schmidt, 1999). Although the processes of cell growth and cell division generally appear to be coupled, classical genetic studies in yeast suggest that these events are indeed separable, with cell growth being dominant and rate limiting over cell cycle progression (Hartwell, 1971; Nurse, 1975).
Despite considerable progress in our knowledge of the signal transduction events that result in proliferation of lymphocytes, we still lack a clear understanding of nuclear events that couple antigen receptor ligation with cell growth and division. However, recent evidence suggests that the Myc family of nuclear oncoproteins (c-, N- and L-Myc) are important factors that couple proliferative signals with the stimulation of cell growth (reviewed by Elend and Eilers, 1999). Myc-family members are basic helix–loop–helix–zipper (bHLHZ) proteins that are known to play an integral role in the proliferation of normal and neoplastic cells. Myc binds E-box DNA sequences as a heterodimer with the bHLHZ protein Max, resulting predominantly in transcriptional activation (reviewed by Grandori et al., 2000). Myc expression rapidly increases within several hours in response to growth factors (Waters et al., 1991; Miyazaki et al., 1995), B-cell receptor (BCR) (Klemsz et al., 1989) or TCR ligation (Lindsten et al., 1988). Although Myc has been implicated in cell cycle control (reviewed by Obaya et al., 1999), recent biological evidence suggests that a highly conserved function of Myc involves the control of cell growth in Drosophila (Johnston et al., 1999), murine and human B lymphocytes (Iritani and Eisenman, 1999; Schuhmacher et al., 1999), T lymphocytes (Buckley et al., 2001) and fibroblasts (Mateyak et al., 1997). Importantly, the involvement of Myc in cell growth control has also been implied from the identity of putative target genes of both c- and N-Myc. The majority of these genes appear to control cellular biometabolism and protein synthesis (Schmidt, 1999; Coller et al., 2000; Greasley et al., 2000; Kim et al., 2000; O’Hagan et al., 2000; Boon et al., 2001; Neiman et al., 2001; Schuhmacher et al., 2001).
The recognition of Myc and cell growth control as a potential nexus for multiple proliferative signaling pathways prompted us to question whether lymphocytes may also negatively regulate cell growth at the level of transcription as a mechanism to slow cell proliferation, or to maintain quiescence. One family of proteins that may subserve a role as negative regulators of antigen receptor signaling is the Mad family of bHLHZ proteins, of which there are four members (Mad1, Mxi1, Mad3 and Mad4) (reviewed by Schreiber-Agus and DePinho, 1998; Grandori et al., 2000). Like Myc-family proteins, Mad-family proteins also heterodimerize with Max and bind E-boxes. However, while Myc–Max heterodimers activate transcription, Mad–Max heterodimers repress transcription by recruiting a chromatin-modifying complex to E-box binding sites (reviewed by Knoepfler and Eisenman, 1999). Expression of mad-family members is also tightly regulated, but in an opposite manner relative to myc genes. Expression of mad-family members generally correlates with terminal differentiation and quiescence in a variety of cell types, including lymphocytes. Consistent with the proposed role of Mad in inhibiting cell proliferation, B lymphocytes from Mad1-null mice exhibit accelerated cell cycle entry following mitogenic stimulation and granulocyte progenitor cells undergo extra rounds of cell division prior to terminal differentiation (Foley et al., 1998). In addition, T lymphocytes and mouse embryonic fibroblasts from Mxi1-null mice exhibit enhanced proliferative responses to mitogenic stimuli compared with control cells (Schreiber-Agus et al., 1998).
To more closely examine the function and mechanism of action of mad genes in primary cells, we utilized transgenesis to express mad genes specifically in T and B lymphocytes during development. We had previously determined that all Mad-family members are differentially expressed during T-cell development, with mad1 expressed exclusively in medullary thymocytes, while mxi1 and mad3 are expressed in cortical thymocytes. mad4 is expressed in both tissues (Queva et al., 1998). In addition, mad1 is the primary Mad-family member expressed in mature germinal center lymphocytes (Foley et al., 1998). We therefore chose to express murine mad1 specifically in T and B cells during development, utilizing the lck proximal promoter and immunoglobulin heavy chain enhancer (Eµ). Our findings suggest that Mad1 functions to modulate cell proliferation and differentiation in part by repressing cell growth.
Results
Generation of T and B lymphocytes that overexpress murine Mad1
To examine the role of Mad1 in lymphocyte development and function, we generated six independent lines of transgenic (Tg) mice containing a transcriptional element whereby the immunoglobulin heavy chain enhancer and lck proximal promoter are juxtaposed, permitting expression of the murine Mad1 protein specifically in B and T lymphocytes and their progenitors (Figure 1A). This expression vector allows expression of heterologous cDNAs in immature and, to a lesser extent, mature peripheral T- and B-lineage cells (Iritani et al., 1997, 1999; Tucker et al., 2002). As shown in Figure 1B, Eµ-lck-mad1 Tg mice express high levels of Mad1 protein in the thymus relative to endogenous levels, and modest levels of Mad1 protein in bone marrow cells and splenocytes, relative to normal littermate control mice (Figures 1B and 7).
Fig. 1. Expression of Mad1 in immature T- and B-lineage cells utilizing the lck proximal promoter and Ig heavy chain enhancer. (A) The Mad1 transcriptional element includes a 3.2 kb fragment of the mouse lck gene that contains the proximal promoter to +37 with respect to the transcriptional start site, a 0.92 kb fragment of the intronic heavy chain enhancer and a 2.1 kb mutated non-translatable version of the human growth hormone gene. The murine mad1 cDNA was inserted into the BamHI site. (B) The Eµ-lck-mad1 construct drives expression of murine Mad1 protein in thymocytes, B-cell progenitors, and weakly in splenocytes. The figure shows an immunoblot of whole-cell lysate from 5 × 106 bone marrow cells (Bm), splenocytes (Sp) and thymocytes (Th) from non-Tg littermate control mice (LMC) and Eµ-lck-mad1 Tg mice. Protein-containing samples were separated using 12% SDS–PAGE, transferred to nitrocellulose and visualized using a murine Mad1-specific antibody (C19). As a loading control, we used antibodies against the highly stable Max protein (Blackwood et al., 1992).
Fig. 7. Overexpression of Mad1 in primary thymocytes alters the expression of predominantly genes involved in controlling cellular growth. DN thymocytes from 6- to 9-week-old Mad1+/RAG2–/– or RAG2–/– mice (4–6 mice per condition) were purified and cRNA arrayed to Affymetrix mouse arrays containing 11 000 genes. (A) Venn diagrams of the number of genes altered in each of three independent experiments. The criteria for decreased gene expression (left) or increased expression (right) were as follows: (i) the ratio of the expression level in the Mad1+/RAG2–/– sample to that in the RAG2–/– sample was <0.5 (left) or >2 (right); (ii) the gene was called ‘present’ by Affymetrix software in the three Mad1+/RAG2–/– samples; and (iii) the difference was >2 SD from the mean. (B) The genes that meet the above criteria for increased or decreased expression in three independent Mad1+/RAG2–/– versus RAG2–/– experiments. An asterisk indicates genes that have previously been shown to be induced by c- or N-Myc in the indicated references (numbers).
