Abstract
NF-E2 is a transcription activator for the regulation of a number of erythroid- and megakaryocytic lineage-specific genes. Here we present evidence that the large subunit of mammalian NF-E2, p45, is sumoylated in vivo in human erythroid K562 cells and in mouse fetal liver. By in vitro sumoylation reaction and DNA transfection experiments, we show that the sumoylation occurs at lysine 368 (K368) of human p45/NF-E2. Furthermore, p45 sumoylation enhances the transactivation capability of NF-E2, and this is accompanied by an increase of the NF-E2 DNA binding affinity. More interestingly, we have found that in K562 cells, the β-globin gene loci in the euchromatin regions are predominantly colocalized with the nuclear bodies promyelocytic leukemia protein (PML) oncogenic domains that are enriched with the PML, SUMO-1, RNA polymerase II, and sumoylatable p45/NF-E2. Chromatin immunoprecipitation assays further showed that the intact sumoylation site of p45/NF-E2 is required for its binding to the DNase I-hypersensitive sites of the β-globin locus control region. Finally, we demonstrated by stable transfection assay that only the wild-type p45, but not its mutant form p45 (K368R), could efficiently rescue β-globin gene expression in the p45-null, erythroid cell line CB3. These data together point to a model of mammalian β-like globin gene activation by sumoylated p45/NF-E2 in erythroid cells.
Mammalian nuclear factor erythroid 2, or NF-E2, is a positively regulatory, DNA binding transcription factor for gene expression in erythroid and megakaryocytic cells (reviewed in reference 2). It was initially purified from murine erythroleukemia (MEL) cells and was later shown to be a heterodimer of basic leucine zipper (bZIP) polypeptides consisting of a larger p45 polypeptide and a smaller subunit belonging to the p18/Maf K family. Of the two subunits, expression of p18/Maf is ubiquitous, while that of p45 is restricted to the erythroid and megakaryocytic lineages (3, 4, 10, 11, 30). Indeed, intact p45 gene and its expression are required for transcriptional activation of the adult β-globin gene in MEL cells (34) and for normal differentiation of the megakaryocytes (55). However, there was little or no effect on erythropoiesis and globin gene expression when the p45 gene was knocked out in mice (54). The generally accepted assumption is that another factor or other factors are capable of substituting for the function of p45 when it is absent during early embryogenesis.
A number of in vitro DNA binding and in vivo expression studies have shown that NF-E2 exerts its functions by first binding, through the bZIP domains of its two subunits, to a class of specific DNA sequences of the consensus sequence T/CGCTAG/CTCAC/T that are present in a number of regulatory elements of erythroid and megakaryocytic genes such as the promoter of porphobilinogen deaminase (PBGD) gene and the different DNase I-hypersensitive sites (HS) of the locus control region of the mammalian β-like globin genes (β-LCR) (15, 43, 44, 46, 59). Indeed, NF-E2 binds in vivo at its cognate recognition motifs in the β-LCR, and this binding correlates well with activation of the β-like globin genes (see references 17, 32, 51, and 52 and references therein).
It appears that NF-E2 exerts its activator function by the recruitment of chromatin remodeling complexes and other factors to the vicinity of its binding sites (5, 24). Indeed, with the activation domain located within its NH2 terminal at positions 1 to 206 (6), p45 interacts with several other proteins, including the general transcription factor TAFII130 (1), the mammalian ubiquitin ligase Itch (13), and CBP, a coactivator with histone acetyltransferase activity (14, 28). CBP acetylates the p18/Maf subunit of NF-E2 and augments its DNA binding and transcription activities (28).
In the following, we present data showing that the p45 subunit of NF-E2 can also be sumoylated, in vitro and in vivo. Sumoylation covalently modifies certain protein substrates with the small ubiquitin-like modifier (SUMO) proteins through the combined usage of the activating enzyme E1 (SAE1/SAE2), conjugating enzyme E2 (UBC9), and E3 ligase. In several cases, sumoylation has been shown to affect the biochemical and biological properties of its substrates (reviewed in reference 31; also see the Discussion below). This modification enhances the DNA binding affinity of NF-E2 and elevates the transactivation capability of NF-E2. Furthermore, it appears that sumoylatable NF-E2 factors are concentrated together with RNA polymerase II (RNAP II) in the nuclear body (NB) promyelocytic leukemia protein (PML) oncogene domains (PODs), within which the majority of the euchromatic β-globin loci are anchored in an erythroid cell-specific manner.
MATERIALS AND METHODS
Cell culture.
The human embryonic-fetal erythroleukemia cell line K562 (ATCC accession no. CCL-234) and mouse adult erythroleukemia cell line CB3 (41) were grown in RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum (HyClone), 50 units/ml of penicillin, and 50 μg/ml of streptomycin (Invitrogen). Cells of the mouse adult erythroleukemia MEL (47), human 293, and HeLa cell lines were cultured in Dulbecco's modified Eagle's medium (Invitrogen). The procedures for the establishment of K562 and CB3 pools stably expressing myc-p45 and myc-p45 (K368R) followed those described in reference 39.
Plasmids and antibodies.
The cDNA encoding human p45 was cloned in pEF-myc (Invitrogen), pEF (Invitrogen), and pGEX-2TK (Amersham Biosciences). The K368R mutation in p45 was generated by PCR-directed mutagenesis. The cDNA encoding human Maf G was cloned in pEF-His (Invitrogen) vector. The DNA inserts of pQE-SUMO-1 (1-97), pCMV-His-SUMO-1, pCMV-SUMO-1 GG, and pCMV-Flag SUMO-1 GG were generated by PCR amplification of pCMV-SUMO-1. pCMV-SUMO-1 AA was generated by PCR amplification of pFlagDsRed2-SUMO-1 AA. pQE-UBC9 was generated by PCR amplification of the insert of pcDNA-UBC9 and cloned into the pQE (QIAGEN) vector. pRBGP2-Luc for transfection was from Masayuki Yamamoto (Tsukuba University, Japan). pFlagDsRed2-SUMO-1 GG and pFlagDsRed2-SUMO-1 AA were provided by B. C. Chung (12). pcDNA-UBC9 was provided by T. P. Yao (Duke University). Polyclonal anti-p45 rabbit antibody was generated in the laboratory. The anti-myc rabbit antibody 9E10, anti-PML mouse antibody PG-M3, anti-NF-E2 rabbit antibody C-19, anti-PML goat antibody A-20, and anti-RNAP II rabbit antibody N20 were purchased from Santa Cruz Inc. Anti-SUMO-1 mouse antibody clone 21C7 and rabbit antibody FL101 were purchased from Zymed Laboratories and Santa Cruz Inc., respectively. Anti-Flag (M2) and anti-tubulin (B-5-1-2) mouse antibodies were purchased from Sigma.
Protein expression.
SUMO-1 and UBC9 were expressed from pQE-SUMO-1 (1-97) and pQE-UBC9, respectively, in Escherichia coli strain XL10-gold and purified by a standard procedure. The recombinant proteins glutathione S-transferase (GST)-p45 and GST-p45 (K368R) were overexpressed in E. coli strain BL21(DE3) pLysS (Novagen) and purified. The two subunits of the sumoylation-activating enzyme E1, SAE1 and SAE2, were coexpressed using a baculovirus expression system. Extracts from cells coinfected with SAE1-carrying and SAE2-carrying viruses were prepared, and the proteins were purified through a Q-Sepharose column (Amersham Pharmacia Biotech). The eluted proteins were dialyzed and concentrated with a Centricon-30 and used in the in vitro sumoylation assay.
In vitro sumoylation and desumoylation assay.
The in vitro SUMO-1 conjugation assay was performed as follows. Briefly, 2 μg of purified GST-p45 or GST-p45 (K368R) protein was incubated with 7.5 μg/ml of SUMO-activating enzyme E1 (SAE1 or SAE2), 50 μg/ml of purified His-SUMO-1, and 50 μg/ml of SUMO-conjugating enzyme His-UBC9 in a 20-μl reaction mixture containing 20 mM HEPES (pH 7.5), 5 mM MgCl2, and 2 mM ATP. After 30 min at 37°C, the reactions were terminated with sodium dodecyl sulfate (SDS) sample buffer and heated at 100°C for 4 min. The reaction products were analyzed by SDS-polyacrylamide gel electrophoresis followed by immunoblotting. For desumoylation assays, we mixed the sumoylated protein with 10 μg of HeLa nuclear extract and incubated for 30 min at the same temperature as was used for the sumoylation reaction.
DNA transfection and luciferase assay.
