Abstract
To determine whether the widespread clinical use of β-lactams has been selective for Citrobacter freundii-derived alleles of plasmid ampC genes, we generated a Bayesian consensus phylogeny of the published ampC sequences and compared the MICs of 16 β-lactam antibiotics for Escherichia coli strains containing cloned copies of the C. freundii ampC alleles. We found that for the majority of compounds investigated, there has been essentially no increase in β-lactam resistance conferred by those alleles. We also found that ampC alleles from the chromosomes of two β-lactam-sensitive C. freundii strains isolated in the 1920s, before the clinical use of antibiotics, were as effective at providing β-lactam resistance in E. coli as were the plasmid-borne alleles from β-lactam-resistant clinical isolates. These results suggest that selection for increased resistance to β-lactam antibiotics has not been a significant force directing the evolution of the C. freundii ampC alleles found in β-lactam-resistant clinical isolates.
Since their introduction nearly 60 years ago, β-lactams have consistently been the most widely used antibiotics, currently accounting for 50% of the antibiotics consumed globally (17). Resistance to β-lactam antibiotics is determined by the production of periplasmically located β-lactamases and by diffusion of the antibiotic through the outer membrane via porins. Indeed, the level of resistance can be accurately predicted from kinetic parameters and concentrations of β-lactamases and from diffusion constants of the drugs (23). The most common β-lactamases among the Enterobacteriaciae are the plasmid-borne class A (10, 21) TEM and SHV β-lactamases. TEM-1, first reported in 1965 (11), confers a high level of resistance to penicillins and early cephalosporins but little resistance to oxyiminocephalosporins and aztreonam (21). Widespread resistance to penicillin stimulated the development of chemically modified β-lactams, including cephalosporins and monobactams, that were less sensitive to hydrolysis by the class A β-lactamases. Beginning in the early 1980s, extended-spectrum β-lactamases (ESBL) derived from TEM-1 and SHV-1 began to appear in response to the widespread use of cephalosporins that had occurred in the previous decade. Indeed, with the exception of the carbapenems, as newer variants of β-lactam antibiotics have been introduced, TEM variants active against those β-lactams have appeared within 2 to 3 years (21). Similarly, as β-lactamase inhibitors, such as clavulanic acid (24) and sulbactam (6, 7), have been introduced, the TEM and SHV β-lactamases have evolved resistance to those inhibitors.
The rapid appearance of resistant TEM β-lactamases following the introduction of new β-lactam antibiotics has been such a consistent pattern that it has conditioned thinking about the evolution of antibiotic resistance and has strongly affected public health policy. The AmpC, or class C (15), β-lactamases have relatively recently moved onto plasmids and become widespread. Their presence allows testing of the generality of the expectation that antibiotic resistance genes will quickly evolve new substrate specificities and increased activities in response to the selection imposed by the clinical use of recently introduced antibiotics.
The AmpC β-lactamases are primarily cephalosporinases that mediate resistance to cephalosporins, oxyiminocephalosporins, and aztreonam (24). ampC genes are widely distributed among the Enterobacteriaceae (16). In typical Escherichia coli and Shigella species, ampC is expressed at such low levels that deletion of ampC does not affect sensitivity to β-lactams, but in Citrobacter freundii and Enterobacter cloacae, ampC is inducible both by penicillins, such as ampicillin, and by cephalosporins (16). Mutations that lead to high-level constitutive expression of ampC in C. freundii and E. cloacae confer clinical resistance to extended-spectrum cephalosporins. Because of their location on host chromosomes, ampC genes were not initially subject to the rapid dissemination allowed by the horizontal transmission of plasmid-borne genes. The first plasmid-borne ampC gene, for CMY-1, was reported in 1989 (4), followed quickly by those for MIR-1 (25) and CMY-2 (A. Bauernfeind, S. Schweigart, K. Dornbusch, and H. Giamarellou, Program Abstr. 30th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 941, 1990). In the last 11 years, over 50 different ampC genes have been reported, including over 20 plasmid-borne ampC genes in clinical isolates worldwide. A recent survey of 20 U.S. hospitals showed that plasmid-borne ampC genes were more prevalent than TEM-type ESBL genes, although they were less common that SHV-type ESBL genes (G. A. Jacoby, P. Han, M. Alvarez, and F. Tenover, Program Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C49, 1995).
Experience with the class A TEM β-lactamases would lead us to expect that during their 11 years of plasmid-borne spread throughout the world, the AmpC β-lactamases should have evolved increased efficiency of β-lactam hydrolysis, a broader substrate range, or both. To test that expectation, we constructed a phylogeny of the ampC β-lactamase genes, paying particular attention to the C. freundii-related ampC β-lactamase genes, and used that phylogeny to deduce the order in which both plasmid-borne and chromosomal ampC alleles evolved. We compared several of the C. freundii enzymes to determine the level of resistance that they produce to 16 β-lactam antibiotics. In order to assess the role of selection imposed by the clinical use of antibiotics, we included in this analysis the ampC genes from two strains of C. freundii that were isolated in the 1920s, well before the antibiotic era.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli K-12 strain JM109 (33) was used as the host for recombinant plasmids. Plasmid pACSE2 was used as the vector for cloning and expression of the different ampC alleles. pACSE2 (5,201 bp) was created in two steps. Step 1 involved replacing the MscI fragment (bp 2938 through 3980 of pACYC184) with bp 6 through 622 of pSE380 (Invitrogen) to yield plasmid pACSE. Step 2 involved amplifying bp 3269 through 167 of pSE380 by using primers with SpeI and XbaI sites and ligating the amplicon, which contains lacIq, into the SpeI site of pACSE. pACSE2 carries the p15A origin of replication (5 to 10 copies/cell); a gene for tetracycline resistance, lacI; and the pTrc promoter-multiple cloning region of pSE380. Expression from the pTrc promoter is regulated by the lacIq gene product. Part of the chloramphenicol resistance gene of pACYC184 is deleted in pACSE2.