Overexpression of murine Mad1 results in impaired T-cell development
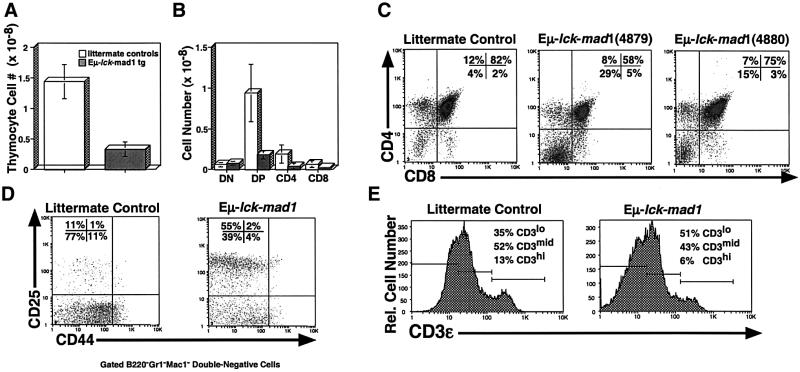
To examine the consequences of overexpressing murine Mad1 on thymocyte development, we purified thymocytes from Tg and littermate control mice, and determined their cellularity and subset representation. As shown in Figure 2A, Eµ-lck-mad1 Tg mice exhibit a 5-fold average decrease in thymocyte cellularity, which corresponds to a decrease in CD4+CD8+ double-positive (DP) and CD4+ or CD8+ single-positive (SP) thymocytes (Figure 2B and C). Further analysis indicates that the decrease in DP thymocytes in Eµ-lck-mad1 Tg mice is associated with a pertubation in T-cell development at the CD44loCD25+ to CD44loCD25– transition (DN3 to DN4), a step in development that is dependent on TCRβ chain rearrangement and pre-TCR formation (Figure 2D). Eµ-lck-mad1 Tg mice also exhibit a decrease in the representation and total number of mature CD3εhi cells, suggesting that high levels of Mad1 protein may compromise the development of SP thymocytes (Figure 2B, C and E). We observe the same phenotypes in all six lines of Eµ-lck-mad1 Tg mice, indicating that the changes are not due to integration artifacts (Figure 2C; data not shown).
Fig. 2. Mad1 overexpression results in decreased thymocyte cellularity and impaired T-lymphocyte development. (A) Total thymocytes were purified from 8- to 16-week-old Eµ-lck-mad1 Tg (4879 line) and normal littermate control mice. The mean and standard error of the total thymocyte cell number from six mice of each genotype are shown. (B) Total thymocyte cell number was multiplied by the percentage of total thymocytes that fall within each quadrant, as determined by flow cytometry using PE-conjugated anti-CD4 or FITC-conjugated anti-CD8. (C) Total thymocytes from Eµ-lck-mad1 Tg (4879 line and 4880 line) and control mice were stained as in (B). A representative flow cytometric histogram from 8-week-old mice is shown. (D) Total thymocytes were stained with biotinylated anti-B220, anti-GR-1, anti-Mac1, anti-CD4, anti-CD8, FITC-conjugated anti-CD25 and PE-conjugated anti-CD44, followed by streptavidin (SA)–tricolor. A representative flow cytometric histogram of gated tricolor-negative cells showing CD44–PE versus CD25–FITC fluorescence is shown. Eµ-lck-mad1 Tg mice are blocked at the CD44–CD25+ cell stage. (E) Total thymocytes were stained with biotinylated anti-CD3ε, followed by SA–tricolor. A representative single-color histogram showing CD3ε expression is shown.
Mad modulates pre-TCR-dependent development and expansion
The decrease in representation and number of DP thymocytes in Eµ-lck-mad1 Tg mice could be due to several effects of Mad1. Mad1 could inhibit proliferation and/or expansion following TCRβ chain rearrangement and pre-TCR formation. Alternatively (or additionally), Mad1 could alter the sensitivity of DP thymocytes to apoptosis. To test the former possibility, we bred Eµ-lck-mad1 Tg mice onto a RAG-2–/– background. Antigen receptor rearrangement does not occur in RAG-2–/– mice due to recombinase-activating gene-2 deficiency (Shinkai et al., 1992). As a result, T- and B-lymphocyte development in RAG-2–/– mice are blocked at the double-negative (DN) and pro-B-cell stages, respectively. Previously, Falk et al. (1996) demonstrated that injection of anti-CD3ε antibodies into recombination-deficient mice engages the CD3 complex on the surface of thymocytes, resulting in the synchronized expansion and maturation of DN cells to the DP stage by mimicking the signals normally delivered by the pre-TCR. Hence, crossing Mad1 Tg mice onto a RAG-2–/– background provides a system for following the effects of Mad1 on a synchronized population of DN cells as they mature to the DP cell stage. We injected RAG-2–/– or RAG-2–/–/Mad1 Tg mice with anti-CD3ε (2C11) antibody, and determined the ability of 2C11 to stimulate cell division, apoptosis and maturation at various time points following injection. As shown in Figure 3A, injection of anti-CD3ε antibody into RAG-2–/– mice results in maturation and expansion of DN cells to the DP cell stage, with appropriate maturation of DN CD44loCD25+ cells to the DN CD44loCD25– cell stage (data not shown). However, the presence of Mad1 Tg protein results in delayed maturation (Figure 3A), decreased fraction of cells in S-phase (Figure 3B), decreased BrdU incorporation (data not shown) and increased sub-G0 apoptotic cells (Figure 3B) at early time points following 2C11 injection. Apoptosis levels in unstimulated RAG-2–/– and RAG-2–/–/Mad1+ mice are indistinguishable, suggesting that the increase in apoptosis observed early following 2C11 injection into RAG-2–/–/Mad1 Tg mice appears to be the result of CD3ε cross-linking in the presence of inappropriate Mad1 expression (Figure 3B; data not shown). While some RAG-2–/–/Mad1 Tg cells still expand and mature to the DP cell stage, these maturing cells express progressively lower levels of Mad1 transgene protein, implying that there are strong selective pressures against Mad1 expression for the development, size increase and expansion of thymocytes following anti-CD3ε injection (Figure 3C). The decrease in Mad1 expression following anti-CD3ε injection is not due to a decrease in activity of the lck proximal promoter, which is highly active in DN and DP thymocytes (Shimizu et al., 2001). Surprisingly, DP cells from Eµ-lck-mad1 transgenic mice on a wild-type background still express Mad1 transgenic protein (data not shown), perhaps due to heterogeneity in the timing and level of transgene expression among individual thymocytes, allowing ‘late’ or lower expressors to mature.