DNA transfection of 293 cells was performed by the calcium phosphate-DNA coprecipitation method. The K562 and CB3 cells were transfected by electroporation. The total amount of transfected DNA was kept constant by the addition of the cloning vectors. The transfection data represent the averages of at least three sets of independent experiments. The relative luciferase activities were indicated as average values with the standard deviations.
Purification of His-tagged proteins from cell extracts.
At 36 h after transfection, the cells were lysed in 1 ml of lysis buffer (6 M guanidinium HCl, 100 mM NaH2PO4, 10 mM Tris-HCl [pH 7.8]). After sonication, the His-tagged proteins were purified from the lysate with 25 μl of Ni-nitrilotriacetic acid (Ni-NTA) magnetic agarose beads (QIAGEN) following the company's protocol.
Preparation of nuclear extract.
To prepare nuclear extract for analysis of p45 sumoylation in vivo, 1010 K562 cells were washed with phosphate-buffered saline (PBS) buffer (10 mM phosphate, 0.15 M NaCl [pH 7.4]) and resuspended in 100 ml of ice-cold sucrose buffer I (0.32 M sucrose, 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris-HCl [pH 8.0], 1 mM dithiothreitol [DTT], and 0.5% NP-40) containing a protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg/ml of leupeptin, and 1 μg/ml of pepstatin). From this step onward, all operations were performed at between 0 and 4°C. The above-described suspension was centrifuged at 500 × g for 10 min. Pellet from the first centrifugation was recovered and resuspended in 100 ml of sucrose buffer I without NP-40. The washing was repeated twice with centrifugation at 500 × g for 10 min. The nuclei pellets were resuspended in 20 ml of a low-salt buffer (20 mM HEPES-NaOH [pH 7.9], 25% glycerol, 1.5 mM MgCl2, 0.02 M KCl, and 0.5 mM DTT). After the addition of 20 ml of high-salt buffer (20 mM HEPES-NaOH [H 7.9], 25% glycerol, 1.5 mM MgCl2, 0.8 M KCl, 0.2 mM EDTA, 1% NP-40, and 0.5 mM DTT), the mixture was rotated at 4°C for 20 min. After centrifugation at 12,000 × g for 15 min, 60 ml of 25 mM HEPES-NaOH [pH 7.6]-25% glycerol-0.1 mM EDTA-0.5 mM DTT was added and mixed well.
Ammonium sulfate precipitation.
The K562 nuclear extract prepared as described above was fractionated by stepwise precipitation with 20% to 50% ammonium sulfate, and each fraction was dialyzed in 500 ml of 20 mM HEPES (pH 7.9)-1 mM DTT-30% glycerol. For immunoprecipitation (IP) and Western blot experiments, the dialyzed fractions were each adjusted to 3 volumes of 20 mM HEPES (pH 7.9)-2 mM DTT-45 mM N-ethylmaleimide-30 mM iodoacetamide-3 mM PMSF-3 μg/ml of leupeptin-3 μg/ml of pepstatin.
Analysis of p45 sumoylation in mouse fetal liver.
To detect sumoylation of p45 and NF-E2 in mouse fetal liver, 14 embryonic day 14.5 (E14.5) mouse fetal livers were gently disrupted by repeated pipetting in ice-cold PBS (10 mM phosphate, 0.15 M NaCl [pH 7.4]). The cells were pelleted, washed twice with ice-cold PBS buffer (pH 7.4), and resuspended in 6 M guanidine hydrochloride-0.1 M NaH2PO4-0.01 M Tris-HCl (pH 8.0). The lysate was precipitated with 10% trichloroacetic acid, washed with 100% alcohol, and resuspended in an IP buffer (20 mM HEPES [pH 7.9], 2 mM DTT, 45 mM N-ethylmaleimide, 30 mM iodoacetamide, 3 mM PMSF, 3 μg/ml of leupeptin, and 3 μg/ml of pepstatin) for immunoprecipitation analysis or in SDS-polyacrylamide gel electrophoresis sample buffer for Western blotting.
Immunoprecipitation and Western blotting.
Flag-SUMO-1-labeled proteins were isolated from the mammalian cell extracts by use of anti-Flag M2 affinity gel (Sigma) following the company's standard protocol. Rabbit anti-p45 and preimmune serum were also used in immunoprecipitation experiments employing ammonium sulfate-fractionated K562 nuclear extract. Western blotting was performed using an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences), with the primary and secondary antibodies prepared in Tris-buffered saline buffer containing 5% dry milk.
EMSA.
The electrophoretic mobility shift assay (EMSA) procedures followed those of Liu et al. (39). Each DNA binding reaction mixture contained 5 μg of nuclear extracts. The oligonucleotides used are as follows, with the underlined sequences being the NF-E2 binding motifs: PBGD (5′-CCTCCAGTGACTCAGCACAGGTTCC-3′ and 3′-GGAGGTCACTGAGTCGTGTCCAAGG-5′), 3′NA (5′-GATCCAGGACTGCTGAGTCATCCTG-3′ and 3′-GTCCTGACGACTCAGTAGGACCTAG-5′), 5′-NA (5′-GATCCGCCAACCATGACTCAGTGCG-3′ and 3′-GCGGTTGGTACTGAGTCACGCCTAG-5′), and HS2 (5′-AGCAATGCTGAGTCATGATGAGTCATGCTG-3′ and 3′-TCGTTACGACTCAGTACTACTCAGTACGAC-5′). They correspond to the NF-E2/AP-1 binding sites in the human PBGD promoter (43), the HS-40 of α-LCR (5′-NA and 3′-NA) (64), and HS2 of the β-LCR (59). These oligonucleotides were synthesized with 5′ overhangs and end labeled using Klenow enzyme with [α-32P]dATP. For supershift analysis, the nuclear extract was incubated with 1 μg of the antisera at room temperature for 10 min prior to use in the binding reaction.
Immunostaining.
The cells were fixed in 4% formaldehyde for 20 min, permeabilized with 0.1% Triton X-100-PBS, and blocked in 10% normal donkey serum (Jackson ImmunoResearch). Antigen localization was done by a first incubation with the appropriate antibodies at 4°C overnight. The secondary antibodies conjugated to Alexa Fluor 488, Alexa Fluor 568, or Alexa Fluor 647 (Molecular Probes) were then applied for another 2 h of incubation at 25°C. The DNA staining was carried out using DAPI (4′,6′diamidino-2-phenylindole) (Molecule Probes).
DNA-FISH.
The fluorescence in situ hybridization (FISH) probe for the human β-globin locus was generated by nick translation of the DNA insert of pμLCR/γ, a gift from Q. Li and G. Stamatoyannopoulos (Washington University). The DNA probe was labeled with biotin by PCR. The protocols used for FISH experiments were similar to those described by Li et al. (38). After hybridization, the biotin-labeled probe was detected with Alexa Fluor 546 (Molecular Probes)-conjugated antibody.
Immuno-DNA-FISH.
To check the relative locations of the human β-globin locus and different NBs, immuno-DNA-FISH was carried out (53) by a combination of the procedures of DNA-FISH and immunofluorescent staining described above. The DNA-FISH has been shown not to significantly alter the immunostaining patterns of NBs, including POD and Cajal bodies (references 53, 60, and 63 and Y. C. Shyu and C.-K. J. Shen, unpublished data).
Image analysis.
DNA FISH and immunostaining analyses were carried out with a Zeiss LSM 510 Meta confocal microscope. Each confocal image was from a single optical section(s). For each set of the analyses, three different pools of the cells were collected for immunostaining and/or DNA FISH. The confocal stacks of all the cells in randomly chosen fields were collected and analyzed. The distances between different signals on the three-dimensional (3D) reconstructed image stacks were measured using LSM 5 image analysis software. To assess the associations of signals from DNA FISH and those from immunostaining using anti-p45, anti-SUMO-1, anti-PML, and anti-RNAP II, the image stacks of the nuclei were captured and reconstructed as 3D images, and then the colocalized signals were counted.
ChIP.
