Abstract
Leishmania-induced macrophage dysfunctions have been correlated with altered signaling events. In this work, we report that SB203580, a specific inhibitor of p38 mitogen-activated protein kinases (MAPK), increases Leishmania donovani survival in human peripheral blood mononuclear macrophages. Consistent with this finding, activation of p38 and c-jun N-terminal kinase (JNK) MAPK signaling pathways by anisomycin significantly reduced parasite survival within these cells. However, the majority of the effect was seen in a 50% reduction in the percentage of macrophages infected, with little effect on the highly infected macrophages. The observed effect was likely to be due to the p38 MAPK pathway since SB203580 was able to completely reverse the effect of anisomycin. These findings suggest that the previously reported p38 MAPK inhibition by Leishmania infection may be partially overcome by anisomycin. Similar effects were observed in pretreated macrophages or in treatment of infected macrophages. These results suggests that p38 MAPK activation may have a potential therapeutic value in the treatment of visceral leishmaniasis.
The parasitic protozoan Leishmania donovani is the causative agent of visceral leishmaniasis in humans. This obligate intracellular parasite resides and multiplies in macrophages. Macrophages are regarded as important players for the initiation and regulation of immune responses upon activation and host defense (46). Macrophage activation for enhanced microbial activity is a critical requirement leading to the successful elimination of Leishmania. Survival within this hostile environment therefore requires rapid physiological adaptation of the organism and involves parasite-mediated modulation of various macrophage cell functions. Leishmania-infected macrophages have been found to have reduced capacity to produce interleukin-1 (IL-1) and tumor necrosis factor α (TNF-α) (7, 35). Other functional alterations in Leishmania-infected macrophages include a diminished oxidative burst (4) and defective expression of IFN-γ-induced major histocompatibility complex class II molecules (36).
Leishmania-induced macrophage deactivation during intracellular infection may be linked to defects in the signaling pathways, which in turn facilitate the parasites' infection and propagation within the cell (34). The antimicrobial state of macrophages is dependent on the appropriate phosphorylation of cellular proteins, and the interference with the phosphorylation process by the parasite is a key way that intracellular survival of Leishmania can be enhanced. There are several lines of evidence that Leishmania interferes with signaling transduction in macrophages. IFN-γ-induced tyrosine phosphorylation of Jak1, Jak2, and Stat are impaired following infection by L. donovani (27). Cell signaling for many functional responses to extracellular stimuli may require activation of protein kinase C (PKC), which induces the expression of the transcriptional regulatory protein c-fos (23). Infection of macrophages with L. major and L donovani or treatment of macrophages with lipophosphoglycan, the major surface glycoconjugate of Leishmania, has been shown to inhibit macrophage PKC-dependent signaling, thereby impairing c-fos gene expression (13, 22, 23, 28). PKC can induce downstream activation of mitogen-activated protein kinases (MAPKs) (40), suggesting the possibility that Leishmania parasites can impair signaling through these enzymes in infected cells.
MAPKs are a group of serine/threonine kinases, the enzymatic activity of which is elicited upon phosphorylation of threonine and tyrosine residues in a Thr-X-Tyr motif in their regulatory domain (42). The MAPK family includes extracellular signal-related kinase 1 and 2 (ERK1/2), c-jun NH2-terminal kinase (JNK), and p38 MAPK. MAPKs phosphorylate selected intracellular proteins, including transcription factors, which subsequently regulate gene expression by transcriptional and posttranscriptional mechanisms (40). Each of these kinases is regulated by other upstream kinases (18). These three families of MAPKs form three parallel signaling cascades activated by distinct or sometimes overlapping sets of stimuli. The ERKs are activated by mitogens and growth factors and mediate signals promoting cell proliferation, differentiation, and survival. JNK and p38 MAPKs are predominantly activated by stress such as osmotic changes and heat shock, but also by inflammatory cytokines TNF-α and IL-1β and bacterial lipopolysaccharide (LPS) (29, 31, 33).
Macrophage responses to phorbol-12 myristate-13-acetate have been shown to be impaired following infection with Leishmania, resulting in a significant decrease in phorbol-12 myristate-13-acetate-induced tyrosine phosphorylation of MAPK and a reduction in the phosphorylation of Elk1, a MAPK substrate (26). Nandan et al. (26) also demonstrated that infection with L. donovani induces phosphotyrosine phosphatases which attenuate MAPK signaling and c-fos induction. NO-dependent killing in macrophages activated by IFN-γ is inhibited by Leishmania (16). It has been observed that specific inhibitors of p38 and ERK pathways block inducible NO synthase (iNOS) and TNF-α in murine macrophages exposed to LPS and IFN-γ (1). The above points suggest that MAPKs may be involved in multiple aspects of the innate response to infection.
In view of the documented ability of Leishmania parasites to inhibit various microbicidal mechanisms, it was of interest to investigate to what extent activation of MAPK pathways would affect L. donovani survival, particularly during the initial stages of infection. The outcome would provide an indication of the extent to which the parasite was dependent on such down-regulation of macrophage function. The disease profile of leishmaniasis is an important immune model in murine systems, and most work on altered macrophage signaling upon Leishmania infection has focused on murine cells (12, 22, 26, 32), and therefore, we investigated the in vitro responses of human-blood-derived peripheral macrophages. Anisomycin has been shown to elicit specific and strong activation of JNK and p38 MAPKs and induce c-fos gene expression (6). Here, we report that anisomycin inhibited parasite survival within macrophages and that the observed killing with anisomycin was dependent on the p38 MAPK pathway.