Fig. 3. Expression of Mad1 inhibits pre-TCR-mediated development and expansion. (A) Eµ-lck-mad1 Tg mice were bred with RAG-2–/– mice to generate Mad1 Tg+/RAG-2–/– progeny. Four- to 8-week-old RAG-2–/– or Mad1+/RAG-2–/– mice were injected with 100 µg of anti-CD3ε (2C11) antibody intraperitoneally in 500 µl of sterile PBS. Thymocytes were harvested at the indicated time points and stained with anti-CD4–PE and anti-CD8–FITC. Representative flow cytometric histograms of cells that fall within a standard forward- and side-scatter lymphocyte gate are shown. A normal control mouse was included in each experiment (upper left panel) to set quadrants. The total cell number is shown in the upper right quadrant, for each time point. (B) RAG-2–/– or Mad1 Tg+/RAG-2–/– mice were injected with 100 µg of anti-CD3ε and thymocytes were harvested 1, 3 and 7 days post-injection and stained with propidium iodide. The percentage of total thymocytes that fall within G0/G1, S or G2 phases of the cell cycle at each time point following anti-CD3ε injection is shown. Sub-G1 cells represent apoptotic cells. (C) RAG-2–/– or Mad1 Tg+/RAG-2–/– mice were injected with 100 µg of anti-CD3ε and thymocytes were harvested 7 or 10 days post-injection. A total of 2 × 106 thymocytes were lysed, boiled and sonicated in 2× sample buffer, and proteins separated on a 12% SDS gel. Proteins were then transferred to a nitrocellulose membrane and probed with anti-Mad1 (C19). An immunoblot indicating murine Mad1 expression at each time point post-anti-CD3ε injection is shown.
Expression of cytoplasmic pre-TCR signaling molecules is not impaired in Mad1 transgenic mice
The intracellular signaling events that govern pre-TCR-dependent maturation and expansion are well defined, and include activation of Src-family protein tyrosine kinases (PTKs) Lck and Fyn, the Syk-family PTKs Syk and ZAP70, recruitment of the adaptor proteins SLP-76 and LAT, as well as activation of Rac (Gomez et al., 2000), Rho, Ras/Raf/MEK/Map kinase pathways (reviewed by Rincon et al., 2001) and Ca2+-dependent pathways. The normal activities of these pathways have been implicated in pre-TCR signaling and development. To determine whether Mad expression in Eµ-lck-mad1 Tg mice could be impairing the expression or function of these important signaling molecules and pathways, we examined the levels and function of signaling proteins in Eµ-lck-mad1 Tg mice. We find that high levels of Mad1 protein do not repress important pre-TCR signaling molecules such as Lck, ZAP70, LAT, SLP-76 and the anti-apoptotic molecule Bcl-2, since expression of these molecules is unchanged in Eµ-lck-mad1 Tg mice (Figure 4A and B). In addition, total tyrosine phosphorylation and phosphorylation of ERK1 and ERK2 occur normally in RAG-2–/–/Mad1 Tg thymocytes following anti-CD3ε stimulation (data not shown). DN thymocytes from Eµ-lck-mad1 Tg mice also flux calcium normally compared with thymocytes from normal littermate control mice (Figure 4C). These results suggest that, within our limits of detection, Mad1 does not appear to impair the expression of signaling proteins or pathways known to be important for pre-TCR and TCR function. Furthermore, as noted below, we do not see any changes in the expression of molecules known to be important for pre-TCR signaling in our Mad1 microarray analysis (Figure 7; data not shown).
Fig. 4. Expression of cytoplasmic pre-TCR signaling molecules is not inhibited by Mad1. (A) A total of 2 × 106 thymocytes from Eµ-lck-mad1 Tg or littermate control mice were lysed, boiled and sonicated in 2× sample buffer, and proteins separated on a 12% SDS gel. Protein were then visualized using anti-Mad1, anti-Bcl-2, anti-Lck antiserum and anti-ZAP70. Immunoblots generated using ECL are shown. (B) Total thymocytes from RAG-2–/– or Mad1 Tg+/RAG-2–/– mice were harvested and processed as in (A). Proteins were visualized using anti-Mad1, anti-LAT and anti-SLP-76, followed by ECL. (C) Total thymocytes from Eμ-lck-mad1 and normal littermate control mice were loaded with indole dye, and stained with anti-CD4–PE and anti-CD8–FITC. A two-dimensional flow cytometric histogram showing the violet to blue ratio (calcium) versus time of gated CD4–CD8– thymocytes following stimulation with ionomycin is shown.
Lymphocytes from Mad1 transgenic mice exhibit impaired proliferation in response to mitogens
To examine the effect of Mad1 overexpression on the expansion of lymphocytes following antigen receptor ligation, we stimulated T or B lymphocytes from Eµ-lck-mad1 Tg and littermate control mice with anti-IgM, anti-CD3ε or lipopolysaccharide (LPS) for 72 h. As shown in Figure 5A, both B and T lymphocytes from Eµ-lck-mad1 Tg mice exhibit impaired proliferation in response to mitogens, as judged by reduced tritiated thymidine uptake relative to normal littermate control mice. To further examine the proliferative capacity of Mad1 Tg lymphocytes, we labeled purified B or T cells with the intracellular fluorescent dye 5-carboxyfluorescein diacetate succinimidyl ester (CFSE), which allows the cumulative number of cell divisions to be estimated due to dilution of fluorescence intensity that occurs with each division (Lyons and Parish, 1994). As shown in Figure 5B, Mad1-expressing cells display significantly decreased dilution of the CFSE dye relative to controls, indicative of a diminished number of cell divisions in both B cells (upper panel) and T cells (lower panel) following BCR or TCR stimulation. These findings are highly consistent with earlier results whereby B lymphocytes from Mad1–/– mice undergo an increased number of divisions relative to wild-type B cells when stimulated with mitogens (Foley et al., 1998), and with the lower thymidine incorporation (Figure 5A), and the diminished expansion of T cells following anti-CD3ε injection into Mad Tg/RAG-2–/– mice (Figure 3A). Hence, modulation of Mad1 protein levels during lymphocyte activation alters the number of cell divisions per stimulation.