C. freundii strains 6879 and 8454 were obtained from the American Type Culture Collection. Both were collected in the 1920s or early 1930s, prior to the clinical introduction of penicillin (28, 32). Cultures of both strains were spread onto Mueller-Hinton agar (Difco). BBL antibiotic disks containing 30 μg of aztreonam, 30 μg of cefepime, 10 μg of meropenem, 10 μg of imipenem, 100 μg of piperacillin, 30 μg of ceftazidime, 30 μg of cefotaxime, or 30 μg of cefuroxime were applied to the plates. Both strains were sensitive to all of these antibiotics because, following overnight incubation at 37°C, inhibition zone diameters were at least 33% greater than the NCCLS (22) threshold for sensitivity.
G. Jacoby supplied E. coli J53/pMG263 (CMY-2). E. Tzelepi and L. S. Tzouvelekis supplied E. coli XL1Blue/pPHPI-5/XL (LAT-1), E. coli XL1Blue/pMEL/XL (LAT-2), E. coli XL1Blue/pMEL-1/XL (LAT-3), and E. coli XL1Blue/pMEL-2/XL (LAT-4). A. Philippon supplied Proteus mirabilis 34955 (CMY-4) and P. mirabilis LAR (CMY-3). A. Fosberry and D. Payne supplied E. coli TC446.891 (BIL-1). D. Sirot provided P. mirabilis CF09 (CMY-3b).
Plasmids pMXCMY2, pMXCMY3, pMXCMY3b, pMXCMY4, pMXLAT3, pMXLAT4, pMX6879, and pMX8454 were created by amplifying the corresponding ampC alleles and cloning them into plasmid pACSE2.
Recombinant DNA methodology.
The ampC alleles were amplified with a Failsafe (Epicentre) polymerase system (buffer A) by using primers P1 (TTGCTTTTAATTACGGAACTGATTT) and P2 (GGAGCTCTAAGTGTAGATGACAGCAGGRAAA), which has a SacI site (boldface) at the 5′ end of the primer. Amplification reactions were carried out by using the following program: 25 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 90 s, followed by 1 cycle at 72°C for 5 min.
The resulting amplicons were purified with Qiagen PCR purification columns according to the manufacturer's instructions and digested with restriction endonucleases BspHI (New England Biolabs) and SacI (New England Biolabs). A BspHI site is located at the ATG start site of the C. freundii ampC gene. Plasmid pACSE2 was digested with restriction endonucleases NcoI and SacI and dephosphorylated with calf intestinal alkaline phosphatase from New England Biolabs.
Digested amplicons and the vector were purified with Qiagen PCR purification columns according to the manufacturer's instructions, combined, ligated with T4 DNA ligase (Gibco BRL), and transformed into strain JM109 by electroporation.
Sequencing.
For each allele that was cloned, the sequence was verified by sequencing an amplicon of the region of the plasmid containing the ampC insert. Amplification reactions were carried out by using Taq PCR Master Mix (Qiagen) under the conditions described above. The amplicons were purified with Qiagen PCR purification columns and sequenced on an ABI Prism 377 DNA sequencer with an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems) according to the manufacturer's protocol.
For the pre-antibiotic era alleles, the sequence was determined for a single strand of both the original amplicon and the cloned copy of the gene. For both alleles, the sequences agreed.
We verified the published sequences of the cloned alleles by sequencing a single strand. When the cloned sequence differed from the published sequence, both strands of the original amplicon generated by primers P1 and P2 were sequenced to determine whether the differences were the result of amplification errors or of errors in the published sequence.
Expression of ampC.
Cloned ampC alleles from pACSE2-based plasmids are expressed from the TAC promoter, which is tightly regulated by the lacI gene product. Expression of the ampC alleles was induced by isopropyl-β-d-thiogalactopyranoside (IPTG). To determine the concentration of IPTG required to induce the expression of ampC at physiological levels, we determined the MIC of piperacillin for strain E. coli J53 carrying plasmid pMG263, a naturally occurring plasmid bearing the CMY-2 allele of ampC. We then determined the concentration of IPTG required to give an equivalent MIC of piperacillin for JM109 carrying plasmid pMXCMY2. IPTG at 62.5 μM induced the expression of CMY-2 to the appropriate level.
Antibiotics and determination of MICs.
The MICs of the following antibiotics were determined: ampicillin (Sigma), piperacillin (Sigma), piperacillin-tazobactam (8:1) (Lederle), temocillin (SmithKline Beecham), ticarcillin (SmithKline Beecham), ticarcillin-clavulanate (30:1) (SmithKline Beecham), cephalothin (Sigma), cefoxitin (Merck & Co.), cefuroxime (Sigma), ceftriaxone (Roche), ceftazidime (Glaxo Wellcome), cefotaxime (Sigma), cefepime (Bristol-Myers Squibb), imipenem (Merck & Co.), meropenem (Zeneca), and aztreonam (Bristol-Myers Squibb). Stock solutions of these drugs were prepared in 0.1 M NaPO4 buffer (pH 7.0) and stored at −80°C in single-use aliquots.
MICs were determined with cultures at a concentration of 105 cells/ml in Mueller-Hinton broth (Difco) containing 62.5 μM IPTG by application of twofold serial dilutions of each antibiotic to each tested strain. Cultures were incubated at 37°C on a rotating shaker for 24 h. The MIC was the lowest concentration of antibiotic that completely blocked the growth of the tested strain.
Phylogenetic methods.