MATERIALS AND METHODS
Reagents and antibodies.
RPMI 1640, M199, fetal calf serum (FCS), penicillin, and streptomycin were purchased from GIBCO/BRL. SB203580 was obtained from Calbiochem (Nottingham, United Kingdom). Anisomycin (Streptomyces griseolus) was obtained from Sigma. Antibodies against phosphorylated and nonphosphorylated p38 MAPK, horseradish peroxidase-conjugated anti-rabbit immunoglobulin G, and PD98059 were obtained from New England Biolabs (Hitchin, Hertfordshire, United Kingdom).
Parasites.
L. donovani (MHOM/ET/67/L82) amastigotes were periodically recovered from the spleens of infected hamsters (provided by V. Yardley and S. L. Croft, London School of Hygiene and Tropical Medicine). Promastigotes were derived through amastigote culture in M199 supplemented with 10% FCS, 2 mM glutamine, penicillin G (100 U/ml), streptomycin sulfate (100 μg/ml), 2 mM glutamate, 1 mM sodium pyruvate, and 10 mM HEPES at 26°C. Promastigotes were used at the stationary phase of growth, which was reached at approximately 7 to 9 days after subculture (39). Parasites were kept in culture by weekly passaging.
Cell preparation and culture.
Peripheral blood mononuclear cells were isolated from buffy coats obtained from healthy donors (National Blood Service, North London Branch, London, United Kingdom) on Histopaque (density, 1.077 g/ml; Sigma). The mononuclear cell fraction was washed three times and suspended in warm RPMI 1640 medium supplemented with 2 mM glutamine, penicillin G (100 U/ml), streptomycin sulfate (100 μg/ml), 2 mM glutamate, 1 mM sodium pyruvate, and 10 mM HEPES. Cell viability was determined using trypan blue (Sigma). Cells (2 × 106) were dispensed into 24-well plates for the preparation of cell lysates, and cells (4 × 105) were dispensed in eight-well chamber slides (Labtek) for infection assays and allowed to adhere for 2 h at 37°C in 5% CO2. Nonadherent cells were washed off, and wells were replenished with supplemented RPMI 1640 containing 10% FCS for 5 to 6 days to generate a more differentiated phenotype.
Infection of macrophages.
Macrophages were either treated pre- or postinfection with a p38 MAPK inhibitor (SB203580), p42/44 MAPK (ERK1/2) inhibitor PD98059, or anisomycin at the indicated concentrations. Cells were then infected at a parasite-to-macrophage ratio of 20:1 for 2 h at 37°C in a humidified atmosphere of 5% CO2, after which noningested promastigotes were washed with warm RPMI. Infected cells were fixed with methanol for 10 min, air dried, and stained with Giemsa's stain (BDH) diluted 1:9 in Sorenson's buffer (4.8 mM Na2HPO4, 1.9 mM KH2PO4). The numbers of infected macrophages and parasite burden (number of parasites per 100 infected macrophages) were determined by counting 500 macrophages on each duplicate of coverslips. Each experiment was repeated at least three times. Results were expressed as mean ± standard error of the mean (SEM) of individual experiments. In other experiments, infected macrophages were treated with anisomycin and/or LPS and supernatants were collected after 18 h for cytokine analyses.
Antileishmanial activity of anisomycin.
Metacyclic promastigotes or amastigotes were seeded into 96-well flat-bottom plates (Nunc) at 106/well to a final volume of 100μl of M199-10% FCS, containing increasing amounts of anisomycin. After incubation for the given times at 26°C, the number of viable leishmaniae/well was determined by adding 10 μl of 5-mg/ml 3-(4,5-dimethylthiazil-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) for 4 h, and the reaction was stopped with 100 μl of 50% isopropanol and 10% sodium dodecyl sulfate (SDS) for 30 min at room temperature. The relative optical density/well was determined by using an automatic microplate reader (Dynatech Labs) at 570 nm.
Preparation of cell extracts for Western blot analysis.
After treatment, cells (2 × 105/sample) were washed twice with RPMI and lysed with ice-cold lysis buffer (50 mM Tris-HCl [pH 7.4], 1 mM EGTA, 150 mM NaCl, 1% [vol/vol] Triton X-100, 1 mM phenylmethylsulfonyl, aprotinin (10 μg/ml), 10 mM EDTA, 1 mM NaF, 1 mM Na3VO4). Lysates were centrifuged at 13,000 × g at 4°C for 10 min, and the resulting supernatants were transferred to fresh tubes and stored at −40°C until required.
Western blot analysis.
Cell lysates were treated with an equal volume of 2× SDS sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 10% [vol/vol] glycerol, 50 mM dithiothreitol, 0.1% [wt/vol] bromphenol blue) and then electrophoresed on a 10% (acrylamide:bisacrylamide, 40:1 [wt/vol]). Proteins were transferred to a polyvinylidene difluoride membrane using a semidry apparatus under standard conditions. The membrane was then incubated successively with and 20 mM Tris (pH 7.6)-0.137 M NaCl-0.1% (vol/vol) Tween-20 (TBST) containing 0.1% (wt/vol) milk powder at room temperature for 1 h, with rabbit antibodies specific for phosphorylated or nonphosphorylated p38 MAPK overnight at 4°C, and then with horseradish peroxidase-labeled anti-rabbit antibody for 1 h. After each incubation, the membrane was extensively washed with TBST, and the immunoreactive band was detected with enhanced-chemiluminescence-detecting reagents and developed with Kodak MR film.