Fig. 5. B- and T-cell proliferation is inhibited by Mad1. (A) Purified T or B lymphocytes were stimulated with either plate-bound 30, 15 or 5 µg/ml anti-CD3ε or 12, 6 or 3 µg/ml anti-IgM (Fab′)2, or LPS at 25 µg/ml for 96 h at 37°C. Cells were pulsed with [3H]thymidine for the last 18 h. A representative bar histogram is shown depicting the average incorporation of [3H]thymidine by Eµ-lck-mad1 Tg versus normal littermate control lymphocytes. (B) Purified T or B lymphocytes were labeled with CFSE dye for 10 min at 37°C. They were then stimulated with either plate-bound 30 µg/ml anti-CD3ε or 12 µg/ml anti-IgM (Fab′)2 for 24 or 48 h at 37°C, and analyzed by flow cytometry. The images are representative flow cytometric histograms showing log CFSE fluorescence for stimulated B or T lymphocytes from Eµ-lck-mad1 Tg (unfilled curve) versus normal littermate control mice (gray filled curve).
Mad1 expression results in smaller cells during thymocyte development
To determine whether Mad1 has an effect on cell growth, we analyzed the size of Eµ-lck-mad1 Tg thymocytes relative to littermate control cells. As shown in Figure 6A, thymocytes from Eµ-lck-mad1 Tg mice are smaller than those of their littermate control counterparts, during the DN, DP, CD4+ SP and CD8+ SP stages of development. This decrease in cell size is only marginally apparent during the DP cell stage, where cell growth is normally minimal, and most apparent during the DN stage of development, where immature thymocytes are significantly larger than their more mature counterparts. Both total DN and CD44–CD25+ DN thymocytes from Mad1 Tg/RAG-2–/– mice are significantly smaller than their counterparts from RAG-2–/– mice alone (Figure 6B). This decrease in cell size is not because Mad1-expressing cells are in a different cell cycle phase than the wild-type cells, since CD44–CD25+ DN cells are predominantly in G0/G1 and gated G0/G1 cells are on average significantly smaller in Mad1 Tg+/RAG-2–/– mice versus RAG-2–/– mice alone (Figure 6C). To further assess the ability of Mad1-expressing thymocytes to grow in size, we examined the ability of thymocytes from Mad1 Tg+/RAG-2–/– mice to enlarge in response to anti-CD3ε injection (see Figure 3). We find that the cells that are capable of enlarging in size 48 h after anti-CD3ε injection have relatively low levels of Mad1 protein compared with the small cells (Figure 6D). Taken together, these results suggest that expression of Mad1 protein inhibits cell growth during thymocyte development. In addition, the finding that Eµ-lck-mad1 cells are detectably smaller indicates that the effects of Mad on cell growth in this system must be greater than any effect on cell division.
Fig. 6. Eµ-lck-mad1 transgenic thymocytes are smaller than littermate control cells throughout T-cell development. (A) Total thymocytes from Eµ-lck-mad1 Tg and normal littermate control mice were stained with FITC-conjugated anti-CD8 and PE-conjugated anti-CD4. Cells were initially gated on a forward- and side-scatter lymphocyte gate to exclude dead cells. The image is a representative flow cytometric histogram showing forward scatter of cells that fall within DN, DP, CD4+CD8– or CD8+CD4– gates. (B) Total thymocytes from Mad1+/RAG-2–/– mice were stained with biotinylated anti-B220, anti-GR-1, anti-Mac1, anti-CD4, anti-CD8, FITC-conjugated anti-CD25 and PE-conjugated anti-CD44, followed by SA–tricolor. The image is a representative flow cytometric histogram showing forward scatter of either total DN thymocytes or CD44–CD25+ DN (CD4–CD8–B220–Gr1–Mac1–) thymocytes. Mad1 Tg thymocytes are smaller than wild-type thymocytes. (C) Total thymocytes from mice were stained with propidium iodide. The image is a representative flow cytometric histogram showing the size of gated G0/G1 cells, as determined by forward light-scatter characteristics. (D) Mad1+/RAG-2–/– mice were injected with anti-CD3ε, and thymocytes harvested 48 h later. Large and small cells were sorted by FACS as shown, and 1 × 106 cells were lysed, boiled and sonicated in 2× sample buffer, and proteins separated on a 12% SDS gel. Proteins were then transferred to a nitrocellulose membrane and probed with anti-Mad1 (C19) or anti-β-actin.
Overexpression of Mad1 predominantly alters expression of genes involved in controlling cell growth
To examine how Mad1 overexpression inhibits lymphocyte proliferation, decreases cell size and induces apoptosis following pre-TCR stimulation, we determined the global changes in expression of genes in Eµ-lck-mad1 Tg thymocytes compared with littermate control thymocytes. Since the representation of thymocyte subpopulations varies between Eµ-lck-mad1 Tg and littermate control mice (Figure 2), we first bred Mad1 mice onto a RAG-2-null background. This allowed us to compare gene expression within a ‘captured’ equivalent population of thymocytes blocked at the DN stage, with or without co-expression of Mad1 transgene. We utilized Affymetrix gene arrays to compare the expression of ∼11 000 genes in Mad1 Tg/RAG-2–/– thymocytes versus RAG-2–/– thymocytes. As shown previously, Mad1 Tg/RAG-2–/– DN thymocytes are smaller in size compared with littermate RAG-2–/– DN thymocytes (Figure 6). Analysis of gene expression in three independent experiments among total DN thymocytes from either Mad1/RAG-2–/– or RAG-2–/– thymocytes suggests in this system that Mad1 represses 55 genes ≥2-fold [>2 standard deviations (SD) from the mean] and stimulates expression of 22 genes ≥2-fold (>2 SD from the mean) (Figure 7A). Among the genes repressed by Mad1, 77% of the known genes fall under the category of cell growth-control genes, including genes involved in protein synthesis, DNA synthesis, glucose metabolism and protein stability (chaperones). Forty-eight percent of the repressed genes appear to be directly involved in protein synthesis, degradation and ribosome biogenesis (Figure 7B). The total number of genes involved in protein synthesis represents <1% of the genes in the array, indicating that the number of ribosomal genes repressed by Mad is truly significant. These results strongly support the notion that Mad-family members act to negatively modulate cell growth during cell proliferation and differentiation.
Among the genes induced by Mad1 in Mad1/RAG-2–/– DN thymocytes versus RAG-2–/– DN thymocytes >2-fold (Figure 7B), perhaps the most notable include N-myc (7.7-fold) and notch1 (2.5-fold). N-Myc has previously been shown to induce expression of predominantly genes encoding ribosomal proteins (Boon et al., 2001), while Notch signaling has been shown to be important for controlling cell fate decisions and apoptosis during T-cell development (reviewed by Deftos and Bevan, 2000). While this could, in principle, reflect the direct induction of N-myc or notch1 by Mad1, this would be inconsistent with earlier work showing that their normal expression patterns (Hasserjian et al., 1996; Queva et al., 1998) and biological effects differ from Mad1. The induction of N-myc and notch1 more likely represents a feedback response of Mad1-expressing thymocytes to severe inhibition of lymphocyte growth and differentiation by Mad1.
