Abstract
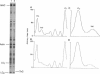
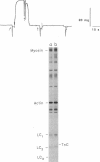
Myosin binding-induced activation of the thin filament was examined in isolated cardiac myocytes and single slow and fast skeletal muscle fibers. The number of cross-bridge attachments was increased by stepwise lowering of the [MgATP] in the Ca(2+)-free solution bathing the preparations. The extent of thin filament activation was determined by monitoring steadystate isometric tension at each MgATP concentration. As pMgATP (where pMgATP is -log [MgATP]) was increased from 3.0 to 8.0, isometric tension increased to a peak value in the pMgATP range of 5.0-5.4. The steepness of the tension-pMgATP relationship, between the region of the curve where tension was zero and the peak tension, is hypothesized to be due to myosin-induced cooperative activation of the thin filament. Results showed that the steepness of the tension-pMgATP relationship was markedly greater in cardiac as compared with either slow or fast skeletal muscle fibers. The steeper slope in cardiac myocytes provides evidence of greater myosin binding-induced cooperative activation of the thin filament in cardiac as compared with skeletal muscle, at least under these experimental conditions of nominal free Ca2+. Cooperative activation is also evident in the tension-pCa relation, and is dependent upon thin filament molecular interactions, which require the presence of troponin C. Thus, it was determined whether myosin-based cooperative activation of the thin filament also requires troponin C. Partial extraction of troponin C reduced the steepness of the tension-pMgATP relationship, with the effect being significantly greater in cardiac than in skeletal muscle. After partial extraction of troponin C, muscle type differences in the steepness of the tension-pMgATP relationship were no longer apparent, and reconstitution with purified troponin C restored the muscle lineage differences. These results suggest that, in the absence of Ca2+, myosin-mediated activation of the thin filament is greater in cardiac than in skeletal muscle, and this apparent cooperativity requires the presence of troponin C on thin filament regulatory strands.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt P. W., Diamond M. S., Schachat F. H. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J Mol Biol. 1984 Dec 5;180(2):379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Reuben J. P., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. II. Role of calcium. J Gen Physiol. 1972 Mar;59(3):305–317. doi: 10.1085/jgp.59.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Roemer D., Schachat F. H. Co-operative activation of skeletal muscle thin filaments by rigor crossbridges. The effect of troponin C extraction. J Mol Biol. 1990 Apr 5;212(3):473–480. doi: 10.1016/0022-2836(90)90326-H. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Stein E. A. Calmodulin-free skeletal-muscle troponin C prepared in the absence of urea. Biochem J. 1981 Apr 1;195(1):205–211. doi: 10.1042/bj1950205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Ridgway E. B. Stretch of active muscle during the declining phase of the calcium transient produces biphasic changes in calcium binding to the activating sites. J Gen Physiol. 1990 Nov;96(5):1013–1035. doi: 10.1085/jgp.96.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Hannon J. D., Martyn D. A., Gordon A. M. Effects of cycling and rigor crossbridges on the conformation of cardiac troponin C. Circ Res. 1992 Oct;71(4):984–991. doi: 10.1161/01.res.71.4.984. [DOI] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Two rigor states in skinned crayfish single muscle fibers. J Gen Physiol. 1976 Sep;68(3):267–280. doi: 10.1085/jgp.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N., Ogawa Y. Increase by trifluoperazine in calcium sensitivity of myofibrils in a skinned fibre from frog skeletal muscle. J Physiol. 1988 Sep;403:407–424. doi: 10.1113/jphysiol.1988.sp017256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Greaser M. L., Moss R. L. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989 May;93(5):855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Kinetics of a Ca(2+)-sensitive cross-bridge state transition in skeletal muscle fibers. Effects due to variations in thin filament activation by extraction of troponin C. J Gen Physiol. 1991 Aug;98(2):233–248. doi: 10.1085/jgp.98.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Myosin light chain 2 modulates calcium-sensitive cross-bridge transitions in vertebrate skeletal muscle. Biophys J. 1992 Aug;63(2):460–468. doi: 10.1016/S0006-3495(92)81614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Thin filament regulation of shortening velocity in rat skinned skeletal muscle: effects of osmotic compression. J Physiol. 1988 Apr;398:165–175. doi: 10.1113/jphysiol.1988.sp017036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Parmacek M. S., Barr E., Pasyk K., Lin W. I., Cochrane K. L., Field L. J., Leiden J. M. Skeletal troponin C reduces contractile sensitivity to acidosis in cardiac myocytes from transgenic mice. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9036–9040. doi: 10.1073/pnas.90.19.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Allen J. D., Greaser M. L. Effects of partial extraction of troponin complex upon the tension-pCa relation in rabbit skeletal muscle. Further evidence that tension development involves cooperative effects within the thin filament. J Gen Physiol. 1986 May;87(5):761–774. doi: 10.1085/jgp.87.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985 Oct;86(4):585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Haworth R. A. Contraction of rabbit skinned skeletal muscle fibers at low levels of magnesium adenosine triphosphate. Biophys J. 1984 Apr;45(4):733–742. doi: 10.1016/S0006-3495(84)84216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. M., Weber A. Cooperativity of the calcium switch of regulated rabbit actomyosin system. Mol Cell Biochem. 1981 Feb 26;35(1):11–15. doi: 10.1007/BF02358183. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B., Mahdavi V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J Clin Invest. 1989 Dec;84(6):1693–1700. doi: 10.1172/JCI114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. S., Gordon A. M., Luo Z. X. Removal of tropomyosin overlap modifies cooperative binding of myosin S-1 to reconstituted thin filaments of rabbit striated muscle. J Biol Chem. 1989 May 25;264(15):8495–8498. [PubMed] [Google Scholar]
- Pan B. S., Solaro R. J. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J Biol Chem. 1987 Jun 5;262(16):7839–7849. [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz D. R., Moss R. L. Influence of a strong-binding myosin analogue on calcium-sensitive mechanical properties of skinned skeletal muscle fibers. J Biol Chem. 1992 Oct 5;267(28):20497–20506. [PubMed] [Google Scholar]
- Sweitzer N. K., Moss R. L. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990 Dec;96(6):1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman L. S., Sawyer D. Calcium binds cooperatively to the regulatory sites of the cardiac thin filament. J Biol Chem. 1990 Jan 15;265(2):931–939. [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Reciprocal coupling between troponin C and myosin crossbridge attachment. Biochemistry. 1989 Aug 8;28(16):6751–6756. doi: 10.1021/bi00442a031. [DOI] [PubMed] [Google Scholar]