Cytokine measurement.
TNF-α was measured by enzyme-linked immunosorbent assay using TNF-α monoclonal antibody (2 μg/ml; Pharmingen) as the capture antibody and biotinylated anti-human TNF-α monoclonal antibody (1 μg/ml; Pharmingen) as the detecting antibody. The development was made with streptavidin-peroxidase conjugate (DAKO) and 3,3′,5,5′-tetramethylbenzinedihydro-chloride tablets (Sigma) as the substrate. Plates were read at 450 nm, and TNF-α concentration was calculated by reference to a standard curve of human TNF-α (NIBSC, South Mimms, United Kingdom).
Statistical analysis.
Except where otherwise stated, the results shown are representative data from a minimum of three similar experiments, which yielded comparable results. Differences between experimental groups were examined using Student's t test for independent means (Fig P for Windows).
RESULTS
Inhibition of p38 MAPK augments L. donovani infection.
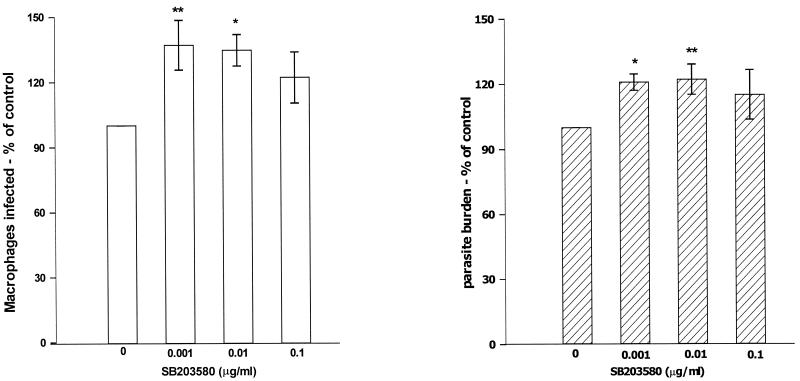
To examine the role of p38 MAPK in leishmania infection, the effect of a selective p38 MAPK inhibitor, SB203580, was assessed. SB203580 is a pyrindyl imidazole which binds with a high affinity to p38 near the ATP binding site, thus rendering p38 MAPK inactive (8). Macrophages were treated with increasing concentrations of SB203580 prior to a 2-h infection with L. donovani promastigotes. Compared to the control, p38 MAPK inhibition induced a significant increase in the subsequent successful infection by L. donovani promastigotes as shown by an increase in the percentage of macrophages infected and parasite burden (number of parasites per 100 infected macrophages) (Fig. 1). When combined, this represented an approximately 50% increase in parasite numbers per macrophage. These concentrations of SB203580 did not induce any toxicity as determined by microscopic observations and trypan blue exclusion (data not shown). Macrophages first infected for 2 h and then subsequently treated with SB203580 for an hour resulted in a slight increment in the percentage of macrophages infected (P < 0.05; 0.01μg/ml) with no significant effect on parasite burden (data not shown).
FIG. 1.
Macrophages pretreated with SB203580 show increased L. donovani infection. Macrophages were treated with increasing concentrations of SB203580 for 1 h and then infected with L. donovani promastigotes for 2 h at a parasite-to-macrophage ratio of 20:1. Shown are means ± SEMs (error bars) of percentage of macrophages infected (A) and parasite burden of L. donovani-infected (B) in comparison with control untreated cells expressed as 100% (n = 4 independent experiments). The range in percentage infected in the control was 11 to 30%, with a parasite burden of 126 to 169 parasites per 100 infected macrophages. Statistical significance: **, P < 0.01 compared to untreated controls; *, P < 0.05 compared to untreated controls.
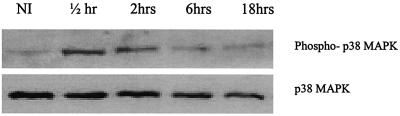
Considering that SB203580 enhances parasite invasion, it is possible that leishmania parasites may not completely inhibit this kinase during infection. We therefore determined whether p38 MAPK is altered during infection by measuring kinetics of its phosphorylation on exposure to L. donovani promastigotes. Compared to noninfected controls, L. donovani promastigotes induced a slight increase in p38 MAPK phosphorylation at 30 min and 2 h, with phosphorylation back to baseline by 18 h of infection (Fig. 2).
FIG. 2.
Activation of p38 MAPK during L. donovani infection. Macrophages were exposed to L. donovani promastigotes for various times as indicated. Whole-cell lysates were prepared and subjected to Western blotting using antibodies specific for phospho-p38 MAPK and p38 as described in Materials and Methods. Comparable results were obtained in two experiments
Anisomycin induces parasite inhibition in infected macrophages.