Discussion
Mad modulates pre-TCR- and TCR-dependent T-lymphocyte development
Successful rearrangement of the TCRβ chain genes and formation of the pre-TCR complex signal maturation, expansion and allelic exclusion of the TCRβ chain locus, as well as TCRα chain transcription during thymocyte development. Here, we show that overexpression of Mad1 results in inhibition of maturation and expansion following pre-TCR formation. Furthermore, we show that Mad1 also alters the sensitivity of DP thymocytes to apoptosis during positive and negative selection (Figure 2; data not shown), an event that may also be dependent on activation of Src-family PTKs (Schmedt et al., 1998). These results suggest that one of the normal functions of Mad-family members may be to modulate the transcriptional response to signaling pathways that are initiated by Src-family PTKs. In thymocytes, we further postulate that Mad-family members may negatively modulate signaling pathways downstream of LAT, SLP-76 and the Ras/Raf pathway since these molecules are required for expansion and maturation following pre-TCR formation.
The presumed role of Mad proteins as antagonists of Myc function also fits well with our observations. For example, in non-lymphoid cells, c-myc has been implicated as being induced by c-Src (Barone and Courtneidge, 1995), Raf (Schulze et al., 2001) and MAPK (Pulverer et al., 1994). In thymocytes, loss of c-myc expression results in a near complete block in development at the late DN stage (Douglas et al., 2001), the same stage of thymocyte development inhibited by Mad1 (Figure 2). We have also shown that Mad1 overexpression can attenuate positive selection (Figure 2C; data not shown), while c-myc has been implicated in enhancing positive selection (Broussard-Diehl et al., 1996; Rudolph et al., 2000). Impairment of lymphocyte development by Mad1 is not due to direct repression of Src-family PTKs, Syk-family PTKs or other known molecules previously implicated in pre-TCR signaling, since their levels are not affected in Eµ-lck-mad1 Tg mice (Figures 4 and 7). Importantly, these data indicate that Mad overexpression appears to phenocopy loss-of-Myc function. As described below, this also relates to their dramatically opposing effects on cell growth and common target genes.
Recently, Rudolph et al. (2001) reported the generation of Tg mice overexpressing human mad1 under the control of the lck proximal promoter. Surprisingly, these mice do not exhibit an effect on pre-TCR signaling, nor is the development of SP thymocytes impaired on a wild-type background. However, they do exhibit a 2-fold reduction in thymocyte cellularity. The discrepancy with the findings described here may reflect differences in the level and/or timing of mad1 expression or possibly the use by Rudolph et al. of human mad1 in their system. We examined six different lines of Eµ-lck-mad1 Tg mice and all lines exhibited impairment of pre-TCR signaling, depending on the level of transgene expression (Figure 2C; data not shown).
Mad and Myc act on an overlapping set of genes
One biochemical model for how Myc and Mad induce their biological functions posits that a balance between Myc–Max and Mad–Max acting on E-boxes within specific promoters controls the expression of a set of genes that are profoundly involved in cell growth and division. Here, we provide biological and transcriptional evidence supporting such a model by demonstrating that expression of Mad1 in primary thymocytes inhibits proliferation, development and cell growth, and results in the repression of a set of genes of which >80% have previously been reported to be induced by Myc (see asterisks in Figure 7B). Furthermore, the majority of genes repressed by Mad1 fall into classes involved in cell growth control (ribosomal genes, translational control, etc.), while the majority of genes stimulated by Myc are involved in enhancing cellular growth. While some genes repressed by Mad have not previously been demonstrated to be Myc-regulated genes, they may be Myc-target genes specific to primary thymocytes, but not to other cell types tested previously. Alternatively, there may be incomplete overlap between Myc and Mad target genes, which would be consistent with the findings of an earlier study using Myc–Mad chimeric proteins (O’Hagan et al., 2000; James and Eisenman, 2002). While our microarray findings do not necessarily identify direct targets of Myc or Mad, this work demonstrates that Mad represses a subset of genes that are stimulated directly, or indirectly, through Myc and are thought to control protein synthesis, stability and biometabolism, which are essential for cell proliferation. In addition, these data are highly consistent with the observed phenotypes of RAG-2–/–/Mad1 Tg versus RAG-2–/– thymi, which include impaired proliferation and differentiation in response to anti-CD3ε stimulation, and reduced cell size.
Mad1 controls cell proliferation in part by regulating cellular growth
Classical genetic studies on cell division in yeast and Drosophila suggest that cell cycle progression is triggered by, or dependent on, an adequate rate of cellular growth (reviewed by Edgar, 1999). These ideas were extended by the discovery that c-Myc is capable of stimulating cell growth in a cell cycle-independent manner in Drosophila cells (Johnston et al., 1999) and B lymphocytes (Iritani et al., 1999; Schuhmacher et al., 1999). The role of endogenous c-Myc in cell growth control has been supported in primary lymphocytes by elegant, tissue-specific gene-targeting experiments whereby c-myc-null B lymphocytes (de Alboran et al., 2001) or thymocytes (Douglas et al., 2001) were shown to be deficient in their ability to enlarge in size in response to mitogenic stimulation. However, a recent study contradicts this idea by showing that c-myc-null T cells enlarge in size but do not proliferate following mitogenic stimulation (Trumpp et al., 2001). While it remains to be determined why there is a discrepancy between these studies, one possibility is that loss of c-Myc negatively affects the rate of growth, permitting cells to still grow in size, but more slowly. Indeed a similar situation was reported for Myc-deficient rat fibroblasts, which were the same size as wild-type fibroblasts, but exhibited a 2- to 3-fold reduction in protein accumulation and rRNA synthesis, which paralleled the decrease in proliferation rate (Mateyak et al., 1997). Another possible explanation for the discrepancy between the c-myc knockout studies in T cells versus thymocytes and B cells is that c-myc-null T cells may have deficiencies in specific aspects of ribosomal biogenesis that subsequently trigger a cell cycle block. Evidence for a cell cycle checkpoint in mammalian cells based on inadequate ribosomal biogenesis or ‘nucleolar stress’ is supported in several studies whereby specific inhibition of ribosomal biogenesis results in defects in cell cycle progression, but not cell growth (Volarevic et al., 2000; Pestov et al., 2001), possibly due to the presence of pre-existing ribosomal storage.
Our finding that overexpression of Mad1 results in the repression of cellular growth in thymocytes, in conjunction with previous findings that Myc enhances cellular growth in thymocytes (Douglas et al., 2001) and B cells, led us to postulate that starting and stopping cell division in response to mitogens may be controlled, at least in part, by the relative levels of Myc and Mad. Together, the coordinate expression of Mad and Myc allows cells to growth arrest or divide with more precision. These studies also support the notion of a size checkpoint whereby cells are blocked in cell cycle progression until they attain a critical size or biosynthetic rate compatible with division into equal-sized daughter cells. Regulating the balance between Myc–Max heterodimers and Mad–Max heterodimers may provide a mechanism to control overactive lymphocyte proliferation in lymphomas or in autoimmune disease, or to enhance clonal expansion of antigen-specific lymphocytes in a primary immune response.
