Abstract
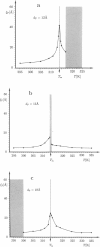
Monte Carlo simulation techniques have been applied to a statistical mechanical lattice model in order to study the coherence length for the spatial fluctuations of the lipid order parameter profiles around integral membrane proteins in dipalmitoyl phosphatidylcholine bilayers. The model, which provides a detailed description of the pure lipid bilayer main transition, incorporates hydrophobic matching between the lipid and protein hydrophobic thicknesses as a major contribution to the lipid-protein interactions in lipid membranes. The model is studied at low protein-to-lipid ratios. The temperature dependence of the coherence length is found to have a dramatic peak at the phase transition temperature. The dependence on protein circumference as well as hydrophobic length is determined and it is concluded that in some cases the coherence length is much longer than previously anticipated. The long coherence length provides a mechanism for indirect lipid-mediated protein-protein long-range attraction and hence plays an important role in regulating protein segregation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Owicki J. C. Self diffusion of interacting membrane proteins. Biophys J. 1989 May;55(5):817–833. doi: 10.1016/S0006-3495(89)82882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Cornell B. A., Ellasz A. W., Perry A. Interactions of helical polypepetide segments which span the hydrocarbon region of lipid bilayers. Studies of the gramicidin A lipid-water system. J Mol Biol. 1977 Jul 5;113(3):517–538. doi: 10.1016/0022-2836(77)90236-4. [DOI] [PubMed] [Google Scholar]
- Cruzeiro-Hansson L., Mouritsen O. G. Passive ion permeability of lipid membranes modelled via lipid-domain interfacial area. Biochim Biophys Acta. 1988 Sep 15;944(1):63–72. doi: 10.1016/0005-2736(88)90316-1. [DOI] [PubMed] [Google Scholar]
- Edholm O., Johansson J. Lipid bilayer polypeptide interactions studied by molecular dynamics simulation. Eur Biophys J. 1987;14(4):203–209. doi: 10.1007/BF00256353. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Houslay M. D., McGill K. A., Birdsall N. J., Metcalfe J. C., Warren G. B. Annular lipids determine the ATPase activity of a calcium transport protein complexed with dipalmitoyllecithin. Biochemistry. 1976 Sep 21;15(19):4145–4151. doi: 10.1021/bi00664a002. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H., Jørgensen K., Mouritsen O. G. Density fluctuations in saturated phospholipid bilayers increase as the acyl-chain length decreases. Biophys J. 1990 Nov;58(5):1099–1107. doi: 10.1016/S0006-3495(90)82452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsen J. H., Mouritsen O. G., Bloom M. Relationships between lipid membrane area, hydrophobic thickness, and acyl-chain orientational order. The effects of cholesterol. Biophys J. 1990 Mar;57(3):405–412. doi: 10.1016/S0006-3495(90)82557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H. The lipid-protein interface in biological membranes. Ann N Y Acad Sci. 1980;348:391–407. doi: 10.1111/j.1749-6632.1980.tb21315.x. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. I. Theoretical description. Biophys J. 1981 Nov;36(2):329–345. doi: 10.1016/S0006-3495(81)84735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. II. Interpretation of experimental results. Biophys J. 1981 Nov;36(2):347–357. doi: 10.1016/S0006-3495(81)84736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Bacteriorhodopsin remains dispersed in fluid phospholipid bilayers over a wide range of bilayer thicknesses. J Mol Biol. 1983 May 15;166(2):203–210. doi: 10.1016/s0022-2836(83)80006-0. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Lookman T., Pink D. A., Grundke E. W., Zuckermann M. J., deVerteuil F. Phase separation in lipid bilayers containing integral proteins. Computer simulation studies. Biochemistry. 1982 Oct 26;21(22):5593–5601. doi: 10.1021/bi00265a032. [DOI] [PubMed] [Google Scholar]