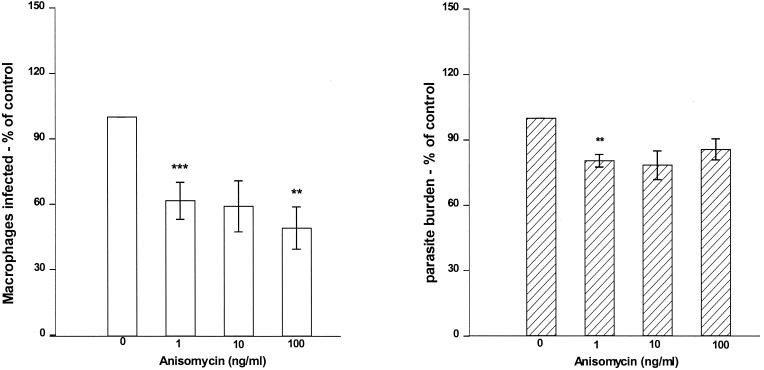
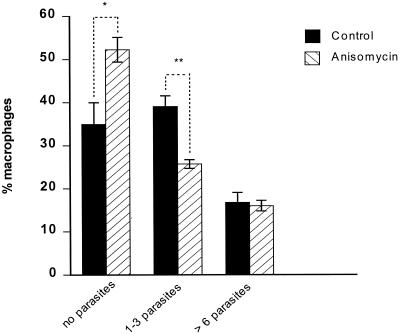
Following the observation that inhibition of p38 MAPK enhances L. donovani infection, we investigated the relevance of activating p38 MAPK on L. donovani infection using anisomycin. Anisomycin acts as a signaling agonist at concentrations at or below 100 ng/ml to selectively activate stress-related MAPKs (p38 MAPK and JNK) (25, 41) with no toxicity. Macrophage monolayers stimulated with anisomycin for 1 h prior to infection with L. donovani promastigotes showed a significant decrease in both the percentages of macrophages infected and parasite burden (Fig. 3). When combined, this represented an approximately 50% reduction in intracellular parasites. This may have been as a result of inhibition of phagocytosis and hence a reduction in parasite uptake. Therefore, in order to examine survival without potential effects on uptake of parasites, L. donovani-infected macrophages were treated with increasing concentrations of anisomycin. Following treatment the percentage of macrophages infected was reduced, although there was no significant change in parasite burden within infected macrophages (Fig. 4). Lack of change in parasite burden could be as a result of anisomycin targeting different populations of infected macrophages. On further observation of such treated macrophages, only those macrophages containing few organisms (one to three parasites) responded to anisomycin treatment (Fig. 5). There was no change in anisomycin-treated macrophages with a higher parasite burden (more than six) compared to the control.
FIG. 3.
Macrophage pretreatment with anisomycin decreases L. donovani infection. Macrophages were treated with increasing concentrations of anisomycin for 1 h and then infected with L. donovani promastigotes for 2 h at a parasite-to-macrophage ratio of 20:1. Data represent means ± SEMs (error bars) of percentages of macrophages infected (A) and parasite burden of L. donovani-infected macrophages (B) in comparison with control untreated cells expressed as 100% (n = 3 independent experiments). The range in percentage infected in the control was between 12 and 45%, with a parasite burden of 145 to 235 parasites per 100 infected macrophages. Statistical significance: ***, P < 0.005 compared to untreated controls; **, P < 0.01 compared to untreated controls
FIG. 4.
Anisomycin reduces L. donovani survival in infected macrophages. Macrophages were infected with L. donovani promastigotes for 2 h at a parasite-to-macrophage ratio of 20:1, washed, and then treated with increasing concentrations of anisomycin for 1 h. Shown are percentage of macrophage infection (A) and parasite burden of L. donovani-infected macrophages (B) in comparison with control untreated cells expressed as 100% (n = 3 independent experiments) (results are presented as means ± SEMs [error bars]). The range in percentage of cells infected in the control was between 9 and 25%, with a parasite burden of 116 to 256 parasites per 100 infected macrophages. Statistical significance: **, P < 0.01 compared to untreated controls; ***, P < 0.005 compared to untreated controls.
FIG. 5.
Anisomycin reduces L. donovani survival in macrophages with low levels of infection. Macrophages were infected with L. donovani promastigotes for 2 h, washed,and then treated with anisomycin (100 ng/ml) for 2 h. The numbers of parasites in macrophages were counted. Data represent means ± SEMs (error bars) of total macrophages (n = 2 independent experiments). *, P < 0.05; **, P < 0.01.
The antileishmanial effects observed may arise from a direct action of anisomycin on the parasites or by an effect of anisomycin on macrophages activated to eliminate parasites. We therefore assessed if anisomycin had any effect on amastigotes, the intracellular stage of Leishmania. Hamster-derived amastigotes were cultured in the presence of anisomycin at concentrations of up to 100 μg/ml at 37°C. After a period of up to 24 h, parasite viability was assayed by the ability of viable parasites to reduce MTT to formazan. Anisomycin had no effect on the viability of amastigotes at concentrations up to 10 μg/ml, suggesting that the observed inhibition was not due to a direct effect on the parasites. Similarly, treatment of the extracellular infective promastigote form with anisomycin at 26°C over a period of 72 h did not have any effect on the promastigote's growth and viability (data not shown).
Anisomycin does not reverse the effect of SB203580 on Leishmania infection.
Anisomycin has been shown to stimulate both the JNK/SAPK and p38 MAPK cascades. Hence, the effects of anisomycin seen so far could be a result of activation of either one or both p38 MAPK and JNK cascades. It was therefore of interest to investigate whether specific p38 MAPK inhibition was able to reverse the effects of anisomycin on Leishmania infection. Macrophages formerly treated with increasing concentrations of SB203580 were infected with L. donovani for 2 h. Uningested parasites were washed off, and infected macrophage monolayers were treated with anisomycin (100 ng/ml). As previously observed, anisomycin reduced the percentage of macrophages compared to control macrophages. Surprisingly, the presence of SB203580 completely prevented this reduction (Fig. 6), indicating the significance of p38 MAPK in the anisomycin-induced killing of L. donovani.