Materials and methods
Expression constructs and transgenic mouse generation
Eµ-lck-mad1 Tg mice were generated by inserting the murine mad1 cDNA into the BamHI cloning site of a transcriptional regulatory element consisting of the immunoglobulin heavy chain enhancer (Eµ) and lck proximal promoter (Iritani et al., 1997). Pronuclei injections and propagation of Eµ-lck-mad1 Tg mice were performed as described previously (Abraham et al., 1991). Mice were genotyped by PCR using primers specific for the hGH exons and introns (Iritani et al., 1997). RAG-2-null mice (Shinkai et al., 1992) were obtained from Taconic Laboratories. Mice were housed under specific-pathogen-free (SPF) conditions in an AAALAC-accredited animal facility and were analyzed between 4 and 20 weeks of age.
Flow cytometric analysis
Cells were isolated from bone marrow, spleen and thymus as described previously, and resuspended in Hank’s balanced salt solution (HBSS) (Gibco BRL, Germantown, MD) plus 3% fetal calf serum (FCS; Hyclone) (Iritani et al., 1997). Erythrocytes were depleted by ammonium chloride lysis. Staining for surface markers using flow cytometry was performed as described previously (Iritani et al., 1999) and 10 000 gated events were collected utilizing a FACsCaliber machine (Becton Dickinson Immunocytometry Systems, San Jose, CA). Data were then analyzed using CellQuest software (Becton Dickinson) or ReproMAC software version 2.07 (Truefacts Software, Seattle, WA). For DNA content analysis, cells were stained with propidium iodide as described previously (Iritani and Eisenman, 1999). For apoptosis analysis, 2 × 106 cells were stained in 50 µl of 1:100 7-aminoactinomycin D and Annexin FITC (Molecular Probes) for 30 min on ice. For cell division experiments, cells were labeled with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester CFSE (Molecular Probes) according to the manufacturer’s instructions. Calcium flux assays were performed on total thymocytes as described previously (Appleby et al., 1992) and analyzed using FACsLSR. Anti-CD3 (2C11) affinity-purified ascites were generously provided by Anne Norment and Michael Bevan. For RAG-2–/–/Mad1 experiments, 100 µg of either purified 2C11 (PharMingen) or ascites were injected intraperitoneally in 0.5 ml of PBS. Mice were killed 24–240 h post-injection.
Proliferation assays
Splenic T cells were purified using mouse T-cell enrichment columns (R &D Systems). Splenic B cells were purified using complement lysis of T cells as described previously (Iritani et al., 1997). T or B cells were cultured in 200 µl of RPMI 1640 medium (Gibco-BRL) supplemented with 10% FCS (Hyclone), 2 mM l-glutamine (Gibco), 100 µM non-essential amino acids (Gibco), 100 U/ml penicillin, 100 U/ml streptomycin (Gibco). Cells were stimulated with the indicated concentration of 2C11 (PharMingen) or (Fab′)2 IgM (Appleby et al., 1995). After 48 h, the cells were incubated with 1 mCi of [3H]thymidine for 18–24 h and harvested onto printed glass fiber filtermats using an LKB-Wallac Cell Harvester. Incorporation of [3H]thymidine was measured using an LKB-Wallac 1205 Betaplate liquid scintillation counter.
Preparation of nucleic acids
DNA was purified from mouse tails as described previously (Foley et al., 1998). For RNA extraction, tissues were collected from mice and immediately frozen on dry ice. Total RNA was then isolated from each tissue using Trizol B (Tel-Test ‘B’ Inc., Friendswood, TX). Genomic DNA was purified from thymocytes as described previously (Anderson et al., 1992). cDNA was synthesized from total RNA using SuperScript Choice kits (Gibco-BRL) and nucleotide primers that contained a sequence recognized by T7 RNA polymerase.
Microarray analysis
cDNA was synthesized as described above. Labeled cRNA was prepared by in vitro transcription using T7 RNA polymerase (MegaScript T7 kit; Ambion, Austin, TX). Affymetrix Mu11KsubA & B GeneChip® arrays were used to interrogate expression of ∼11 000 murine genes/expressed sequence tags (ESTs) for three pairs of MadRag and Rag samples. Initial array analysis for each sample was performed using the Affymetrix Microarray Suite (AMS) software employing default analysis parameters and a global scaling factor of 1000. For genes/ESTs with negative or small ‘average difference’ (AveDiff) values, AveDiff was set to 20. In an effort to minimize experimental variation, pairwise comparisons were made using Mad1/RAG-2–/– and RAG-2–/– sample pairs that were prepared and processed in parallel. For each of three comparison sets, data from Mu11Ksub A & B arrays were combined and an expression ratio (R) was calculated for individual genes/ESTs, where R = AveDiffMadRag/AveDiffRag. Data filtering and normalization were similar to those used by Coller et al. (2000). Genes/ESTs that the AMS algorithm called ‘absent’ in both MadRag and Rag sample pairs, or where |AveDiffMadRag – AveDiffRag| ≤ 100, were filtered from further analysis. An exception was made for mad, where in two of three comparisons the high level of mad expression in the MadRag samples produced extensive saturation of the array probe signals, which resulted in an erroneous ‘absent’ call when the AMS gene-call algorithm was applied. Ratio values for each filtered data set were log2 transformed and normalized using mean = 0 and SD = 1. Genes/ESTs were considered significantly overexpressed in MadRag if R≥ 2 for each of the three pairwise comparisons. Likewise, genes/ESTs were considered underexpressed in MadRag if R≤ 0.5 for each of the three pairwise comparisons.
Protein analysis
A total of 2 × 107 thymocytes, splenocytes or total bone marrow cells were boiled and sonicated in 25 µl of PBS and 25 µl of 2× sample buffer. Protein-containing samples were separated using 10–12% SDS–PAGE, transferred to nitrocellulose and visualized using either murine Mad1-specific antibody (C19; Santa Cruz), Lck antiserum (a gift from Roger Perlmutter), ZAP70-specific antibody (485; Santa Cruz), anti-phosphotyrosine (4G10; UBI), anti-Bcl-2 (Santa Cruz), LAT antiserum (a gift from Lawrence Samelson), SLP-76 antiserum (a gift from Steve Levin) or anti-phospho ERK (7383; Santa Cruz), followed by peroxidase anti-rabbit (Vector labs) or peroxidase anti-sheep (Santa Cruz) and enhanced chemiluminescence (ECL; Amersham) as described previously (Iritani et al., 1999). As a loading control, we used antibodies against the highly stable Max protein (Blackwood et al., 1992).