NF-E2-binding at specific chromatin regions was analyzed by a mammalian chromatin immunoprecipitation (ChIP) approach described previously (17). The primers for PCR of the four HS sites were as described before. Those for the two intergenic regions were as follows: for intergenic site a, the upstream primer was 5′ (9801)-TCGCTAAGAGCACAGAGAGA-3′ and the downstream primer was 5′ (10400)-CCTATAGCCTAGGCATTGTG-3′, and for intergenic site b, the upstream primer was 5′ (2291)-CAGCCCGAGTGAGTTAATAC-3′ and the downstream primer was 5′ (2740)-TCCAGCATACAGACTGCCAA-3′ (numbers refer to those in the NCBI database [V01317]). In general, confluent cells (2 × 106) were fixed at room temperature in 60 ml of RPMI 1640 medium containing 1% formaldehyde for 10 min. After sonication, the protein-DNA complexes were immunoprecipitated with anti-human p45 and anti-myc antibodies, respectively. The precipitated chromatin DNAs were purified and amplified by PCRs (95°C for 7 min followed by 34 cycles at 94°C for 30 s, 55°C for 40 s, and 72°C for 40 s).
Northern blot analysis.
The Northern blot hybridization procedures essentially followed those of Liu et al. (39). The total RNAs were extracted with commercial TRIzol reagent (Invitrogen). Amounts (5 μg) of each RNA sample were separated on a 1% agarose-6% formaldehyde gel, transferred to a nylon membrane, and hybridized with radioactive DNA probes amplified by PCR. The blots were subjected to autoradiography or direct quantitation in a PhosphorImager (Fuji).
RESULTS
Sumoylation of p45 in vitro.
Inspection of the sequence of human p45 revealed the presence of a tetrapeptide, TKME, around lysine 368 (K368) near the C-terminal bZIP domain. This sequence conforms to ψKXE, the consensus of sumoylation sites found in most sumoylated proteins (Fig. 1A). To test whether p45 is a previously unrecognized substrate for SUMO-1 modification, we carried out in vitro sumoylation assays as described in Materials and Methods. As shown in Fig. 1B, a higher-molecular-weight form of the p45 protein was detected in reaction mixtures containing the substrate and all the components required for the sumoylation (Fig. 1B, lane 2). The apparent molecular weight of the band suggested that a single molecule of His-SUMO-1 was attached to p45 under our in vitro sumoylation conditions. Matrix-assisted laser desorption ionization mass analysis showed that this band indeed contained both SUMO-1 and GST-p45 (data not shown).
FIG. 1.
Sumoylation of p45/NF-E2 in vitro. (A) Schematic representation of the domain organization of p45. The region (positions 1 to 114) interacting with CBP, the PY motif interacting with the WW domain, and the bZIP domain are indicated. Also shown are the phosphorylation site at S169 and the sumoylation site at K368; the sequence around K368 is compared with the consensus motif of sumoylation (ψKXE). (B) Sumoylation reaction in vitro. The reaction was carried out as described in Materials and Methods. The GST-p45 substrate and the SUMO-1-GST-p45 generated in the reaction were analyzed by immunoblotting (IB) with anti-p45 (upper panel) or anti-SUMO-1 (lower panel). (C) Requirement of K368 for in vitro sumoylation. The products of sumoylation and/or subsequent desumoylation reactions using GST-p45 and GST-p45 (K368R) as the substrates were analyzed by immunoblotting with anti-p45. SUMO-1-GST-p45 could only be generated from the reaction in lane 5.
To verify that K368 of p45 was indeed the sumoylation site for the conjugating enzyme, we replaced it with an arginine (R) by site-directed mutagenesis and repeated the sumoylation reactions with GST fusions of either wild-type or mutant p45 (K368R) (Fig. 1C). In similarity to the data shown in Fig. 1B, the wild-type p45 fusion protein could be sumoylated in vitro (lane 5, Fig. 1C) and the sumoylated band disappeared upon desumoylation (lane 4, Fig. 1C). In contrast, the mutant form could not be sumoylated (lane 2, Fig. 1C). Taken together, these data suggest that the p45 subunit of human NF-E2 could be sumoylated in vitro and that the major site of its sumoylation is at K368.
Sumoylation of p45 in vivo.
First, we cotransfected K562 cells with pEF-p45 and pCMV-His-SUMO-1. The whole-cell extract was fractionated through Ni-NTA agarose, and the retained fraction containing His-tagged proteins was immunoblotted with anti-p45. As shown in Fig. 2A, a 55-kDa band could be easily detected when both plasmids were used in transfection (lane 1, Fig. 2A). Interestingly, use of pCMV-His-SUMO-1 alone also resulted in a faint 55-kDa band on the blot (lane 2, Fig. 2A), suggesting that some endogenous p45 proteins of K562 were sumoylated with addition of the transfected His-SUMO-1.
FIG. 2.
Sumoylation of p45/NF-E2 in vivo. (A) Sumoylation of p45 in transfected K562 cells. The transfections of K562 cells were carried out using pEF-p45 and pCMV-His-SUMO-1 as described in Materials and Methods. The whole-cell extracts were then prepared and incubated with Ni-NTA agarose to isolate His-tagged protein(s). The retained fractions were analyzed by immunoblotting (IB) with anti-p45. (B) Requirement of K368 for sumoylation of human p45/NF-E2 in vivo. 293 cells were transfected with different combinations of the expression plasmids. The whole-cell extracts (WCE) were then prepared, IP with anti-Flag, and immunoblotted (IB) with anti-p45. Note that sumoylated p45 could only be detected in the 293 extract containing myc-p45 and FlagDsRed-SUMO-1 GG (lane 1, upper panel). The WCE prior to IP were also analyzed by Western blotting with anti-p45 (middle panel) and anti-SUMO-1 (lower panel), respectively. The arrowheads point to the positions of the sumoylated p45 on the gel. (C) Sumoylation of endogenous p45 in K562 cells. The nuclear extract was prepared from K562 cells, fractionated by precipitation with 20% to 50% ammonium sulfate, and analyzed by immunoblotting with anti-p45 (left panel) or with anti-SUMO-1 (middle panel). The 20% ammonium sulfate fraction was also immunoprecipitated with rabbit preimmune serum (left lane, right panel) or rabbit anti-p45 (right lane, right panel) and then hybridized with mouse anti-SUMO-1. (D) Sumoylation of endogenous p45 in mouse E14.5 fetal liver. The whole-cell extract was prepared from mouse E14.5 fetal livers as described in Materials and Methods and then analyzed by Western blotting using anti-p45 (lane 1) or anti-SUMO-1 (lane 2) as the probe. The extract was also immunoprecipitated with rabbit preimmune serum (lane 3) or rabbit anti-p45 (lane 4). The immunoprecipitates were then analyzed by Western blotting using mouse anti-SUMO-1 antibody as the probe.
The sumoylation in vivo was further studied by cotransfection experiments using 293 cells and one or more of the expression plasmids pEF-myc-p45, pEF-myc-p45 (K368R), pFlagDsRed-SUMO-1 GG, and pFlagDsRed-SUMO-1 AA. At 48 h posttransfection, the extracts were immunoprecipitated with anti-Flag antibody and hybridized with anti-p45 (Fig. 2B). As shown, a band of 75 kDa was specifically detected only when wild-type p45 and tagged SUMO-1GG were coexpressed (lane 1, Fig. 2B). This band corresponds to the combined molecular weight of myc-p45 and FlagDsRed-SUMO-1GG substrate. The FlagDsRed-SUMO-1 AA contains Gly (G)-to-Ala (A) mutation at G97, which is essential for the ability of SUMO-1 to be conjugated to the substrates. Replacement of FlagDsRed-SUMO-1 GG with FlagDsRed-SUMO-1 AA (lane 2, Fig. 2B) or of myc-p45 with myc-p45 (K368R) (lane 4, Fig. 2B) resulted in the loss of the 75-kDa band. This data suggested that in similarity to the in vitro reaction results, sumoylation of p45 in vivo also occurs predominately at the K368 residue. The whole-cell extract was hybridized with anti-SUMO-1 and anti-p45 as controls (middle and lower panels, Fig. 2B).
We have investigated whether the endogenous p45 of the erythroid K562 cells is sumoylated in vivo. First, to increase the signal-to-noise ratio of detection of the endogenous p45 and its modified form in K562 cells, K562 nuclear extract was prepared and fractionated by step-wise precipitation using 20% to 50% ammonium sulfate. These fractions were then analyzed by Western blotting with anti-p45 and anti-SUMO-1 antibodies. As shown in Fig. 2C, a band of 55 kDa, mainly in the 20% and 30% ammonium sulfate fractions, was detected on blots reacted with either antibody. A doublet around the molecular weight of 45 kDa was detected by anti-p45 (left panel, Fig. 2C) but not by anti-SUMO-1 (middle panel, Fig. 2C). The electrophoretic mobility of the 55-kDa species was consistent with the addition of a single SUMO-1 chain to p45. The 20% ammonium sulfate fraction was further immunoprecipitated with rabbit anti-p45 and preimmune serum, respectively, and then reacted with mouse anti-SUMO-1 (right panel, Fig. 2C). As seen, only the sample immunoprecipitated with anti-p45 gave a 55-kDa band on the Western blot (right lane of the right panel, Fig. 2C). The data of Fig. 2C indicated that a subpopulation, albeit minor (5 ∼ 10%), of the endogenous p45 molecules in the human erythroid K562 cells was monosumoylated.