The sequences of 59 genes that are closely related to the plasmid-borne CMY-2 ampC gene were identified by a BLAST search (1, 2) of the nonredundant protein database at the National Center for Biotechnology Information. Those protein sequences were aligned with the sequences of CMY-2, the sequence of CMY-3b (8), and the deduced amino acid sequences of AmpC from pre-antibiotic era C. freundii strains Cfre8454 and Cfre6879 by using the multiple alignment program ClustalX (14, 30, 31). The Gonet 250 similarity matrix was used with a gap opening penalty of 35 and a gap extension penalty of 0.75 for the pairwise alignment stage and a gap opening penalty of 15 and a gap extension penalty of 0.3 for the multiple alignment stage. When sequencing revealed errors in the published sequences, the corrected sequences were used in the alignment.
The corresponding DNA coding sequences (accession numbers are given in Table 1) were aligned by introducing triplet gaps between codons corresponding to gaps in the aligned protein sequences with the program CodonAlign (version 1.0). CodonAlign for Macintosh and for PC (Windows) computers and a source code that can be compiled for other platforms are available at no charge at http://www.rochester.edu/College/BIO/labs/HallLab/index.html.
TABLE 1.
Symbols on phylogenetic trees and accession numbers
| Symbol on phylogenetic trees | Nucleotide sequence accession no. | Plasmid or chromosome | Organism, if gene is on chromosome |
|---|---|---|---|
| AbauRYC | AJ009979 | Chromosome | Acinetobacter baumannii |
| ACC-1 | AJ133121 | Plasmid | |
| ACT-1 | U58495 | Plasmid | |
| AhydCepH | AHY276030 | Chromosome | Aeromonas hydrophila |
| AsobAER14 | U10250 | Chromosome | Aeromonas sobria |
| AsobCepS | X80277 | Chromosome | Aeromonas sobria |
| BIL-1 | X74512a | Plasmid | |
| Cfre6879 | AF349569 | Chromosome | Citrobacter freundii |
| Cfre8454 | AF349570 | Chromosome | Citrobacter freundii |
| CfreGC3 | D85910 | Chromosome | Citrobacter freundii |
| CfreGN346 | D13207 | Chromosome | Citrobacter freundii |
| CfreH224 | Y15129 | Chromosome | Citrobacter freundii |
| CfreI113 | X76636 | Chromosome | Citrobacter freundii |
| CfreOS60 | X03866 | Chromosome | Citrobacter freundii |
| CMY-1 | X92508 | Plasmid | |
| CMY-2 | X91840 | Plasmid | |
| CMY-2b | U77414 | Plasmid | |
| CMY-3 | Y16783 | Plasmid | |
| CMY-3b | None | Chromosome | Proteus mirabilis |
| CMY-4 | Y16785 | Plasmid | |
| CMY-5 | Y17716 | Plasmid | |
| CMY-6 | AJ011293 | Plasmid | |
| CMY-7 | AJ011291 | Plasmid | |
| CMY-8 | AF167990 | Plasmid | |
| CMY-9 | AB049588 | Unknown | Escherichia coli |
| DHA-1 | Y16410 | Plasmid | |
| DHA-2 | AF259520 | Plasmid | |
| Eare1 | AF211348 | Chromosome | Enterobacter aerogenes |
| EcloCHE | AJ278994 | Chromosome | Enterobacter cloacae |
| EcloGC1 | D44479 | Chromosome | Enterobacter cloacae |
| EcloGN747 | AB016611 | Chromosome | Enterobacter cloacae |
| EcloMHN1 | X08082 | Chromosome | Enterobacter cloacae |
| EcloOUDh | AJ278995 | Chromosome | Enterobacter cloacae |
| EcloP99 | X07274 | Chromosome | Enterobacter cloacae |
| EcolK12 | AE000487 | Chromosome | Escherichia coli K-12 |
| FOX-1 | X77455 | Plasmid | |
| FOX-2 | Y10282 | Plasmid | |
| FOX-3 | Y11068 | Plasmid | |
| FOX-4 | AJ277535 | Plasmid | |
| FOX-5 | AY007369 | Plasmid | |
| HalvHA1 | AF180953 | Chromosome | Hafnia alvei |
| HalvHA10 | AF180961 | Chromosome | Hafnia alvei |
| HalvHA4 | AF180955 | Chromosome | Hafnia alvei |
| HalvHA8 | AF180959 | Chromosome | Hafnia alvei |
| LAT-1 | X78117a | Plasmid | |
| LAT-2 | X97039a | Plasmid | |
| LAT-3 | Y15411a | Plasmid | |
| LAT-4 | Y15412a | Plasmid | |
| LlacYK90 | X56660 | Chromosome | Lysobacter lactamgenus |
| MIR-1 | M37839 | Plasmid | |
| MmorSLM01 | Y10283 | Chromosome | Morganella morganii |
| MOX-1 | D13304 | Plasmid | |
| MOX-2 | AJ276453 | Plasmid | |
| Oant2171 | AJ299421 | Chromosome | Ochrobactrum anthropi |
| Oant82116 | AJ295342 | Chromosome | Ochrobactrum anthropi |
| PaerPAO1 | AE004827 | Chromosome | Pseudomonas aeruginosa |
| PimmA5 | X83586 | Chromosome | Psychrobacter immobilis |
| PstuVDG96 | Y17315 | Chromosome | Providencia stuartii |
| SmarSLS73 | AJ271368 | Chromosome | Serratia marcescens |
| SmarSR50 | X52964 | Chromosome | Serratia marcescens |
| SmarSRT1 | AB008454 | Chromosome | Serratia marcescens |
| SmarSST1 | AB008455 | Chromosome | Serratia marcescens |
| YentIP97 | X63149 | Chromosome | Yersinia enterocolitica |
Sequence corrected from the published sequence. See Table 2.