Acknowledgments
Acknowledgements
We are grateful to Carol Ware, Angel Nelson and Warren Ladiges of the Nathan Shock Transgenic Center, University of Washington, for Eµ-lck-mad1 pronuclear injections. We thank Michael Bevan, Jose Alberola-Ila, Anne Norment and Steve Levin for generously providing mice and reagents. We also thank Paul Neiman, Chen Dong, Anne Norment, Ananda Goldrath, Phillip Wong, Grant McArthur and Steve Levin for helpful discussions and comments on this manuscript. This work was supported by NIH/NIAID Mentored Clinical Investigator Award 1 K08 AJ01445-01 and University of Washington/HHMI pilot and faculty start-up grants (to B.M.I.) and NIH/NCI grants (to R.N.E.). R.N.E. is a Research Professor of the American Cancer Society.
References
- Abraham K.M., Levin,S.D., Marth,J.D., Forbush,K.A. and Perlmutter,R.M. (1991) Delayed thymocyte development induced by augmented expression of p56lck. J. Exp. Med., 173, 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aifantis I., Gounari,F., Scorrano,L., Borowski,C. and von Boehmer,H. (2001) Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-κB and NFAT. Nat. Immunol., 2, 403–409. [DOI] [PubMed] [Google Scholar]
- Anderson S.J., Abraham,K.M., Nakayama,T., Singer,A. and Perlmutter,R.M. (1992) Inhibition of T-cell receptor β-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J., 11, 4877–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby M.W., Gross,J.A., Cooke,M.P., Levin,S.D., Qian,X. and Perlmutter,R.M. (1992) Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell, 70, 751–763. [DOI] [PubMed] [Google Scholar]
- Appleby M.W., Kerner,J.D., Chien,S., Maliszewski,C.R., Bondada,S. and Perlmutter,R.M. (1995) Involvement of p59fynT in interleukin-5 receptor signaling. J. Exp. Med., 182, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone M.V. and Courtneidge,S.A. (1995) Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature, 378, 509–512. [DOI] [PubMed] [Google Scholar]
- Blackwood E.M., Luscher,B. and Eisenman,R.N. (1992) Myc and Max associate in vivo. Genes Dev., 6, 71–80. [DOI] [PubMed] [Google Scholar]
- Boon K. et al. (2001) N-myc enhances the expression of a large set of genes functioning in ribosome biogenesis and protein synthesis. EMBO J., 20, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard-Diehl C., Bauer,S.R. and Scheuermann,R.H. (1996) A role for c-myc in the regulation of thymocyte differentiation and possibly positive selection. J. Immunol., 156, 3141–3150. [PubMed] [Google Scholar]
- Buckley A.F., Kuo,C.T. and Leiden,J.M. (2001) Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat. Immunol., 2, 698–704. [DOI] [PubMed] [Google Scholar]
- Coller H.A., Grandori,C., Tamayo,P., Colbert,T., Lander,E.S., Eisenman,R.N. and Golub,T.R. (2000) Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling and adhesion. Proc. Natl Acad. Sci. USA, 97, 3260–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alboran I.M., O’Hagan,R.C., Gartner,F., Malynn,B., Davidson,L., Rickert,R., Rajewsky,K., DePinho,R.A. and Alt,F.W. (2001) Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity, 14, 45–55. [DOI] [PubMed] [Google Scholar]
- Deftos M.L. and Bevan,M.J. (2000) Notch signaling in T cell development. Curr. Opin. Immunol., 12, 166–172. [DOI] [PubMed] [Google Scholar]
- Douglas N.C., Jacobs,H., Bothwell,A.L. and Hayday,A.C. (2001) Defining the specific physiological requirements for c-Myc in T cell development. Nat. Immunol., 2, 307–315. [DOI] [PubMed] [Google Scholar]
- Edgar B.A. (1999) From small flies come big discoveries about size control. Nat. Cell Biol., 1, E191–E193. [DOI] [PubMed] [Google Scholar]
- Elend M. and Eilers,M. (1999) Cell growth: downstream of Myc—to grow or to cycle? Curr. Biol., 9, R936–R938. [DOI] [PubMed] [Google Scholar]
- Falk I., Potocnik,A.J., Barthlott,T., Levelt,C.N. and Eichmann,K. (1996) Immature T cells in peripheral lymphoid organs of recombinase-activating gene-1/-2-deficient mice. Thymus dependence and responsiveness to anti-CD3 epsilon antibody. J. Immunol., 156, 1362–1368. [PubMed] [Google Scholar]
- Foley K.P., McArthur,G.A., Queva,C., Hurlin,P.J., Soriano,P. and Eisenman,R.N. (1998) Targeted disruption of the MYC antagonist MAD1 inhibits cell cycle exit during granulocyte differentiation. EMBO J., 17, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M., Tybulewicz,V. and Cantrell,D.A. (2000) Control of pre-T cell proliferation and differentiation by the GTPase Rac-I. Nat. Immunol., 1, 348–352. [DOI] [PubMed] [Google Scholar]
- Grandori C., Cowley,S.M., James,L.P. and Eisenman,R.N. (2000) The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol., 16, 653–699. [DOI] [PubMed] [Google Scholar]
- Greasley P.J., Bonnard,C. and Amati,B. (2000) Myc induces the nucleolin and BN51 genes: possible implications in ribosome biogenesis. Nucleic Acids Res., 28, 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L.H. (1971) Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J. Mol. Biol., 59, 183–194. [DOI] [PubMed] [Google Scholar]
- Hasserjian R.P., Aster,J.C., Davi,F., Weinberg,D.S. and Sklar,J. (1996) Modulated expression of notch1 during thymocyte development. Blood, 88, 970–976. [PubMed] [Google Scholar]
- Iritani B.M. and Eisenman,R.N. (1999) c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc. Natl Acad. Sci. USA, 96, 13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani B.M., Forbush,K.A., Farrar,M.A. and Perlmutter,R.M. (1997) Control of B cell development by Ras-mediated activation of Raf. EMBO J., 16, 7019–7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani B.M., Alberola-Ila,J., Forbush,K.A. and Perlmutter,R.M. (1999) Distinct signals mediate maturation and allelic exclusion in lymphocyte progenitors. Immunity, 10, 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James L. and Eisenman,R.N. (2002) Myc and Mad bHLHZ domains possess identical DNA-binding specificities but only partially overlapping functions in vivo. Proc. Natl Acad. Sci. USA, 99, 10429–10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.A., Prober,D.A., Edgar,B.A., Eisenman,R.N. and Gallant,P. (1999) Drosophila myc regulates cellular growth during development. Cell, 98, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Li,Q., Dang,C.V. and Lee,L.A. (2000) Induction of ribosomal genes and hepatocyte hypertrophy by adenovirus-mediated expression of c-Myc in vivo. Proc. Natl Acad. Sci. USA, 97, 11198–11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M.J., Justement,L.B., Palmer,E. and Cambier,J.C. (1989) Induction of c-fos and c-myc expression during B cell activation by IL-4 and immunoglobulin binding ligands. J. Immunol., 143, 1032–1039. [PubMed] [Google Scholar]