The endogenous p45 of the mouse E14.5 fetal liver also appeared to be sumoylated in vivo. As shown in Fig. 2D, when the whole-cell extract prepared from the fetal liver was probed with anti-p45, both the p45 doublet and a 55-kDa band were seen on the Western blot (lane 1, Fig. 2D). However, only the 55-kDa band was seen when anti-SUMO-1 antibody was used (lane 2, Fig. 2D). When the extract was first IP with rabbit anti-p45 or rabbit preimmune serum and then subjected to Western blotting analysis using mouse anti-SUMO-1 as the probe, only the former IP sample exhibited the 55-kDa band (compare lanes 3 and 4, Fig. 2D). The data of Fig. 2C and 2D have demonstrated that a subpopulation, albeit minor (5 ∼ 10%), of the endogenous p45 molecules in the human erythroid K562 cells and the mouse erythroid fetal liver cells is monosumoylated.
Biological effects of p45 sumoylation.
Next, we examined the possible biological effects of p45 sumoylation on the transactivation capability and DNA binding affinity of NF-E2.
(i) Transactivation.
The transactivation capability of NF-E2 as affected by sumoylation of its p45 subunit was assessed. For this, we used as the reporter a pRBGP2-luciferase (Luc) construct (29), which contains tandemly arranged NF-E2/AP1 binding sites linked to the TATA box of the β-globin promoter (top map, Fig. 3A). Increasing amounts (0.5 to 4 μg) of pEF-myc-p45 or pEF-myc-p45 (K368R) were cotransfected with 2 μg of pBRGB2-Luc into 293 cells. Indeed, as shown previously (29), the exogenous p45 transactivated the promoter up to 7.7-fold (black bars, Fig. 3A). On the other hand, the exogenous p45 (K368R) transactivated the promoter only 1.7-fold (white bars, Fig. 3A). Interestingly, coexpression of the wild-type conjugation enzyme UBC9 with p45 further increased the reporter activity, by as much as 17-fold, in 293 cells (black bars, Fig. 3B). Again, this increase was not observed when the plasmid pEF-myc-p45 (K368R) was used (white bars, Fig. 3B). Furthermore, the inclusion of pCMV-SUMO-1 GG (black bars, Fig. 3C and 3D), but not pCMV-SUMO-1 AA (white bars, Fig. 3C and 3D), in the cotransfection mixture also further increased the transactivation level by twofold to threefold when pEF-myc-p45 (Fig. 3C), but not pEF-myc-p45 (K368R) (Fig. 3D), was used. The above-described up-regulation by UBC9 and SUMO-1 GG was not observed when pEF-myc was used (data not shown). These results demonstrated that sumoylation of p45 significantly enhanced the transactivation capability of NF-E2.
FIG. 3.
Enhancement of transactivation capability of p45/NF-E2 by sumoylation. (A) Reporter transactivation by myc-p45 (black bars) and myc-p45 (K368R) (white bars), respectively. 293 cells were transfected with 2 μg of pRBGP2-Luc (map shown on top) and different amounts of the expression plasmid pEF-myc-p45 or pEF-myc-p45 (K368R). The luciferase activities were measured and calculated. Their relative values are shown in the bar histogram. Data shown are expressed as means ± standard errors of the means from at least three independent assays, each carried out in duplicate. The amounts of myc-p45 and myc-p45 (K368R) in different transfectants were compared by immunoblotting. (B) Enhancement of p45-mediated transactivation by coexpressed UBC9. 293 cells were transfected with 2 μg of pRBGP2-Luc, 2 μg of pEF-myc-p45, or 2 μg of pEF-myc-p45 (K368R) and different amounts of the expression plasmid pcDNA-UBC9. Note that the transactivation mediated by myc-p45 (black bars), but not myc-p45 (K368R) (white bars), was enhanced with coexpression of UBC9. The amounts of myc-p45 and myc-p45 (K368R) in different transfectants were compared by immunoblotting. (C) Enhancement of p45-mediated transactivation by coexpressed SUMO-1. 293 cells were transfected with 2 μg of pRBGP2-Luc, 2 μg of pEF-myc-p45, and different amounts of the expression plasmid pCMV-SUMO-1 GG or pCMV-SUMO-1 AA. Note that the transactivation mediated by myc-p45 was enhanced by coexpression of SUMO-1 GG (black bars) but not by that of SUMO-1-AA (white bars). (D) Requirement of K368 for enhancement of p45-mediated transactivation by SUMO. Parallel transfections were done as described above for panel C except that pEF-myc-p45 was replaced with pEF-myc-p45 (K368R). The amounts of myc-p45 (K368R) in different transfectants were estimated by immunoblotting. We have also analyzed the transactivation capability of p45/NF-E2 in K562 cells as affected by coexpression of UBC9 or SUMO-1. Results similar to those seen with 293 cells were obtained, but the effects were smaller due to the low activity of the cytomegalovirus promoter in K562 cells (data not shown).
(ii) DNA binding affinity.
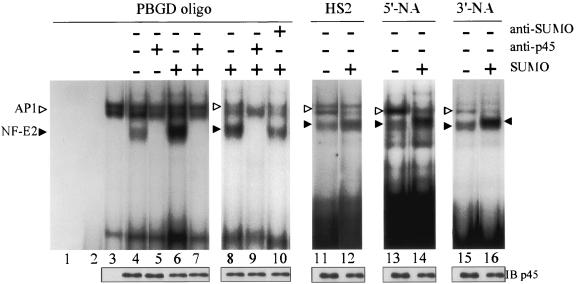
Next, we tested whether sumoylation of p45 would increase the DNA binding affinity of NF-E2 to its cognate binding motifs. To achieve this, we carried out EMSA using specific oligonucleotide probes and nuclear extracts prepared from 293 cells transfected with different combinations of plasmids expressing p45, Maf G, and SUMO-1, respectively. As shown for the oligonucleotide containing the NF-E2 binding site of the PBGD promoter, coexpression of p45 and Maf G resulted in two clusters of complexes (lane 4, Fig. 4). Of the two, the lower one could be abolished with the use of anti-p45 (lane 5, Fig. 4), indicating that it was indeed the NF-E2/DNA complex(es). This pattern is similar to those revealed in many previous studies (for references, see the introduction above), which also showed that the upper band consisted of at least the AP1/DNA complexes. Interestingly, however, additional expression of SUMO-1 in 293 cells resulted in increased levels of the lower NF-E2/DNA complex and often concomitant decreased levels of the upper AP1/DNA complex (for example, compare lane 6 to lane 4 in Fig. 4). A similarly increased level of the NF-E2/DNA complex upon cotransfection with pCMV-Flag-SUMO-1 GG was observed with the use of three other NF-E2/AP1-binding site-containing oligonucleotides (lanes 12, 14, and 16, Fig. 4). Interestingly, the upper portion of the NF-E2/DNA band could be super shifted with the use of anti-SUMO-1 (compare lane 8 to lane 10 in Fig. 4). This is consistent with the notion that the complex contains sumoylated p45/NF-E2. Under the transfection conditions used, the small subunit of NF-E2, MafG, could not be modified by SUMO-1 (M. Yamamoto and H. Motohashi, personal communication). The data of Fig. 4 thus suggest that NF-E2 containing sumoylated p45 has higher DNA binding affinity towards its cognate binding sites than the unmodified form. This is likely one of the reasons that sumoylation enhanced the transactivation capability of NF-E2.
FIG. 4.
Effects of p45 sumoylation on NF-E2/DNA binding. EMSA was carried out using extracts prepared from 293 cells cotransfected with pEF-p45 plus pEF-His-Maf G (lanes 4, 5, 11, 13, and 15) or with pEF-p45 plus pEF-His-Maf G plus pCMV-SUMO-1 (lanes 6, 7, 8 to 10, 12, 14, and 16). Nuclear extracts were prepared from 293 or transfected 293 cells and used for EMSA with different oligonucleotides (oligo) as the probes. Lane 1, probe only; lane 2, probe plus anti-p45; lane 3, probe plus 293 extract. The samples in lanes 5, 7, and 9 were the same as those in lanes 4, 6, and 8 except that anti-p45 was included in the binding reactions. The sample in lane 10 was the same as that in lane 8 except that anti-SUMO-1 was included in the binding reaction. The amounts of p45 protein in the different extracts used for EMSA were estimated by Western blotting (IB-p45). The filled and blank arrowheads point to the positions of the NF-E2/DNA complex and AP1/DNA complex, respectively, on the gel. Note the slightly slower migration of a portion of the NF-E2/DNA complex(es) in the SUMO (+) sample lanes (for example, compare lane 15 to lane 16). This portion is likely the complex formed between the DNA oligonucleotide and NF-E2 containing sumoylated p45.