Phylogenies were constructed by the Bayesian method (19, 20, 27) as implemented with the program MrBayes (version 1.04). MrBayes is available from http://brahms.biology.rochester.edu/software.html. The Bayesian method seeks the most likely trees given the data (the alignment) and the evolutionary model. The posterior probabilities of the phylogenies, branch lengths, and substitution parameters cannot be calculated directly; however, they can be approximated by the Markov chain Monte Carlo (MCMC) process by sampling trees from the posterior probability distribution. A variant of the MCMC process called the metropolis-coupled Markov chain Monte Carlo (MCMCMC) process runs several chains, some of which are heated. A heated Markov chain has the posterior probability of a tree raised to some power i. Heated Markov chains can more easily cross deep likelihood valleys. The effect of heating is to fill in valleys and lower peaks; hence, a heated Markov chain can better explore the parameter space. Using the MCMCMC algorithm, a swap of the states between two chains is attempted at each step. If the swap is accepted, then the states for the two chains are exchanged. If a swap occurs between a heated chain and the cold chain, the cold chain might cross a large valley that it would normally cross with only a very small probability. A more detailed discussion of the method and its implementation is provided in reference 13.
Trees were saved every 100 generations. At the end of the run, a consensus tree with branch lengths was calculated from the saved trees. As the chains ran, the ln likelihoods of the trees converged on a stable value. Only trees saved well after the ln likelihoods had converged were used to calculate the consensus tree.
The evolutionary model was the general time reversible model (29), and among-site variation in the evolutionary rate was estimated separately for first, second, and third positions of sites within codons. Four chains, with a “temperature” of 0.2 for the heated chains, were run for 1,010,000 generations, with sampling of trees every 100 generations. The ln likelihoods of the trees had converged on a constant value by generation 10,000, i.e., after saving of 100 trees. The consensus tree was calculated from the final 10,000 trees visited, well after convergence had occurred.
One of the advantages of Bayesian inference of phylogeny is that the results are easy to interpret. For example, the sum of the posterior probabilities of all trees is 1. Moreover, the posterior probability of any single clade is simply the sum of the posterior probabilities of all trees that contain that clade. The consensus tree calculated by MrBayes does not include the posterior probabilities of the clades; thus, the entire set of trees was imported into PAUP* (version 4.0b4a; Sinauer Associates, Sunderland, Mass.), and the same trees used by MrBayes to calculate a consensus were used to calculate a 50% majority rule consensus in PAUP*. The resulting tree shows the posterior probabilities of the clades. The consensus tree calculated by MrBayes was imported into PAUP* for the purposes of displaying and printing the tree.
RESULTS
Sequence corrections.
Analysis of the sequences for LAT-1, LAT-2, LAT-3, LAT-4, and BIL-1 revealed several errors in the published sequences for their alleles (Table 2).
TABLE 2.
Corrections to published DNA sequences
| Allele | Type of sequence | Sequence (nucleotide)a |
|---|---|---|
| BIL-1 | Published | C (283) G (297) C (665) G (666) C (926) G (927) C (945) G (946) C (974) G (975) |
| Correct | G CG CG CG C G C | |
| LAT-1 | Published | C (23) C (99) G (168) C (288) T (289) G (290) A (358) T (555) |
| Correct | G TA TG CC C | |
| LAT-2 | Published | C (288) T (289) G (290) T (555) A (636) G (637) A (825) |
| Correct | T G CC G CG | |
| LAT-3 | Published | C (99) C (288) T (289) G (290) G (294) G (358) T (476) G (704) T (1015) G (1115) |
| Correct | TT GCCCAA A C | |
| LAT-4 | Published | C (99) C (288) T (289) G (290) T (1015) C (1115) |
| Correct | TT GCA T |
Nucleotides are numbered according to the coding sequence. The first base of the coding sequence is nucleotide 1.
Correction of those sequences showed that the sequences for BIL-1, LAT-2, and CMY-2 are identical (BIL-1-LAT-2-CMY-2). LAT-1 is identical to LAT-4, and LAT-4 and LAT-1 differ from CMY-2 by one nonsynonymous substitution. LAT-3 is the same as CMY-6. The differences are summarized in Table 3.
TABLE 3.
Nucleotide and amino acid substitutions in mobilized C. freundii ampC alleles
| Sitea | Nucleotide (amino acid) substitution in the following allele:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CMY-2-BIL-1-LAT-2 | CMY-3 | CMY-3b | CMY-4 | CMY-5 | CMY-6-LAT-3 | CMY-7 | LAT-4-LAT-1 | |
| 125 | A (E) | G (G) | ||||||
| 313 | C (L) | T (F) | ||||||
| 393 | C | T | ||||||
| 511 | G (A) | T (S) | ||||||
| 539 | C (A) | A (E) | ||||||
| 555 | C | |||||||
| 594 | C | |||||||
| 661 | T (W) | C (R) | C (R) | C (R) | ||||
| 662 | G (W) | T (L) | ||||||
| 704 | A (Q) | G (R) | ||||||
| 1088 | G (S) | A (N) | A (N) | |||||
| 1140 | G | A | A | |||||
Nucleotides are numbered according to the coding sequence. The first base of the coding sequence is nucleotide 1. CMY-2 is used as the reference sequence.
Phylogenetic analysis.
Figure 1 shows a Bayesian consensus phylogeny of the ampC genes. The phylogeny was rooted by using the alleles for Oant82116 and Oant2171 as the outgroup. These alleles come from the chromosome of Ochrobactrum anthropi, a species in the alpha group of the Proteobacteria, whereas all of the other ampC genes come from the chromosomes of species in the gamma group. This phylogeny is largely consistent with the taxonomic groupings of the gamma group. All of the Enterobacteriaceae are contained within a single clade, the members of which are descended from a single common ancestor. The Aeromonadaceae and Pseudomonadaceae are contained within a different single clade. For clades that had a probability of less than 90%, the most probable clade was used, and the probability of that clade is shown at its root.