- Knoepfler P.S. and Eisenman,R.N. (1999) Sin meets NuRD and other tails of repression. Cell, 99, 447–450. [DOI] [PubMed] [Google Scholar]
- Lindsten T., June,C.H. and Thompson,C.B. (1988) Multiple mechanisms regulate c-myc gene expression during normal T cell activation. EMBO J., 7, 2787–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A.B. and Parish,C.R. (1994) Determination of lymphocyte division by flow cytometry. J. Immunol. Methods, 171, 131–137. [DOI] [PubMed] [Google Scholar]
- Mateyak M.K., Obaya,A.J., Adachi,S. and Sedivy,J.M. (1997) Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ., 8, 1039–1048. [PubMed] [Google Scholar]
- Miyazaki T., Liu,Z.J., Kawahara,A., Minami,Y., Yamada,K., Tsujimoto,Y., Barsoumian,E.L., Perlmutter,R.M. and Taniguchi,T. (1995) Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc and lck cooperate in hematopoietic cell proliferation. Cell, 81, 223–231. [DOI] [PubMed] [Google Scholar]
- Neiman P.E., Ruddell,A., Jasoni,C., Loring,G., Thomas,S.J., Brandvold,K.A., Lee,R., Burnside,J. and Delrow,J. (2001) Analysis of gene expression during myc oncogene-induced lymphomagenesis in the bursa of Fabricius. Proc. Natl Acad. Sci. USA, 98, 6378–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld T.P., de la Cruz,A.F., Johnston,L.A. and Edgar,B.A. (1998) Coordination of growth and cell division in the Drosophila wing. Cell, 93, 1183–1193. [DOI] [PubMed] [Google Scholar]
- Nurse P. (1975) Genetic control of cell size at cell division in yeast. Nature, 256, 547–551. [DOI] [PubMed] [Google Scholar]
- Obaya A.J., Mateyak,M.K. and Sedivy,J.M. (1999) Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene, 18, 2934–2941. [DOI] [PubMed] [Google Scholar]
- O’Hagan R.C. et al. (2000) Gene-target recognition among members of the myc superfamily and implications for oncogenesis. Nat. Genet., 24, 113–119. [DOI] [PubMed] [Google Scholar]
- Pestov D.G., Strezoska,Z. and Lau,L.F. (2001) Evidence of p53-dependent cross-talk between ribosomal biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G1/S transition. Mol. Cell. Biol., 21, 4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenis M. and Schmidt,E.V. (1999) Coordination of cell growth with cell division. Curr. Opin. Genet. Dev., 9, 76–80. [DOI] [PubMed] [Google Scholar]
- Pulverer B.J., Fisher,C., Vousden,K., Littlewood,T., Evan,G. and Woodgett,J.R. (1994) Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene, 9, 59–70. [PubMed] [Google Scholar]
- Queva C., Hurlin,P.J., Foley,K.P. and Eisenman,R.N. (1998) Sequential expression of the MAD family of transcriptional repressors during differentiation and development. Oncogene, 16, 967–977. [DOI] [PubMed] [Google Scholar]
- Rincon M., Flavell,R.A. and Davis,R.J. (2001) Signal transduction by MAP kinases in T lymphocytes. Oncogene, 20, 2490–2497. [DOI] [PubMed] [Google Scholar]
- Rudolph B., Hueber,A.O. and Evan,G.I. (2000) Reversible activation of c-Myc in thymocytes enhances positive selection and induces proliferation and apoptosis in vitro. Oncogene, 19, 1891–1900. [DOI] [PubMed] [Google Scholar]
- Rudolph B., Hueber,A.O. and Evan,G.I. (2001) Expression of Mad1 in T cells leads to reduced thymic cellularity and impaired mitogen-induced proliferation. Oncogene, 20, 1164–1175. [DOI] [PubMed] [Google Scholar]
- Schmedt C., Saijo,K., Niidome,T., Kuhn,R., Aizawa,S. and Tarakhovsky,A. (1998) Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature, 394, 901–904. [DOI] [PubMed] [Google Scholar]
- Schmidt E.V. (1999) The role of c-myc in cellular growth control. Oncogene, 18, 2988–2996. [DOI] [PubMed] [Google Scholar]
- Schreiber-Agus N. and DePinho,R.A. (1998) Repression by the Mad(Mxi1)–Sin3 complex. BioEssays, 20, 808–818. [DOI] [PubMed] [Google Scholar]
- Schreiber-Agus N., Meng,Y., Hoang,T., Hou,H.,Jr, Chen,K., Greenberg,R., Cordon-Cardo,C., Lee,H.W. and DePinho,R.A. (1998) Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature, 393, 483–487. [DOI] [PubMed] [Google Scholar]
- Schuhmacher M., Staege,M.S., Pajic,A., Polack,A., Weidle,U.H., Bornkamm,G.W., Eick,D. and Kohlhuber,F. (1999) Control of cell growth by c-Myc in the absence of cell division. Curr. Biol., 9, 1255–1258. [DOI] [PubMed] [Google Scholar]
- Schuhmacher M. et al. (2001) The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res., 29, 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze A., Lehmann,K., Jefferies,H.B., McMahon,M. and Downward,J. (2001) Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev., 15, 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C. et al. (2001) Progression of T cell lineage restriction in the earliest subpopulation of murine adult thymus visualized by the expression of lck proximal promoter activity. Int. Immunol., 13, 105–117. [DOI] [PubMed] [Google Scholar]
- Shinkai Y. et al. (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell, 68, 855–867. [DOI] [PubMed] [Google Scholar]
- Trumpp A., Refaeli,Y., Oskarsson,T., Gasser,S., Murphy,M., Martin,G.R. and Bishop,J.M. (2001) c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature, 414, 768–773. [DOI] [PubMed] [Google Scholar]
- Tucker S.N., Jessup,H.K., Fujii,H. and Wilson,C.B. (2002) Enforced expression of the Ikaros isoform IK5 decreases the numbers of extrathymic intraepithelial lymphocytes and natural killer 1.1+ T cells. Blood, 99, 513–519. [DOI] [PubMed] [Google Scholar]
- Volarevic S., Stewart,M.J., Ledermann,B., Zilberman,F., Terracciano,L., Montini,E., Grompe,M., Kozma,S.C. and Thomas,G. (2000) Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science, 288, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Waters C.M., Littlewood,T.D., Hancock,D.C., Moore,J.P. and Evan,G.I. (1991) c-myc protein expression in untransformed fibroblasts. Oncogene, 6, 797–805. [PubMed] [Google Scholar]