Erythroid cell-specific association of human β-globin locus with PODs containing sumoylated p45/NF-E2 and RNA polymerase II. (i) Costaining of anti-p45, anti-SUMO-1, and anti-PML.
The expression of genes has been linked to their spatial positions in the nucleus relative to the NBs (references 16 and 35 and references therein). We have combined immunofluorescence staining with DNA-FISH to examine whether the regulation of the human β-like globin genes is related to the distributions and spatial arrangements of the locus and specific NBs, in particular in relation to the sumoylation of p45 described above. As seen in Fig. 5A, panel a, the p45 factors in K562 cells are distributed mostly as nuclear dots, in similarity to the p45-staining pattern of the MEL cells (22). However, each K562 cell contains two to more than four p45-containing nuclear substructures that are relatively larger, with the diameters greater than 0.2 μm. We termed them the “p45 bodies.” Depending on the growth conditions, approximately 60 to 90% (n = 94) of these “p45 bodies” are present in the euchromatin regions of the K562 nuclei, as exemplified in Fig. 5A, panel a. Use of anti-SUMO-1 also detected a partially overlapping dotted pattern of K562 nuclear staining (Fig. 5A, panel b) that was revealed by the use of anti-PML (Fig. 5A, panels d and f). These distribution patterns of SUMO-1 and PML are similar to those observed previously by others (21, 26). As with the p45 bodies, we noticed that there also existed 4 to 10 relatively larger SUMO-1-staining nuclear substructures, or SUMO-1 bodies, the diameters of which were larger than 0.5 μm (arrowhead, Fig. 5A, panel b). Interestingly, most if not all (94/94) of the p45 bodies colocalized with the SUMO-1 bodies, as exemplified in Fig. 5A, panel e.
FIG. 5.
Immunofluorescence staining of K562 cells. (A) K562 cells were immunostained for the nuclear distributions of p45 (panel a), SUMO-1 (panel b), and PML (panel c). The merged patterns of panels b and c, a and b, and a, b, and c are shown in panels d, e, and f, respectively. The scale bars are 2 μm. (B) K562 pools overexpressing myc-p45 or myc-p45 (K368R) were immunostained with anti-myc (panels a, c, d, and f) or anti-SUMO-1 (panels b, c, e, and f), respectively. Note the colocalization of p45 and SUMO-1 in K562-myc-p45 but not in K562-myc-p45 (K368R).
It appeared that only the sumoylatable p45 molecules resided within the p45 bodies. We transfected K562 cells with pEF-myc-p45 and pEF-myc-p45 (K368R), respectively, and analyzed the immunofluorescent staining patterns of the exogenous p45 molecules with the use of anti-myc antibody. As exemplified in Fig. 5B, in similarity to the results seen with endogenous p45 (Fig. 5A), the exogenous myc-p45 molecules were also distributed as nuclear dots and larger bodies (Fig. 5B, panel a). More than 90% (39/43) of the relatively larger, myc-p45 bodies also colocalized with the endogenous SUMO-1 bodies (Fig. 5B, panel c). In contrast, 85% (31/36) or more of the myc-p45 (K368R) bodies were found not to colocalize with the SUMO-1 bodies, as exemplified in Fig. 5B, panels d to f. Thus, an intact sumoylation site of p45 is essential for the nuclear colocalization of the p45 bodies with the SUMO-1 bodies-POD.
(ii) Immuno-DNA-FISH of the human β-like globin locus.
The K562 cell line we used has a modal chromosome number of 67 (range, 64 to 69); three of the chromosomes are chromosome 11. To examine the positioning of the human β-globin locus in the 3D nucleus relative to the above-described NBs, we first validated the DNA-FISH technique by mapping the β-like globin locus on the metaphase chromosomes of K562 cells. Indeed, with use of the μLCR/γ fragments derived from the β-globin locus and WCB11 (Vysis) as the probes, the β-like globin loci could be specifically detected on the three pairs of the metaphase chromosome 11 of K562 (data not shown). In consistency with this, two to three μLCR/γ hybridizing signals could be seen after DNA-FISH of the interphase K562 cells (see below; also data not shown).
DNA-FISH was then combined with the use of different antibodies for immunostaining of the NBs. As exemplified in Fig. 6A, SUMO-1 bodies also existed in nonerythroid cells such as HeLa cells, which did not show significant (<5%; n = 3/53) association with the human β-globin locus identified by DNA-FISH. In interesting contrast, as exemplified in Fig. 6B, more than 95% (72/76) of the human β-globin loci in K562 cells were colocalized with the p45 bodies and consequently the SUMO-1 bodies. In consistency with this, 95% (56/59) of the human β-globin loci were colocalized with the anti-PML-stained substructures or POD (Fig. 6C).
FIG. 6.
Immuno-DNA-FISH. Immuno-DNA-FISH experiments using HeLa cells (A) and K562 cells (B, C, and D) were carried out as described in Materials and Methods. The merged patterns of DAPI staining and DNA-FISH of the β-globin locus are shown in panels a. Note the colocalization patterns of β-globin locus-p45 body-SUMO-1 body, β-globin locus-PML body-SUMO-1 body, and β-globin locus-PML body-RNAP II body for the K562 cells shown in panels B, C, and D, respectively.
Recently, actively transcribing β-globin loci in the mouse erythroid cells were found to be located in the so-called “RNA polymerase II factories” (48). To see whether the β-globin locus-associated p45/SUMO-1 bodies in human erythroid cells are also enriched with RNAP II, we first carried out immunostaining of K562 cells with the antibody N-20, which recognized predominantly phosphorylated RNAP II but also, to a much less extent, the unphosphorylated RNAP II. As seen in Fig. 6D, panel b, the RNAP II distribution pattern was similar to those previously observed (see references 48, 61, and 65 and references therein), with quite a few relatively large bodies similar to the “RNAP II factories” described in reference 48. Interestingly, the human β-globin loci of K562 (Fig. 6D, panel a) colocalized significantly (42/55) with the RNAP II bodies as well (Fig. 6D, panel d). Consistent with this, most of the β-globin locus-associated RNAP II bodies also significantly colocalized or were in close proximity with POD (Fig. 6D, panels c and d). Use of 8WG16, which recognized mainly the unphosphorylated form of RNAP II, gave similar colocalization patterns of POD, p45 bodies, and the β-globin locus with RNAP II, suggesting that both RNAP IIo and RNAP IIa were concentrated in the β-globin locus-associated POD in K562 cells (data not shown). On the basis of the data of Fig. 5 and 6, we conclude that in the globin-expressing K562 cells, but not in the inactive nonerythroid cells, there is a significant one-to-one relationship of colocalization of the human β-globin locus, the SUMO-1-concentrated POD, the p45 bodies, and the RNAP II factories.
Binding in vivo of wild-type p45, but not p45 (K368R), to the β-LCR in K562 cells.
In view of the data of Fig. 4, 5, and 6, it is interesting, and important as well, to check whether the mutant p45 (K368R)/NF-E2 could be recruited to the β-LCR of K562 and bind to the different HS sites, as previously shown for the wild-type or endogenous p45/NF-E2. For this, the ChIP method (17) was used.
K562 cells were transfected with pEF-myc, pEF-myc-p45, or pEF-myc-p45 (K368R) and then subjected to analysis by ChIP. As seen in Fig. 7, binding of the endogenous p45 at HS1-HS4 sites of the β-LCR, but not at the intergenic regions (regions a and b), could be detected in K562 cells transfected with pEF-myc (K562-myc lanes, Fig. 7B to G). Interestingly, the exogenous myc-p45 molecules were also associated with the four HS sites of β-LCR in K562 cells transfected with pEF-myc-p45 (K562-myc-p45 lanes, Fig. 7B to E). In contrast, few or no PCR signals could be seen in the ChIP samples prepared from K562 cells transfected with pEF-myc-p45 (K368R) and precipitated with anti-myc [see K562-myc-p45 (K368R) lanes, Fig. 7B to E]. Thus, together with the data of Fig. 4 to 6, the ChIP analysis of Fig. 7 strongly supports the scenario that an intact sumoylation site at K368 of p45/NF-E2, which confers stronger DNA binding affinity of NF-E2 than the K368R mutant, is a priori for the association among NF-E2, the SUMO-1 body/POD, and the human β-like globin locus in the erythroid K562 cells.