FIG. 1.
Bayesian consensus phylogeny of the ampC genes. Probabilities of clades that are less than 90% are shown, except for the C. freundii clade. Abbreviations for taxon labels, accession numbers, gene locations (plasmid or chromosome), and organism names are given in Table 1.
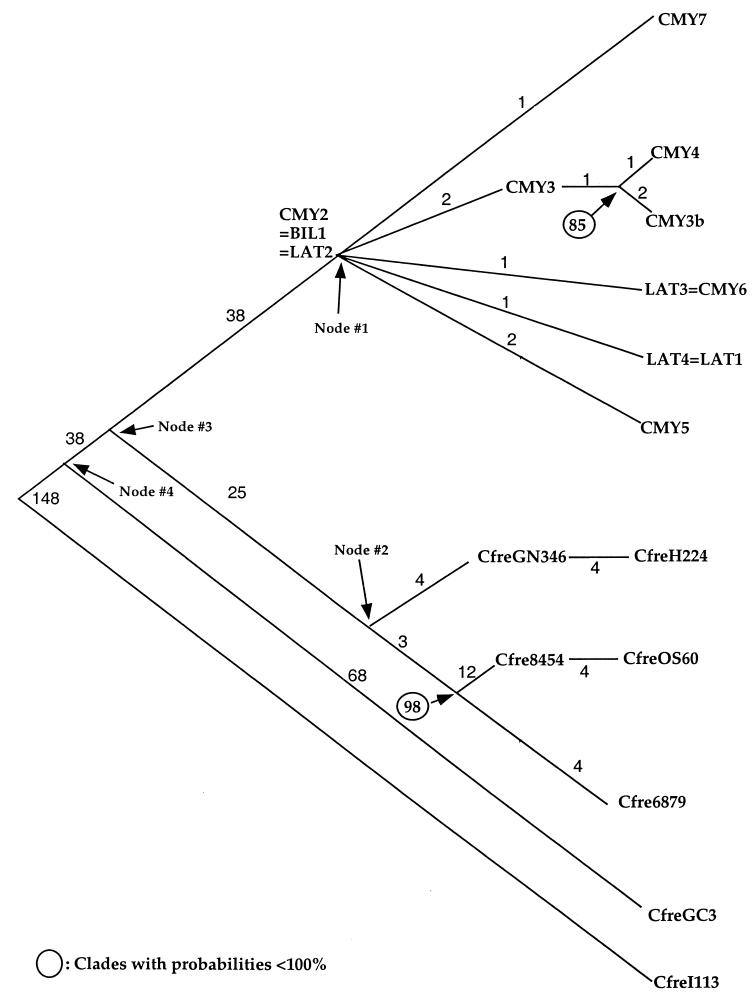
Figure 2 shows a Bayesian consensus phylogeny of the C. freundii ampC alleles. CfreI113 was used for the outgroup because it is the outgroup for this clade in the multispecies ampC phylogeny shown in Fig. 1. The Bayesian estimates for the number of nucleotide changes that have occurred along each of the branches are shown, as are the probabilities of clades that are less than 100%.
FIG. 2.
Bayesian consensus phylogeny of the C. freundii ampC alleles. Probabilities of clades that are less than 100% are shown. Bayesian estimates for the number of nucleotide changes that have occurred along each of the branches are also shown. Abbreviations for taxon labels, accession numbers, gene locations (plasmid or chromosome), and organism names are given in Table 1.
Phenotypic analysis.
The MICs of ampicillin, ticarcillin, ticarcillin-clavulanate, piperacillin, pipercillin-tazobactam, temocillin, cephalothin, cefoxitin, cefuroxime, ceftriaxone, cefotaxime, ceftazidime, cefepime, imipenem, meropenem, and aztreonam were determined for each strain carrying the cloned C. freundii ampC alleles (Table 4). Each of the alleles was cloned into the same vector and transformed into the same host background so that the resistance levels conferred by the alleles could be directly compared. Because the CMY-2-BIL-1-LAT-2 allele is the ancestor of the other plasmid-borne alleles, any evolution of increased activity toward an antibiotic would result in an MIC higher than that for CMY-2-BIL-1-LAT-2; therefore, the MIC for the CMY-2-BIL-1-LAT-2 allele should be used as a reference for comparing the MICs for the other alleles.
TABLE 4.