- MacDonald A. L., Pink D. A. Thermodynamics of glycophorin in phospholipid bilayer membranes. Biochemistry. 1987 Apr 7;26(7):1909–1917. doi: 10.1021/bi00381a019. [DOI] [PubMed] [Google Scholar]
- Marcelja S. Chain ordering in liquid crystals. II. Structure of bilayer membranes. Biochim Biophys Acta. 1974 Oct 29;367(2):165–176. doi: 10.1016/0005-2736(74)90040-6. [DOI] [PubMed] [Google Scholar]
- Marcelja S. Lipid-mediated protein interaction in membranes. Biochim Biophys Acta. 1976 Nov 11;455(1):1–7. doi: 10.1016/0005-2736(76)90149-8. [DOI] [PubMed] [Google Scholar]
- Mendelsohn R., Dluhy R., Taraschi T., Cameron D. G., Mantsch H. H. Raman and Fourier transform infrared spectroscopic studies of the interaction between glycophorin and dimyristoylphosphatidylcholine. Biochemistry. 1981 Nov 10;20(23):6699–6706. doi: 10.1021/bi00526a027. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Jippo T., Kataoka R. Thermodynamic properties of the lipid bilayer transition. Pseudocritical phenomena. Biophys J. 1983 May;42(2):137–144. doi: 10.1016/S0006-3495(83)84379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984 Aug;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owicki J. C., McConnell H. M. Theory of protein-lipid and protein-protein interactions in bilayer membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4750–4754. doi: 10.1073/pnas.76.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owicki J. C., Springgate M. W., McConnell H. M. Theoretical study of protein--lipid interactions in bilayer membranes. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1616–1619. doi: 10.1073/pnas.75.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink D. A., Chapman D. Protein-lipid interactions in bilayer membranes: a lattice model. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1542–1546. doi: 10.1073/pnas.76.4.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink D. A., Green T. J., Chapman D. Raman scattering in bilayers of saturated phosphatidylcholines. Experiment and theory. Biochemistry. 1980 Jan 22;19(2):349–356. doi: 10.1021/bi00543a016. [DOI] [PubMed] [Google Scholar]
- Pink D. A. Theoretical studies of phospholipid bilayers and monolayers. Perturbing probes, monolayer phase transitions, and computer simulations of lipid-protein bilayers. Can J Biochem Cell Biol. 1984 Aug;62(8):760–777. doi: 10.1139/o84-098. [DOI] [PubMed] [Google Scholar]
- Riegler J., Möhwald H. Elastic interactions of photosynthetic reaction center proteins affecting phase transitions and protein distributions. Biophys J. 1986 Jun;49(6):1111–1118. doi: 10.1016/S0006-3495(86)83740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero A., Hudson B. Critical density fluctuations in lipid bilayers detected by fluorescence lifetime heterogeneity. Biophys J. 1989 Jun;55(6):1111–1124. doi: 10.1016/S0006-3495(89)82908-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler D. M., Worcester D. L. Neutron scattering studies of photosynthetic membranes in aqueous dispersion. J Mol Biol. 1982 Aug 15;159(3):485–499. doi: 10.1016/0022-2836(82)90297-2. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Distance dependence of the diffusion coefficient. Biophys J. 1989 Sep;56(3):615–622. doi: 10.1016/S0006-3495(89)82708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Seelig J. Deuterium order parameters in relation to thermodynamic properties of a phospholiped bilayer. A statistical mechanical interpretation. Biochemistry. 1975 Jun 3;14(11):2283–2287. doi: 10.1021/bi00682a001. [DOI] [PubMed] [Google Scholar]
- Scott H. L. Monte Carlo calculations of order parameter profiles in models of lipid-protein interactions in bilayers. Biochemistry. 1986 Oct 7;25(20):6122–6126. doi: 10.1021/bi00368a043. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Sperotto M. M., Ipsen J. H., Mouritsen O. G. Theory of protein-induced lateral phase separation in lipid membranes. Cell Biophys. 1989 Feb;14(1):79–95. doi: 10.1007/BF02797393. [DOI] [PubMed] [Google Scholar]