FIG. 7.
ChIP assay. ChIP was used to assay the association of myc-p45 and myc-p45 (K368) with the β-like globin locus in transfected K562 cells. (A) Map of the globin locus and the regions analyzed for p45/NF-E2 binding. (B, C, D, E, F, and G) DNAs precipitated from K562 cell transfected with pEF-myc (gray-shaded bars), pEF-myc-p45 (black bars), and pEF-myc-p45 (K368R) (white bars), respectively, were analyzed by PCR using primers specific for HS1 (B), HS2 (C), HS3 (D), and HS4 (E) of β-LCR, two intergenic regions (region a [panel F] and region b [panel G]), and the β-actin gene. The samples of the input (In) and those precipitated with the use of anti-p45 (p45), anti-myc (myc), and preimmune serum (pre), respectively, are individually indicated underneath the gel panels. The DNA amounts used for PCR were determined by the intensities of the β-actin signals. The PCR signals were first normalized to those from the β-actin region. The different target/β-actin ratios were then further normalized again the target/β-actin ratios of the input samples and used to plot the histographs. The relative intensities of the positive signals were given as the increases (fold) over those from the preimmune samples. Each histogram consists of averages of data derived from two to three sets of PCR analysis conducted with the use of chromatin DNAs precipitated from at least two different K562 pools. The differences (fold) are given as means ± standard errors of the means.
Rescue of β-globin gene expression in CB3 cells by wild-type p45 but not by p45 (K368R).
The physiological function of p45 sumoylation in the transactivation of the endogenous β-like globin locus in erythroid cells was directly tested by the use of CB3 cells. CB3 is a derivative of the mouse adult erythroleukemic cell line MEL. Due to a proviral integration event, CB3 does not express p45. This has led to the little expression of βmajor-globin and α-globin genes in CB3, even with dimethyl sulfoxide (DMSO) induction. Interestingly, the βmajor-globin and α-globin expression in CB3 cells could be partially restored upon expression of exogenous p45/NF-E2 (7, 34, 41).
CB3 cells were stably transfected with pEF-p45 or with pEF-p45 (K368R). Pools from independent transfections were selected, and the expression levels of the exogenous p45, the endogenous globin genes, and two housekeeping genes in these cell pools were analyzed by Northern or Western blotting. As shown in Fig. 8, expression of the adult βmajor-globin and α-globin genes, but not embryonic ε-globin genes, was inducible by DMSO in MEL cells (lanes 1 and 2, Fig. 8) but not in CB3 cells (lanes 7 and 8, Fig. 8). Stable transfection with pEF-p45/NF-E2 rescued the expression of the adult globin genes, as exemplified for two independent CB3 (p45) pools (lanes 3 to 6). In contrast, the mutant p45 (K368R) could not rescue the globin gene expression, as exemplified by analysis of two independent CB3 [p45 (K368R)] pools (lanes 9 to 12). The data of Fig. 8 demonstrated that an intact K368 site of p45, and by implication the sumoylation of p45, is essential for transactivation of the endogenous globin genes in erythroid cells by NF-E2.
FIG. 8.
Rescue of adult globin gene expression in CB3 cells. RNAs and protein extracts were isolated from MEL (lanes 1 and 2), CB3 (lanes 7 and 8), and CB3 pools stably integrated with pEF-p45 (lanes 3 to 6) or pEF-p45 (K368R) (lanes 9 to 12), with or without prior induction by DMSO for 72 h. The RNAs were subjected to analysis by reverse transcription-PCR for estimation of the gene expression levels of adult βmajor-globin and α-globin and embryonic ε-globin. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as the control. Western blotting (IB) was used to estimate the levels of p45 and tubulin proteins. Note that, as already been observed previously by others (7, 34), the expression level of the βmajor-globin gene is often higher in CB3(p45) than in MEL without DMSO induction.
DISCUSSION
In this study, we have identified a new and functionally important modification of the transcription activator NF-E2. Human NF-E2 is known to be acetylated at its small subunit Maf G (28), and its large subunit p45 could be phosphorylated in vitro (9). We have now demonstrated that p45 could also be sumoylated in vitro and in vivo. Our data also suggest that sumoylation of p45 plays important regulatory roles in the transactivation capability of NF-E2 as well as the nuclear positioning of its target genes.
p45 sumoylation enhanced the transactivation capability of NF-E2 in transfected cells (Fig. 3). More physiologically important is that the ability of human p45/NF-E2 to rescue the expression of the endogenous globin genes of CB3 cells was hampered by mutation at the K368 sumoylation site of p45 (Fig. 8). In most of the previous studies, SUMO-1 modification correlated with the activation or repression of transcription (see a review in reference 31). Sumoylation of HSF1 and HSF2 led to their relocalization to the stress granules or to POD, and it resulted in the concomitant activation of the DNA binding and transcriptional activities of these proteins (25, 27). At the moment, the details of the enhancement of human NF-E2-mediated transactivation by p45 sumoylation are not well defined. It is at least in part due to the enhanced binding of human NF-E2 to its cognate recognition motifs upon p45 sumoylation (Fig. 4), an effect similar to the acetylation of Maf G (28). Another possibility is that the sumoylated p45/NF-E2 molecules are colocalized mainly with their target gene loci in the same nuclear subcompartments in order to function. Indeed, as suggested by our combined DNA-FISH-immunofluorescence staining (Fig. 6) and ChIP (Fig. 7) analyses, sumoylation of p45 very likely plays a critical role in the anchoring of NF-E2-regulated gene loci, such as the β-like globin gene cluster, within close proximity of specific NBs such as POD.
NBs are one class of eukaryotic nuclear substructures defined immunologically by the presence of one or more “signature proteins.” The NBs, interacting with each other through partial overlapping or interconnection with different types of NBs (40, 62), have been suggested to function as the storage sites of transcription factors and splicing factors, the assembly stations of the corresponding transcription-splicing complexes, and more recently also as the places within or near which active transcription-splicing could go on (references 23, 40, 42, 48, 56, and 62 and references therein).
Among the known NBs, POD or the PML bodies have been implicated in several cellular pathways, including the control of apoptosis (reviewed in reference 50), viral pathogenicity (reviewed in reference 49), and regulation of the higher-order chromatin structure (18). Nevertheless, in comparison to other well-studied NBs such as Cajal bodies or the speckles, it is still unclear whether PODs play an active role in nuclear events, i.e., whether they form as the result of nuclear events or are simply random accumulations of excess nuclear factors (45). With respect to the issue mentioned above, it is interesting that human p45/NF-E2 molecules are also distributed in the nucleus as discrete dots and “p45 bodies” (reference 22 and this study), the latter of which were demonstrated to overlap with the SUMO-1-containing PODs as well as the RNAP II bodies and factories in the erythroid cells (Fig. 5 and 6). Furthermore, as determined on the basis of their costaining patterns with anti-PML and anti-SUMO-1, more than 95% of the β-globin loci in K562 cells colocalize with a POD (Fig. 6C). The β-globin locus also colocalizes with RNAP II factories (Fig. 6D) and CBP (data not show). The PODs have been shown to be capable of serving as the sites of transcription because of the presence of transcription factors and chromatin remodeling proteins, including coactivators such as CBP, RNAP II, and nascent RNAs, either undefined or gene specific, at the periphery of and/or within the PODs (33, 36, 58, 61). Thus, the β-globin locus joins the major histocompatibility complex (53) and TP53 locus (57) to utilize the PODs as the nuclear sites of transcription complex assembly and/or active transcription. There are two relevant studies whose results are consistent with this scenario. First, as already mentioned, CBP interacts with NF-E2 in vivo (14, 28). Furthermore, RNA-FISH and coimmunofluorescence staining showed recently that the mouse β-globin loci of the E18.5 erythroid fetal liver cells are localized within the RNAP II factories (48).