FIG. 6.
SB203580 reverses anisomycin-induced parasite reduction. Macrophages were treated with increasing concentrations of SB203580 for 1 h prior to a 2-h infection with L. donovani at a parasite-to-macrophage ratio of 20:1. Infected macrophages were then treated with anisomycin (0.1 μg/ml) for 1 h, fixed,and stained, and infected macrophages were determined microscopically. Data represent means ± SEMs (error bars) of percentages of macrophages infected in comparison with control untreated cells expressed as 100% (n = 4 independent experiments). Statistical significance: *, P < 0.05 compared to untreated controls; ***, P < 0.005 compared to controls.
Effect of ERK inhibitor on L. donovani infection.
To examine whether ERK was involved in L. donovani infection, macrophages were preincubated with PD98059 1 h prior to infection. PD98059 is a selective and potent inhibitor of ERK and mediates its effects by binding to and inactivating the ERK-specific MAPK kinase, MEK (2, 10). It has no effects on any of the components of JNK and p38 MAPK cascades (30). Inhibition of ERK showed an increase in the percentage of macrophages (1.0 μM, P < 0.05) (Fig. 7). This increase was not as pronounced as that seen with SB203580. Furthermore, the presence of PD98059 did not inhibit anisomycin-induced parasite inhibition. There was no significant change in the parasite burden (data not shown).
FIG. 7.
PD098059 does not inhibit anisomycin-induced killing. Macrophages were treated with increasing concentrations of PD098059 for 1 h prior to a 2-h infection with L. donovani. Infected macrophages were then treated with anisomycin (0.1 μg/ml) for 1 h, fixed, and stained, and the macrophages infected were determined microscopically. Data represent means ± SEMs (error bars) in comparison with control untreated cells expressed as 100% (n = 3 independent experiments). Statistical significance: *, P < 0.05 compared to untreated controls; **, P < 0.01 compared to untreated controls, ***, P < 0.005 compared to untreated controls.
Anisomycin-induced killing does not increase with time.
Anisomycin induced killing within 2 h; therefore, we tested whether this effect was altered over longer periods of time. Macrophages infected with L. donovani promastigotes for 18 h and treated with anisomycin (100 ng/ml) for 1, 6, 12, 24, 48, and 72 h resulted in 93.7% ± 2.0% (P = 0.08), 57.2% ± 8.7% (P < 0.05), 44.1% ± 2.6% (P < 0.02), 49.6% ± 4.0% (P < 0.02), 51.3% ± 1.0% (P < 0.001), and 48.2% ± 6.8% (P < 0.02) inhibition of percentage of macrophages infected, respectively, compared to control. These results indicate that the effects of anisomycin were not reduced by longer infection periods of 18 h, nor was a further killing observed beyond 6 h and up to 48 h.
LPS does not enhance anisomycin-induced inhibition.
IFN-γ and LPS cooperatively enhance killing of intracellular Leishmania in macrophages, an action that is dependent on TNF-α and NO secretion (14-16). It has been well established that among the MAPKs, p38 MAPK plays an important role in LPS-induced TNF-α production in many different cell types (19, 20) We therefore assessed whether LPS would synergize with anisomycin and cooperatively enhance killing of intracellular Leishmania. Macrophages (18 h infected) were treated with various concentrations of anisomycin and LPS. As shown in Fig. 8A, the presence of LPS did not further enhance anisomycin-induced parasite inhibition. Infection of macrophages with L. donovani has been shown to interfere with cytokine production (9). Anisomycin, unlike LPS was unable to induce the production of TNF-α (Fig. 8B). The presence of anisomycin moderately enhanced LPS-induced TNF-α production. In addition, TNF-α production from these cells treated with a combination of anisomycin and LPS confirmed that anisomycin was not toxic to the cells at the concentrations used.
FIG. 8.
Effect of combined LPS and anisomycin treatment. Macrophages were infected with L. donovani promastigotes for 18 h, washed, and then treated with increasing concentrations of anisomycin and LPS for another 18 h. (A) The numbers of parasites infected were counted microscopically. Statistical significance: *, P < 0.05 compared to untreated controls; **, P < 0.01 compared to untreated controls. (B) Supernatants were also harvested and levels of TNF-α determined by enzyme-linked immunosorbent assay. Statistical significance: *, P < 0.05 compared to untreated controls; **, P < 0.01 compared to untreated controls; ***, P < 0.005 compared to untreated controls.
Anisomycin phosphorylates p38 MAPK in infected macrophages.
As we have previously observed, SB203580 inhibited anisomycin-induced parasite inhibition (Fig. 6), indicating that activation of p38 MAPK was involved in anisomycin-induced parasite inhibition. We therefore used Western blotting to examine the activation of p38 MAPK by anisomycin in L. donovani-infected macrophages. Positive controls consisted of naïve uninfected macrophages stimulated with anisomycin for the same times as infected macrophages. Activation of p38 MAPK requires phosphorylation at threonine and tyrosine residues. Immunoblot analyses using anti-phospho-specific p38 antibodies were performed. As shown in Fig. 8A and C, naïve macrophages treated with 100-ng/ml anisomycin had a maximal activation of p38 MAPK between 15 min and 2 h. Whereas anisomycin induced a strong and transient phosphorylation of p38 MAPK in naïve macrophages, there was a superinduction of phosphorylated p38 MAPK in L. donovani-infected macrophages (Fig. 9B and C).
FIG. 9.