MICs for strains carrying cloned C. freundii ampC alleles
| β-Lactama | MIC (μg/ml) for strain carrying the following alleles:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| pACSE2 | CMY-2-BIL-1-LAT-2 | CMY-3 | CMY-3b | CMY-4 | LAT-3-CMY-6 | LAT-4-LAT-1 | Cfre6879 | Cfre8454 | |
| AMP | 2 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | 1,024 | ≥2,048 | ≥2,048 |
| PIP | 2 | 256 | 256 | 128 | 256 | 512 | 512 | 512 | 512 |
| TZP | 1 | 32 | 64 | 64 | 64 | 32 | 16 | 64 | 64 |
| TEM | 4 | 32 | 32 | 32 | 64 | 64 | 64 | 32 | 32 |
| TIC | 4 | 512 | 2,048 | 2,048 | 2,048 | 1,024 | 1,024 | 1,024 | 1,024 |
| TIM | 4 | 256 | 1,024 | 512 | 512 | 512 | 512 | 512 | 256 |
| CEF | 4 | 1,024 | 2,048 | 2,048 | 2,048 | 2,048 | 2,048 | 1,024 | 2,048 |
| FOX | 8 | 512 | 256 | 512 | 128 | 256 | 256 | 1,024 | 256 |
| CXM | 8 | 256 | 2,048 | ≥4,096 | 2,048 | 1,024 | 2,048 | 512 | 512 |
| CRO | 0.0625 | 128 | 128 | 64 | 128 | 256 | 256 | 256 | 256 |
| CTX | 0.125 | 64 | 128 | 64 | 256 | 128 | 256 | 128 | 128 |
| CAZ | 0.25 | 128 | 256 | 256 | 256 | 256 | 256 | 512 | 128 |
| FEP | 0.0625 | 1 | 1 | 2 | 0.5 | 0.5 | 1 | 8 | 1 |
| IPM | 0.125 | 0.5 | 0.25 | 0.25 | 0.5 | 0.25 | 0.25 | 0.5 | 0.25 |
| MEM | 0.0156 | 0.0625 | 0.0313 | 0.0625 | 0.0625 | 0.0625 | 0.0625 | 0.125 | 0.0625 |
| ATM | 0.125 | 16 | 256 | 128 | 128 | 64 | 128 | 256 | 128 |
Abbreviations for β-lactams: AMP, ampicillin; PIP, piperacillin; TZP, piperacillin-tazobactam (8:1); TEM, temocillin; TIC, ticarcillin; TIM, ticarcillin-clavulanate (30:1); CEF, cephalothin; FOX, cefoxitin; CXM, cefuroxime; CRO, ceftriaxone; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; IPM, imipenem; MEM, meropenem; and ATM, aztreonam. For drug-inhibitor combinations, the MIC is expressed as the drug concentration.
For the antibiotics tested, there was very little difference between the MICs for JM109/pMXCMY2 and those for the other strains containing the cloned alleles. There was a 4- to 16-fold increase in the cefuroxime and aztreonam MICs relative to those for JM109/pMXCMY2.
DISCUSSION
Any phylogeny is ultimately dependent upon the sequences from which it is constructed and is no more accurate than the data on which it is based. The errors that we found in the published ampC sequences serve as a caution to those constructing phylogenies. Extra caution is especially called for when one is using sequences determined without automated sequencing, because transcription errors are easy to make and fairly common. This caution is of particular importance in a phylogeny with short branches, because even a few errors in a single sequence can significantly alter the topology and branch lengths of the phylogeny.
Because there are several hundred nucleotide changes along the branches of the multispecies ampC phylogeny (Fig. 1) and because the phylogeny largely agrees with the taxonomic groupings of the Proteobacteria, we can be confident that even if some errors in sequencing data exist, this phylogeny provides a valid description of the relationships among the ampC alleles.
Phylogenetic analyses attempt to reconstruct historical genetic events. Such analyses seek the most parsimonious explanation, i.e., the explanation that requires the least number of events, to explain a tree that has been deduced from sequence or morphological comparisons. Because the phylogeny of the chromosomal ampC alleles closely matches the phylogeny of the species harboring those alleles, it is more parsimonious to believe that plasmid-borne alleles originated from chromosomal alleles rather than the other way around. A clade is a group that shares a common ancestor, and that common ancestor is represented by a node on the tree from which one can move outward (toward the tips of the tree) and reach any member of the clade. When a clade consists of plasmid-borne alleles, e.g., CMY-2, -2b, -3, -3b, -4, -5, and -7 and LAT-3 and -4, the inference is that the common ancestor was also plasmid borne. That common ancestor, indicated by node 1 in Fig. 2, is CMY-2. The chromosomal alleles in strains CfreGN346, CfreH224, Cfre8454, CfreOS60, and Cfre6879 also constitute a clade, whose common ancestor is indicated by node 2 in Fig. 2. Nodes 1 and 2 share a common ancestor indicated by node 3, while CfreGC3 and node 3 share a common ancestor indicated by node 4. If the allele at node 3 was chromosomal, then somewhere along the branch between node 3 and node 1 an allele was mobilized to a plasmid. If the ancestor at node 3 was plasmid borne, then somewhere along the branch between node 3 and node 4 an allele was mobilized to a plasmid. For that to be true, a plasmid-borne descendant of node 3 would have had to have moved into the Citrobacter chromosome along the branch between node 3 and node 2; thus, two events (moving from chromosome to plasmid and moving from plasmid to chromosome) would be required to explain the tree. The more parsimonious explanation is that a single event, moving from chromosome to plasmid, occurred along the branch from node 3 to node 1.
Using that phylogenetic reasoning, we have determined that the ampC gene has been mobilized at least six times. The LAT alleles and most of the CMY alleles are descended from a C. freundii ampC gene. This group includes CMY-3b, which was found in the chromosome of P. mirabilis (8) and which must have entered that chromosome by horizontal transfer from C. freundii. ACT-1 and MIR-1 are descended from an ampC gene of E. cloacae. DHA-1 and DHA-2 are descended from Morganella morganii ampC (3, 26). An M. morganii chromosomal ampC allele that is identical to the plasmid-borne DHA-1 allele has been reported (26), suggesting that the ampC gene from M. morganii was mobilized twice. ACC-1 is descended from Hafnei alvei ampC. The FOX alleles are descended from an Aeromonas sobria ampC gene. CMY-1, CMY-8, and CMY-9 and the MOX alleles were also mobilized from the Aeromonadacaea group, although there is not sufficient resolution in that portion of the tree to determine the species from which they came. The phylogeny shows that although the ampC gene has been mobilized at least six times, with the exception of Morganella, it has only been mobilized once from any given species. This finding suggests to us that once an antibiotic resistance gene from a given species is mobilized, it is less likely that the gene will be successfully mobilized again from that species than from a different species. Once a gene has been mobilized and is free to move horizontally, it will spread through nearby members of that species and perhaps other species. Those species, in effect, constitute an ecological niche for the population of plasmid-borne alleles. For another allele to be mobilized and to spread, it must compete successfully with the first allele to be mobilized When phylogenetic analysis detects a single mobilization, it means either that no alleles mobilized later were able to compete with the allele mobilized first and thus left no descendants or that an allele mobilized later outcompeted and completely displaced an allele mobilized earlier from the population before it was detected, in which event the allele mobilized earlier left no descendants. Just as phylogenetic analysis cannot detect the existence of species that left no descendants, it cannot detect mobilization events that left no descendants.