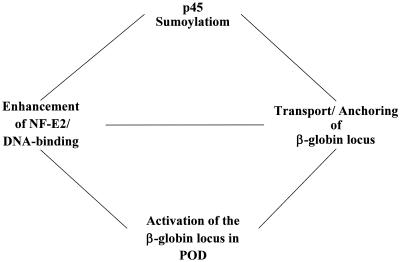
Base on the results detailed above, we propose a model of human β-globin gene regulation in relation to the sumoylation of p45/NF-E2. Much information has been produced regarding the molecular and cellular basis of mammalian β-globin switch (8, 19, 20, 37). The switch involves the physical and functional interactions between the multiple protein complexes formed at the β-LCR and the different β-like globin promoters. In particular, the complexes formed at the several DNase I-HS sites act together as a “holo-complex” to regulate the chromatin structure and transcription of the β-globin locus. The β-LCR and promoter complexes consist of various DNA binding factors and coregulators, including NF-E2, which appears to be required for loading of RNAP II from β-LCR to the β-globin promoter (see the introduction above for references). Here we suggest that the effective concentration of appropriately modified NF-E2 is critical for active transcription of the β-globin locus (Fig. 9). In particular, p45 sumoylation, perhaps in conjunction with acetylation of the small Maf G subunit, enhances the DNA binding affinity of NF-E2 to its cognate recognition motifs in the β-LCR. This in turn facilitates the chromatin remodeling and transcription of the β-globin locus by effective recruitment of various cofactors and RNAP II to the LCR and subsequently to the β-like globin promoters. More importantly, we suggest that in order for optimal expression in the erythroid cells to occur, the β-globin locus needs to be brought into and anchored with the nuclear body POD, an event mediated by the sumoylated p45/NF-E2. Within the POD of erythroid cells, the assembly and functioning of the active transcription complexes of the locus then take place. Since NF-E2 also regulates the α-globin locus through binding at its upstream regulatory element HS-40 (reference 64 and references therein), POD could be one of the key nuclear subdomains within which the coordinate regulation of α-like and β-like globin gene loci occurs. It should be noted that 72% of the β-globin loci in K562 cells are colocalized with the speckles (Y.-C. Shyu and C.-K.J. Shen, unpublished data).
FIG. 9.
Model of transcriptional activation of human β-like globin genes by p45/NF-E2 sumoylation. It is proposed that sumoylation of p45 plays an important role in the activation of the human-like β-globin locus by regulation of the NF-E2/DNA binding affinity and its function to anchor the gene locus within the POD.
Acknowledgments
We thank B.-C. Chung, Chi-Hung Lin, Qiliang Li, George Stamatoyannopoulos, Masayuki Yamamoto, and T. P. Yao for plasmids used in the study. We also thank H. Motohashi for sharing unpublished data.
This research was supported by a grant from the National Health Research Institute and by the Academia Sinica, Taipei, Taiwan, Republic of China.
REFERENCES
- 1.Amrolia, P. J., L. Ramamurthy, D. Saluja, N. Tanese, S. M. Jane, and J. M. Cunningham. 1997. The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the alpha- and beta-globin gene loci in an erythroid cell line. Proc. Natl. Acad. Sci. USA 94:10051-10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, N. C. 1998. The NF-E2 transcription factor. Int. J. Biochem. Cell Biol. 30:429-432. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, N. C., H. Erdjument-Bromage, M. B. Davidson, P. Tempst, and S. H. Orkin. 1993. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature 362:722-728. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, N. C., K. J. Kotkow, P. A. Ney, H. Erdjument-Bromage, P. Tempst, and S. H. Orkin. 1993. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc. Natl. Acad. Sci. USA 90:11488-11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong, J. A., and B. M. Emerson. 1996. NF-E2 disrupts chromatin structure at human beta-globin locus control region hypersensitive site 2 in vitro. Mol. Cell. Biol. 16:5634-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean, T. L., and P. A. Ney. 1997. Multiple regions of p45 NF-E2 are required for beta-globin gene expression in erythroid cells. Nucleic Acids Res. 25:2509-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blank, V., M. J. Kim, and N. C. Andrews. 1997. Human MafG is a functional partner for p45 NF-E2 in activating globin gene expression. Blood 89:3925-3935. [PubMed] [Google Scholar]
- 8.Bulger, M., and M. Groudine. 1999. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 13:2465-2477. [DOI] [PubMed] [Google Scholar]
- 9.Casteel, D., M. Suhasini, T. Gudi, R. Naima, and R. B. Pilz. 1998. Regulation of the erythroid transcription factor NF-E2 by cyclic adenosine monophosphate-dependent protein kinase. Blood 91:3193-3201. [PubMed] [Google Scholar]
- 10.Caterina, J. J., D. Donze, C. W. Sun, D. J. Ciavatta, and T. M. Townes. 1994. Cloning and functional characterization of LCR-F1: a bZIP transcription factor that activates erythroid-specific, human globin gene expression. Nucleic Acids Res. 22:2383-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan, J. Y., X. L. Han, and Y. W. Kan. 1993. Isolation of cDNA encoding the human NF-E2 protein. Proc. Natl. Acad. Sci. USA 90:11366-11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, W. Y., W. C. Lee, N. C. Hsu, F. Huang, and B. C. Chung. 2004. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J. Biol. Chem. 279:38730-38735. [DOI] [PubMed] [Google Scholar]
- 13.Chen, X., S. Wen, M. N. Fukuda, N. R. Gavva, D. Hsu, T. O. Akama, T. Yang-Feng, and C.-K. J. Shen. 2001. Human ITCH is a coregulator of the hematopoietic transcription factor NF-E2. Genomics 73:238-241. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, X., M. J. Reginato, N. C. Andrews, and M. A. Lazar. 1997. The transcriptional integrator CREB-binding protein mediates positive cross talk between nuclear hormone receptors and the hematopoietic bZip protein p45/NF-E2. Mol. Cell. Biol. 17:1407-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox, T. C., M. J. Bawden, A. Martin, and B. K. May. 1991. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 10:1891-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cremer, T., and C. Cremer. 2001. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2:292-301. [DOI] [PubMed] [Google Scholar]
- 17.Daftari, P., N. R. Gavva, and C.-K. J. Shen. 1999. Distinction between AP1 and NF-E2 factor-binding at specific chromatin regions in mammalian cells. Oncogene 18:5482-5486. [DOI] [PubMed] [Google Scholar]
- 18.de Jong, L., M. A. Grande, K. A. Mattern, W. Schul, and R. van Driel. 1996. Nuclear domains involved in RNA synthesis, RNA processing, and replication. Crit. Rev. Eukaryot. Gene Expr. 6:215-246. [DOI] [PubMed] [Google Scholar]
- 19.de Laat, W., and F. Grosveld. 2003. Spatial organization of gene expression: the active chromatin hub. Chromosome Res. 11:447-459. [DOI] [PubMed] [Google Scholar]
- 20.Engel, J. D., and K. Tanimoto. 2000. Looping, linking, and chromatin activity: new insights into beta-globin locus regulation. Cell 100:499-502. [DOI] [PubMed] [Google Scholar]
- 21.Eskiw, C. H., G. Dellaire, J. S. Mymryk, and D. P. Bazett-Jones. 2003. Size, position and dynamic behavior of PML nuclear bodies following cell stress as a paradigm for supramolecular trafficking and assembly. J. Cell Sci. 116:4455-4466. [DOI] [PubMed] [Google Scholar]
- 22.Francastel, C., W. Magis, and M. Groudine. 2001. Nuclear relocation of a transactivator subunit precedes target gene activation. Proc. Natl. Acad. Sci. USA 98:12120-12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gall, J. G. 2000. Cajal bodies: the first 100 years. Annu. Rev. Cell Dev. Biol. 16:273-300. [DOI] [PubMed] [Google Scholar]
- 24.Gong, Q. H., J. C. McDowell, and A. Dean. 1996. Essential role of NF-E2 in remodeling of chromatin structure and transcriptional activation of the epsilon-globin gene in vivo by 5′ hypersensitive site 2 of the beta-globin locus control region. Mol. Cell. Biol. 16:6055-6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodson, M. L., Y. Hong, R. Rogers, M. J. Matunis, O. K. Park-Sarge, and K. D. Sarge. 2001. Sumo-1 modification regulates the DNA binding activity of heat shock transcription factor 2, a promyelocytic leukemia nuclear body associated transcription factor. J. Biol. Chem. 276:18513-18518. [DOI] [PubMed] [Google Scholar]