Time-dependent activation of p38 MAPK by anisomycin. Naïve (A) and L. donovani-infected (B) macrophages were treated with anisomycin (0.1 μg/ml) for up to 6 h. Whole-cell lysates were prepared and subjected to Western blotting using antibodies specific for phospho-p38 MAPK and p38 as described in Materials and Methods. The extent of phosphorylation was quantified using a densitometer with ImageQuant software(C). Comparable results were obtained in two experiments.
DISCUSSION
The capacity of Leishmania donovani to suppress macrophage function has been linked to alterations in key signaling cascades (28, 34, 45). The objective of this study was therefore to investigate the effect of activation or depletion of MAPKs on parasite survival within human macrophages in vitro. MAPKs are critical components in the coordination of incoming signals generated by a variety of intracellular and extracellular mediators. Treatment of macrophages with a specific p38 MAPK inhibitor, SB203580 increased the percentage of macrophages infected and parasite burden in these cells. Facilitation of L. donovani survival by inhibiting p38 MAPK may occur either at the level of reducing the ability of the macrophage to kill invading parasites or by reducing the ability of the macrophage to subsequently direct immune responses. In vitro effects were on killing rather than uptake because effects were seen on macrophages previously infected and then treated. It has previously been demonstrated that MAPK inhibitors do not alter phagocytosis (3).
It was found that parasites in macrophages infected for 18 h were still susceptible to killing by anisomycin treatment for up to 72-hrs indicating amastigote susceptibility. Not only that but while the killing effect of p38 activation could be observed at between 2 and 6 h following anisomycin treatment no further effects on survival at 36 h were seen. This therefore indicates that inhibition of p38 signaling pathway would be beneficial for L. donovani survival. Previous evidence for deactivation of MAPK by a cellular phosphotyrosine phosphatase in Leishmania-infected cells (26) suggests that the parasite does use this mechanism. Prive et al. also demonstrated that attachment and entry of L. donovani promastigotes to macrophages induced a slight dephosphorylation of p38 MAPK (32). This is in correlation with our observations in which initial exposure to L donovani induced p38 MAPK phosphorylation, which was gradually dephosphorylated by 18 h. These data suggest that impairment of p38 MAPK early during host-parasite interaction may be important in the establishment of infection by L. donovani.
Macrophage pretreatment with anisomycin, a potent activator of JNK/SAPK and p38 MAPKs, prior to infection, significantly decreased infection levels compared to those observed in untreated controls. The concentrations used were not toxic to macrophages (5, 21). There was also a reduction in parasite survival when anisomycin was added to already-infected cells, demonstrating that activation of JNK/p38 MAPK impeded parasites from maintaining a successful invasion rather than altering uptake. Reduced parasite infection was seen in a group of macrophages that had low parasite numbers. The highly infected cells did not clear infection as indicated in the lack of very significant decreases in parasite burden. Parasites in highly infected macrophages may have other mechanisms to control the macrophage or may be able to inhibit MAPK more effectively. In addition it is possible that different populations of macrophages may respond to MAPK modulation in different ways. This warrants investigations at a single-cell level.
Considering that anisomycin strongly elicits high and significant activation of both JNK/SAPK and p38 MAPKs but not ERKs (6), the role played by each of these pathways was distinguished by using SB203580, a p38 MAPK-specific inhibitor. Anisomycin was unable to reverse the effects of p38 MAPK inhibition by SB203580 in infected macrophages. This suggests that the influence of anisomycin is through p38 or at least that p38 is required for JNK/SAPK MAPK action. p38 MAPK activation rather than JNK/SAPK may specifically be inhibited upon L. donovani infection. Inhibition of the ERK pathway with PD059089 increased parasite survival either through increased uptake or decreased killing. This is in contradiction to Feng et al. (12) who found that L. donovani lipophosphoglycan stimulated ERK to inhibit the production of IL-12 and NO, hence promoting survival. In accordance with their findings, inhibition of ERK should therefore be expected to decrease parasite survival, contrary to our observations. PD059089 did not have an effect on anisomycin-induced parasite inhibition. Western blots revealed time-dependent anisomycin-induced p38 MAPK activation in uninfected macrophages. The presence of anisomycin in infected macrophages resulted in a superinduction of phosphorylated p38 MAPK compared to that observed in control uninfected macrophages Anisomycin-induced augmentation of phospho-p38 MAPK in infected macrophages may reflect other parasite effects causing elimination of negative feedback influences perhaps originating from downstream kinases. Collectively, these findings provide evidence that L. donovani may inhibit MAPKs both before anisomycin's level of action and perhaps downstream of it.
The antileishmanial effects of anisomycin were also observed in macrophages that were infected for a longer period of time. This indicates that L. donovani requires active inhibition of p38 MAPK to ensure longer-term survival in macrophages. Interestingly, treating the infected macrophages with LPS, which also activates stress kinases, including p38 MAPK (20), did not result in parasite inhibition. The presence of LPS also failed to enhance anisomycin-induced parasite inhibition. Anisomycin failed to induce TNF-α production in infected macrophages but augmented LPS-induced TNF-α production. Although inhibition of MAPK pathways may reduce the levels of TNF-α produced in some systems, there is no reason why MAPK activation alone, in the absence of, for instance, NF-κB activation, would be expected to induce TNF-α (37, 43). This also suggests that there was no experimental contamination with activating substances such as LPS. The increase in TNF-α when LPS was added would be consistent with a role in making NF-κB sites available (38). Higher concentrations of anisomycin (100 ng/ml) with LPS attenuated TNF-α production. This may be a consequence of anisomycin eliciting heterologous desensitization, whereby the addition of LPS fails to elicit a response due the loss of its ability to activate p38 MAPK in desensitized cells (17). The effects of anisomycin on IL-12 production are worth investigating in the future, as Leishmania stimulates ERK MAPK to inhibit this cytokine and promote parasite survival (12).