From the multispecies phylogeny shown in Fig. 1, we focused our attention on the C. freundii clade because there are numerous plasmid-borne and chromosomal alleles within this clade, including pre-antibiotic era alleles. In addition, there is good agreement between the historical information about the origin and spread of the C. freundii ampC alleles and the phylogeny (Fig. 2). The phylogeny shows that the C. freundii ampC gene has been mobilized once. This finding is consistent with the fact that all of the plasmid-borne C. freundii ampC alleles have very similar sequences. The phylogeny shows that CMY-2-BIL-1-LAT-2 is the most recent common ancestor of all of the plasmid-borne C. freundii ampC alleles. CMY-2 and BIL-1 were the first reported plasmid-borne ampC alleles from C. freundii (5, 12). Therefore, the historical data also suggest that CMY-2-BIL-1-LAT-2 is the ancestor of the other C. freundii ampC alleles that have been found on plasmids since then.
It has been suggested that the discovery of plasmids bearing class C β-lactamases was delayed because, in the past, resistance to cephamycins was generally attributed to the up-regulation of chromosomal β-lactamases, such as AmpC (5). The finding that CMY-2 and BIL-1 have the same nucleotide sequence supports this suggestion. CMY-2 and BIL-1 were reported within 1 year of each other (5, 12). CMY-2 was found in a Klebsiella pneumoniae isolate collected in Greece, and BIL-1 was found in an E. coli isolate that originated in Pakistan. Although we have no way of estimating how long the C. freundii ampC gene was mobilized before this discovery was made, the fact that CMY-2 or BIL-1 was found in two different species of bacteria in two different areas at about the same time means that it was mobilized somewhat earlier than it was found.
At the sequence level, the evolution of the plasmid-borne C. freundii AmpC β-lactamases resembles the evolution of the TEM β-lactamases. Like the AmpC enzymes, most of the TEM enzymes vary from their ancestor, TEM-1, by only a few amino acids. (For a table of the amino acid sequences of the TEMs, see http://www.lahey.org/studies/webt.htm.) The evolution of the chromosomal ampC alleles, however, contrasts with the evolution of the TEMs. Because they were not subject to selection by the clinical use of antibiotics, it is surprising that the pre-antibiotic era ampC alleles from strains Cfre6879 and Cfre8454 lie either at or near the tips of the phylogeny rather than at a deep node. Cfre6879 lies at the tip of the phylogeny, while Cfre8454 lies at an internal node of the phylogeny, four nucleotide substitutions away from CfreOS60. The chromosomal alleles in this clade and the mobilized alleles in the CMY-2 clade are about the same distance (25 to 38 substitutions) from their most recent common ancestor.
Phenotypically, the evolution of the TEM enzymes and that of the C. freundii AmpC enzymes are very different. The evolution of the TEM enzymes has produced numerous phenotypes that give the TEM enzymes activity against extended-spectrum β-lactams (21) and resistance to β-lactamase inhibitors (18). On the other hand, the resistance levels conferred by the pre-antibiotic era alleles of ampC are essentially the same as the resistance levels conferred by the ampC alleles that have been found on plasmids and isolated from clinically resistant strains of bacteria. This means that, except for resistance to aztreonam and cefuroxime, the AmpC enzymes are not evolving phenotypically. Two explanations immediately come to mind: first, the C. freundii AmpC enzymes may not have potential for evolving new phenotypes; second, because they are already capable of conferring resistance to most β-lactams, there may be little pressure for increased resistance. The first explanation could explain why the AmpC enzymes have not yet evolved the ability to confer resistance to imipenem or meropenem. AmpC may well lack the potential to evolve truly novel carbapenem-hydrolyzing activity. That possibility would be entirely consistent with the failure to find any carbapenem-hydrolyzing AmpC β-lactamases in nature. There may well be a real distinction between the potential to evolve a completely novel activity and the potential to improve upon an existing activity. Low selection pressure could explain the lack of an increase in resistance conferred by CMY-2-derived alleles toward most β-lactams. Because C. freundii ampC alleles could already confer high levels of resistance to most β-lactam antibiotics before those antibiotics ever came into clinical use, there probably has not been a need for increased activity against them. Alternatively, the potential for improved activity toward most β-lactams other than carbapenems may be physically limited as well. Bulychev and Mobashery (9) have shown that the activity of AmpC β-lactamase from E. cloacae toward its preferred substrates, cephaloridine and cephalosporin C, is diffusion limited and therefore not subject to further improvement. Both the pre-antibiotic era chromosomal alleles and the plasmid-borne alleles encode enzymes with significant activities toward all of the tested drugs, except for the carbapenems and cefepime. It is possible that those enzymes are at or near the diffusion limit with respect to those substrates.
Cefepime stands out as the sole tested drug toward which all the enzymes exhibit some activity, but insufficient activity to confer significant resistance. We speculate that these AmpC β-lactamases do indeed have the potential to evolve improved activity toward cefepime, activity that will confer resistance, and yet none have achieved that potential because the relatively recent introduction of cefepime (U.S. Food and Drug Administration approval in 1996) has not allowed selection over a sufficient period of time.