- 26.Hakli, M., U. Karvonen, O. A. Janne, and J. J. Palvimo. 2005. SUMO-1 promotes association of SNURF (RNF4) with PML nuclear bodies. Exp. Cell Res. 304:224-233. [DOI] [PubMed] [Google Scholar]
- 27.Hong, Y., R. Rogers, M. J. Matunis, C. N. Mayhew, M. L. Goodson, O. K. Park-Sarge, K. D. Sarge, and M. Goodson. 2001. Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. J. Biol. Chem. 276:40263-40267. [DOI] [PubMed] [Google Scholar]
- 28.Hung, H. L., A. Y. Kim, W. Hong, C. Rakowski, and G. A. Blobel. 2001. Stimulation of NF-E2 DNA binding by CREB-binding protein (CBP)-mediated acetylation. J. Biol. Chem. 276:10715-10721. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi, K., K. Itoh, H. Motohashi, N. Hayashi, Y. Matuzaki, H. Nakauchi, M. Nishizawa, and M. Yamamoto. 1995. Activity and expression of murine small Maf family protein MafK. J. Biol. Chem. 270:7615-7624. [DOI] [PubMed] [Google Scholar]
- 30.Igarashi, K., K. Kataoka, K. Itoh, N. Hayashi, M. Nishizawa, and M. Yamamoto. 1994. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature 367:568-572. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, E. S. 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73:355-382. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, K. D., H. M. Christensen, B. Zhao, and E. H. Bresnick. 2001. Distinct mechanisms control RNA polymerase II recruitment to a tissue-specific locus control region and a downstream promoter. Mol. Cell 8:465-471. [DOI] [PubMed] [Google Scholar]
- 33.Kiesslich, A., A. von Mikecz, and P. Hemmerich. 2002. Cell cycle-dependent association of PML bodies with sites of active transcription in nuclei of mammalian cells. J. Struct. Biol. 140:167-179. [DOI] [PubMed] [Google Scholar]
- 34.Kotkow, K. J., and S. H. Orkin. 1995. Dependence of globin gene expression in mouse erythroleukemia cells on the NF-E2 heterodimer. Mol. Cell. Biol. 15:4640-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamond, A. I., and J. E. Sleeman. 2003. Nuclear substructure and dynamics. Curr. Biol. 13:R825-R828. [DOI] [PubMed] [Google Scholar]
- 36.LaMorte, V. J., J. A. Dyck, R. L. Ochs, and R. M. Evans. 1998. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc. Natl. Acad. Sci. USA 95:4991-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, Q., K. R. Peterson, X. Fang, and G. Stamatoyannopoulos. 2002. Locus control regions. Blood 100:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, Y. C., C. Lee, D. Sanoudou, T. H. Hseu, S. Y. Li, C. C. Lin, and T. H. Hsu. 2000. Interstitial colocalization of two cervid satellite DNAs involved in the genesis of the Indian muntjac karyotype. Chromosome Res. 8:363-373. [DOI] [PubMed] [Google Scholar]
- 39.Liu, J. J., S. C. Hou, and C.-K. J. Shen. 2003. Erythroid gene suppression by NF-kappa B. J. Biol. Chem. 278:19534-19540. [DOI] [PubMed] [Google Scholar]
- 40.Liu, Q., and G. Dreyfuss. 1996. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 15:3555-3565. [PMC free article] [PubMed] [Google Scholar]
- 41.Lu, S. J., S. Rowan, M. R. Bani, and Y. Ben-David. 1994. Retroviral integration within the Fli-2 locus results in inactivation of the erythroid transcription factor NF-E2 in Friend erythroleukemias: evidence that NF-E2 is essential for globin expression. Proc. Natl. Acad. Sci. USA 91:8398-8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matera, A. G. 1999. Nuclear bodies: multifaceted subdomains of the interchromatin space. Trends Cell Biol. 9:302-309. [DOI] [PubMed] [Google Scholar]
- 43.Mignotte, V., J. F. Eleouet, N. Raich, and P. H. Romeo. 1989. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc. Natl. Acad. Sci. USA 86:6548-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moi, P., and Y. W. Kan. 1990. Synergistic enhancement of globin gene expression by activator protein-1-like proteins. Proc. Natl. Acad. Sci. USA 87:9000-9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negorev, D., and G. G. Maul. 2001. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene 20:7234-7242. [DOI] [PubMed] [Google Scholar]
- 46.Ney, P. A., B. P. Sorrentino, K. T. McDonagh, and A. W. Nienhuis. 1990. Tandem AP-1-binding sites within the human beta-globin dominant control region function as an inducible enhancer in erythroid cells. Genes Dev. 4:993-1006. [DOI] [PubMed] [Google Scholar]
- 47.Nudel, U., J. E. Salmon, M. Terada, A. Bank, R. A. Rifkind, and P. A. Marks. 1977. Differential effects of chemical inducers on expression of beta globin genes in murine erythroleukemia cells. Proc. Natl. Acad. Sci. USA 74:1100-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osborne, C. S., L. Chakalova, K. E. Brown, D. Carter, A. Horton, E. Debrand, B. Goyenechea, J. A. Mitchell, S. Lopes, W. Reik, and P. Fraser. 2004. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat. Genet. 36:1065-1071. [DOI] [PubMed] [Google Scholar]
- 49.Regad, T., and M. K. Chelbi-Alix. 2001. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene 20:7274-7286. [DOI] [PubMed] [Google Scholar]
- 50.Salomoni, P., and P. P. Pandolfi. 2002. The role of PML in tumor suppression. Cell 108:165-170. [DOI] [PubMed] [Google Scholar]
- 51.Sawado, T., J. Halow, M. A. Bender, and M. Groudine. 2003. The beta-globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 17:1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawado, T., K. Igarashi, and M. Groudine. 2001. Activation of beta-major globin gene transcription is associated with recruitment of NF-E2 to the beta-globin LCR and gene promoter. Proc. Natl. Acad. Sci. USA 98:10226-10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiels, C., S. A. Islam, R. Vatcheva, P. Sasieni, M. J. Sternberg, P. S. Freemont, and D. Sheer. 2001. PML bodies associate specifically with the MHC gene cluster in interphase nuclei. J. Cell Sci. 114:3705-3716. [DOI] [PubMed] [Google Scholar]
- 54.Shivdasani, R. A., and S. H. Orkin. 1995. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc. Natl. Acad. Sci. USA 92:8690-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivdasani, R. A., M. F. Rosenblatt, D. Zucker-Franklin, C. W. Jackson, P. Hunt, C. J. Saris, and S. H. Orkin. 1995. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 81:695-704. [DOI] [PubMed] [Google Scholar]
- 56.Spector, D. L. 1993. Macromolecular domains within the cell nucleus. Annu. Rev. Cell Biol. 9:265-315. [DOI] [PubMed] [Google Scholar]
- 57.Sun, Y., L. K. Durrin, and T. G. Krontiris. 2003. Specific interaction of PML bodies with the TP53 locus in Jurkat interphase nuclei. Genomics 82:250-252. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi, Y., V. Lallemand-Breitenbach, J. Zhu, and H. de The. 2004. PML nuclear bodies and apoptosis. Oncogene 23:2819-2824. [DOI] [PubMed] [Google Scholar]
- 59.Talbot, D., and F. Grosveld. 1991. The 5′HS2 of the globin locus control region enhances transcription through the interaction of a multimeric complex binding at two functionally distinct NF-E2 binding sites. EMBO J. 10:1391-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verschure, P. J., I. van Der Kraan, E. M. Manders, and R. van Driel. 1999. Spatial relationship between transcription sites and chromosome territories. J. Cell Biol. 147:13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Mikecz, A., S. Zhang, M. Montminy, E. M. Tan, and P. Hemmerich. 2000. CREB-binding protein (CBP)/p300 and RNA polymerase II colocalize in transcriptionally active domains in the nucleus. J. Cell Biol. 150:265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, I. F., N. M. Reddy, and C.-K. J. Shen. 2002. Higher order arrangement of the eukaryotic nuclear bodies. Proc. Natl. Acad. Sci. USA 99:13583-13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, J., C. Shiels, P. Sasieni, P. J. Wu, S. A. Islam, P. S. Freemont, and D. Sheer. 2004. Promyelocytic leukemia nuclear bodies associate with transcriptionally active genomic regions. J. Cell Biol. 164:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen, S. C., K. Roder, K. Y. Hu, I. Rombel, N. R. Gavva, P. Daftari, Y. Y. Kuo, C. Wang, and C.-K. J. Shen. 2000. Loading of DNA-binding factors to an erythroid enhancer. Mol. Cell. Biol. 20:1993-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng, C., E. Kim, S. L. Warren, and S. M. Berget. 1997. Dynamic relocation of transcription and splicing factors dependent upon transcriptional activity. EMBO J. 16:1401-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]