In conclusion, our findings suggest that activation and inhibition of p38 MAPK pathway in host macrophages regulates Leishmania infection. The exact mechanism of activation of p38 MAPK by anisomycin to induce parasite inhibition remains to be identified. Anisomycin stimulates p38 MAPK in a Ras-independent manner, indicating that anisomycin-stimulated signaling occurs downstream of PKC (17, 44). The two well-described mediators of parasite killing within activated macrophages are oxygen radicals formed by the respiratory burst NADPH oxidase (24) and nitrogen radicals formed by iNOS (15). p38 MAPK and ERK are involved in the activation of p47phox, a component of NADPH oxidase which catalyzes the oxidative burst (11, 47). Previous studies have also implicated p38 MAPK in the induction of iNOS (1), suggesting a different mechanism that anisomycin may employ. Further investigation of what effect such macrophage activation may have in vivo is needed, since in vivo, other effects of macrophage activation may be seen. Further understanding of interference by Leishmania parasites on MAPK signaling pathways and functions displayed by macrophages are of importance and will allow the identification of specific molecules involved in the cross talk between parasites and host cells. Effectors of these cascades may lead to the development of candidate chemotherapy targets. We are currently investigating the use of anisomycin in vivo. Such an effect would also clearly have effects on most cell types. This report demonstrates that the therapies that might activate MAPKs in infected macrophages could contribute to Leishmania clearance but will probably need to be coupled with complementary macrophage activation mechanisms or a direct antiparasitic compound.
Acknowledgments
We thank Juanco for financial support of Muthoni Junghae.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Ajizian, S. J., B. K. English, and E. A. Meals. 1999. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J. Infect. Dis. 179:939-944. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 3.Ayala, J. M., Goyal, S., Liverton, N. J., Claremon, D. A., O'Keefe, S. J., Hanlon, W. A. 2000. Serum-induced monocyte differentiation and monocyte chemotaxis are regulated by the p38 MAP kinase signal transduction pathway. J. Leukoc. Biol. 67:869-875 [PubMed] [Google Scholar]
- 4.Buchmuller-Rouiller, Y., and J. Mauel. 1987. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect. Immun. 55:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cano, E., C. A. Hazzalin, and L. C. Mahadevan. 1994. Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun. Mol. Cell. Biol. 14:7352-7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cano, E., and L. C. Mahadevan. 1995. Parallel signal processing among mammalian MAPKs. Trends Biochem. Sci. 20:117-122. [DOI] [PubMed] [Google Scholar]
- 7.Carrera, L., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 9.Descoteaux, A., and G. Matlashewski. 1989. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages Mol. Cell. Biol. 9:5223-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Benna, J., J. Han, J. W. Park, E. Schmid, R. J. Ulevitch, and B. M. Babior. 1996. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch. Biochem. Biophys. 334:395-400. [DOI] [PubMed] [Google Scholar]
- 12.Feng, G. J., H. S. Goodridge, M. M. Harnett, X. Q. Wei, A. V. Nikolaev, A. P. Higson, and F. Y. Liew. 1999. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163:6403-6412. [PubMed] [Google Scholar]
- 13.Frankenburg, S., V. Leibovici, N. Mansbach, S. J. Turco, and G. Rosen. 1990. Effect of glycolipids of Leishmania parasites on human monocyte activity. Inhibition by lipophosphoglycan. J. Immunol. 145:4284-4289. [PubMed] [Google Scholar]
- 14.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 15.Green, S. J., M. S. Meltzer, J. B. Hibbs, Jr., and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 16.Green, S. J., L. F. Scheller, M. A. Marletta, M. C. Seguin, F. W. Klotz, M. Slayter, B. J. Nelson, and C. A. Nacy. 1994. Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol. Lett. 43:87-94. [DOI] [PubMed] [Google Scholar]
- 17.Hazzalin, C. A., R. Le Panse, E. Cano, and L. C. Mahadevan. 1998. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol. Cell. Biol. 18:1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Her, J. H., S. Lakhani, K. Zu, J. Vila, P. Dent, T. W. Sturgill, and M. J. Weber. 1993. Dual phosphorylation and autophosphorylation in mitogen-activated protein (MAP) kinase activation. Biochem. J. 296:25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 20.Lee, J. C., and P. R. Young. 1996. Role of CSB/p38./RK stress response kinase in LPS and cytokine signaling mechanisms. J. Leukoc. Biol. 59:152-157. [DOI] [PubMed] [Google Scholar]
- 21.Mahadevan, L. C., and D. R. Edwards. 1991. Signalling and superinduction. Nature 349:747-748. [DOI] [PubMed] [Google Scholar]
- 22.McNeely, T. B., and S. J. Turco. 1987. Inhibition of protein kinase C activity by the Leishmania donovani lipophosphoglycan. Biochem. Biophys. Res. Commun. 148:653-657. [DOI] [PubMed] [Google Scholar]