Perhaps as new antibiotics are introduced into the clinical setting or perhaps after the carbapenems and cefepime have been used more extensively, the AmpC enzymes will evolve new phenotypes and gain the ability to confer resistance to antibiotics that they originally could not hydrolyze.
Acknowledgments
We are grateful to G. Jacoby, E. Tzelepi, L. S. Tzouvelekis, A. Philippon, A. Fosberry, D. Payne, and D. Sirot for providing strains. We thank one of the reviewers for many helpful suggestions.
This study was supported by grant GM60761 from the National Institutes of Health.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnaud, G., G. Arlet, C. Verdet, O. Gaillot, P. H. Lagrange, and A. Philippon. 1998. Salmonella enteritidis: AmpC plasmid-mediated inducible beta-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob. Agents Chemother. 42:2352-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauernfeind, A., Y. Chong, and S. Schweighart. 1989. Extended broad spectrum beta-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 17:316-321. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind, A., I. Stemplinger, R. Jungwirth, and H. Giamarellou. 1996. Characterization of the plasmidic beta-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob. Agents Chemother. 40:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudes, H., F. Jude, E. B. Chaibi, C. Arpin, C. Bebear, R. Labia, and C. Quentin. 1999. Molecular characterization of TEM-59 (IRT-17), a novel inhibitor-resistant TEM-derived beta-lactamase in a clinical isolate of Klebsiella oxytoca. Antimicrob. Agents Chemother. 43:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blazquez, J., M. R. Baquero, R. Canton, I. Alos, and F. Baquero. 1993. Characterization of a new TEM-type beta-lactamase resistant to clavulanate, sulbactam, and tazobactam in a clinical isolate of Escherichia coli. Antimicrob. Agents Chemother. 37:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bret, L., C. Chanal-Claris, D. Sirot, E. B. Chaibi, R. Labia, and J. Sirot. 1998. Chromosomally encoded AmpC-type beta-lactamase in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 42:1110-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulychev, A., and S. Mobashery. 1999. Class C β-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin substrates. Antimicrob. Agents Chemother. 43:1743-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta, N., and P. Kontomichalou. 1965. Penicillinase synthesis controlled by infectious R factors in Enterobacteriacae. Nature 208:239-241. [DOI] [PubMed] [Google Scholar]
- 12.Fosberry, A. P., D. J. Payne, E. J. Lawlor, and J. E. Hodgson. 1994. Cloning and sequence analysis of blaBIL-1, a plasmid-mediated class C beta-lactamase gene in Escherichia coli BS. Antimicrob. Agents Chemother. 38:1182-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, B. G. 2001. Phylogenetic trees made easy: a how-to manual for molecular biologists. Sinauer Associates, Sunderland, Mass.
- 14.Higgins, D. G., and P. M. Sharp. 1988. CLUSTAL: a package for performing multiple sequence alignement on a microcomputer. Gene 73:237-244. [DOI] [PubMed] [Google Scholar]
- 15.Jaurin, B., and T. Grundstrom. 1981. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc. Natl. Acad. Sci. USA 78:4897-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg, F., and S. Normark. 1986. Contribution of chromosomal beta-lactamases to beta-lactam resistance in enterobacteria. Rev. Infect. Dis. 8(Suppl. 3):S292-S304. [DOI] [PubMed] [Google Scholar]
- 17.Livermore, D. M. 1996. Are all beta-lactams created equal? Scand. J. Infect. Dis. Suppl. 101:33-43. [PubMed] [Google Scholar]
- 18.Martinez, J. L., E. Cercenado, M. Rodriguez-Creixems, M. F. Vincente-Perez, A. Delgado Iribarren, and F. Baquero. 1987. Resistance to beta-lactam/clavulanate. Lancet ii:1473. [DOI] [PubMed] [Google Scholar]
- 19.Mau, B., and M. Newton. 1997. Phylogenetic inference for binary data on dendrograms using Markov chain Monte Carlo. J. Comput. Graph. Stat. 6:122-131. [Google Scholar]
- 20.Mau, B., M. Newton, and B. Larget. 1999. Bayesian phylogenetic inference via Markov chain Monte Carlo methods. Biometrics 55:1-12. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros, A. A. 1997. Evolution and dissemination of beta-lactamases accelerated by generations of beta-lactam antibiotics. Clin. Infect. Dis. 24:S19-S45. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial susceptibility testing; ninth informational supplement. NCCLS document M100-S9, vol. 19. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Nikaido, H., and S. Normark. 1987. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol. Microbiol. 1:29-36. [DOI] [PubMed] [Google Scholar]
- 24.Nordmann, P. 1998. Trends in beta-lactam resistance among Enterobacteriaceae. Clin. Infect. Dis. 27(Suppl. 1):S100-S106. [DOI] [PubMed] [Google Scholar]
- 25.Papanicolaou, G. A., A. A. Medeiros, and G. A. Jacoby. 1990. Novel plasmid-mediated beta-lactamase (MIR-1) conferring resistance to oxyimino- and alpha-methoxy beta-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 34:2200-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel, L., M. Guibert, D. Girlich, T. Naas, and P. Nordmann. 1999. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rannala, B., and Z. H. Yang. 1996. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. E 43:304-311. [DOI] [PubMed] [Google Scholar]
- 28.Sarles, W. B., and B. W. Hammer. 1933. Species of Escherichia-Aerobacter organisms responsible for some defects in dairy products. J. Bacteriol. 26:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavaré, L. 1986. Some probabilistic and statistical problems on the analysis of DNA sequences. Lect. Math. Life Sci. 17:57-86. [Google Scholar]
- 30.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positiion specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werkman, C. H., and G. F. Gillen. 1932. Bacteria producing trimethylene glycol. J. Bacteriol. 23:167-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]