- 23.Moore, K. J., S. Labrecque, and G. Matlashewski. 1993. Alteration of Leishmania donovani infection levels by selective impairment of macrophage signal transduction. J. Immunol. 150:4457-4465. [PubMed] [Google Scholar]
- 24.Murray, H. W. 1982. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J. Immunol. 129:351-357. [PubMed] [Google Scholar]
- 25.Nahas, N., T. F. Molski, G. A. Fernandez, and R. I. Sha'afi. 1996. Tyrosine phosphorylation and activation of a new mitogen-activated protein (MAP)-kinase cascade in human neutrophils stimulated with various agonists. Biochem. J. 318:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nandan, D., R. Lo, and N. E. Reiner. 1999. Activation of phosphotyrosine phosphatase activity attenuates mitogen-activated protein kinase signaling and inhibits c-FOS and nitric oxide synthase expression in macrophages infected with Leishmania donovani. Infect. Immun. 67:4055-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandan, D., and N. E. Reiner. 1995. Attenuation of gamma interferon-induced tyrosine phosphorylation in mononuclear phagocytes infected with Leishmania donovani: selective inhibition of signaling through Janus kinases and Stat1. Infect. Immun. 63:4495-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivier, M., R. W. Brownsey, and N. E. Reiner. 1992. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc. Natl. Acad. Sci. USA 89:7481-7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell. Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 30.Pang, L., T. Sawada, S. J. Decker, and A. R. Saltiel. 1995. Inhibition of MAP kinase kinase blocks the differentiation of PC-12. cells induced by nerve growth factor. J. Biol. Chem. 270:13585-13588. [DOI] [PubMed] [Google Scholar]
- 31.Paul, A., S. Wilson, C. M. Belham, C. J. Robinson, P. H. Scott, G. W. Gould, and R. Plevin. 1997. Stress-activated protein kinases: activation, regulation and function. Cell. Signal. 9:403-410. [DOI] [PubMed] [Google Scholar]
- 32.Prive, C., and A. Descoteaux. 2000. Leishmania donovani promastigotes evade the activation of mitogen-activated protein kinases p38., c-Jun N-terminal kinase, and extracellular signal-regulated kinase-1/2 during infection of naive macrophages. Eur. J. Immunol. 30:2235-2244. [DOI] [PubMed] [Google Scholar]
- 33.Raingeaud, J., S. Gupta, J. S. Rogers, M. Dickens, J. Han, R. J. Ulevitch, and R. J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 34.Reiner, N. E. 1994. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol. Today 15:374-381. [DOI] [PubMed] [Google Scholar]
- 35.Reiner, N. E. 1987. Parasite accessory cell interactions in murine leishmaniasis. I. Evasion and stimulus-dependent suppression of the macrophage interleukin 1 response by Leishmania donovani. J. Immunol. 138:1919-1925. [PubMed] [Google Scholar]
- 36.Reiner, N. E., W. Ng, T. Ma, and W. R. McMaster. 1988. Kinetics of gamma interferon binding and induction of major histocompatibility complex class II mRNA in Leishmania-infected macrophages. Proc. Natl. Acad. Sci. USA 85:4330-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutault, K., C. A. Hazzalin, and L. C. Mahadevan. 2001. Combinations of ERK and p38 MAPK inhibitors ablate tumor necrosis factor-alpha (TNF-alpha) mRNA induction. Evidence for selective destabilization of TNF-alpha transcripts. J. Biol. Chem. 276:6666-6674. [DOI] [PubMed] [Google Scholar]
- 38.Saccani, S., S. Pantano, and G. Natoli. 2002. p38.-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat. Immunol. 3:69-75. [DOI] [PubMed] [Google Scholar]
- 39.Sacks, D. L. 1989. Metacyclogenesis in Leishmania promastigotes. Exp. Parasitol. 69:100-103. [DOI] [PubMed] [Google Scholar]
- 40.Seger, R., and E. G. Krebs. 1995. The MAPK signaling cascade. FASEB J. 9:726-735. [PubMed] [Google Scholar]
- 41.Shu, J., M. Hitomi, and D. Stacey. 1996. Activation of JNK/SAPK pathway is not directly inhibitory for cell cycle progression in NIH3T3 cells. Oncogene 13:2421-2430. [PubMed] [Google Scholar]
- 42.Su, B., and M. Karin. 1996. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 8:402-411. [DOI] [PubMed] [Google Scholar]
- 43.Swantek, J. L., M. H. Cobb, and T. D. Geppert. 1997. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 17:6274-6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torocsik, B., and J. Szeberenyi. 2000. Anisomycin uses multiple mechanisms to stimulate mitogen-activated protein kinases and gene expression and to inhibit neuronal differentiation in PC12. phaeochromocytoma cells. Eur. J. Neurosci. 12:527-532. [DOI] [PubMed] [Google Scholar]
- 45.Turco, S. J. 1999. Adversarial relationship between the leishmania lipophosphoglycan and protein kinase C of host macrophages. Parasite Immunol. 21:597-600. [DOI] [PubMed] [Google Scholar]
- 46.Unanue, E. R., and P. M. Allen. 1987. The basis for the immunoregulatory role of macrophages and other accessory cells. Science 236:551-557. [DOI] [PubMed] [Google Scholar]
- 47.Zu, Y. L., J. Qi, A. Gilchrist, G. A. Fernandez, D. Vazquez-Abad, D. L. Kreutzer, C. K. Huang, and R. I. Sha'afi. 1998. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-alpha or FMLP stimulation. J. Immunol. 160:1982-1989. [PubMed] [Google Scholar]