Abstract
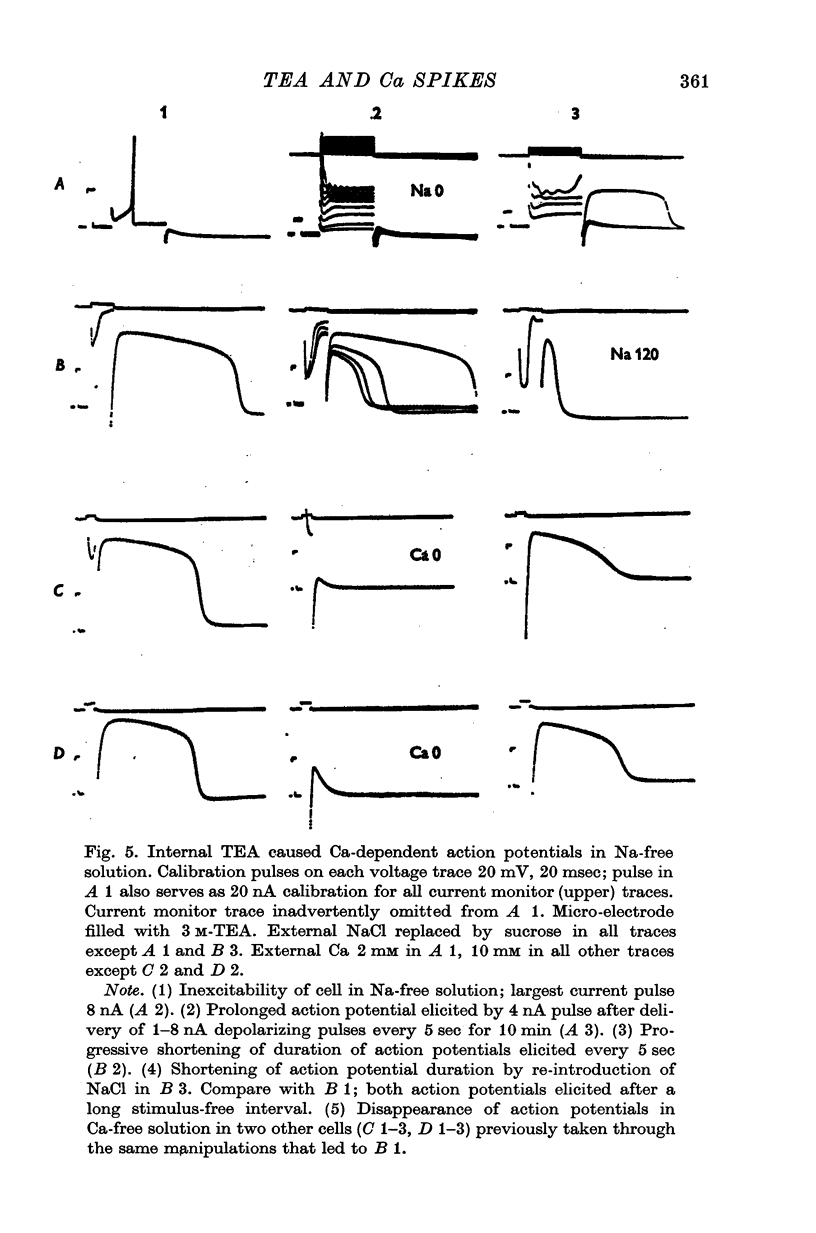
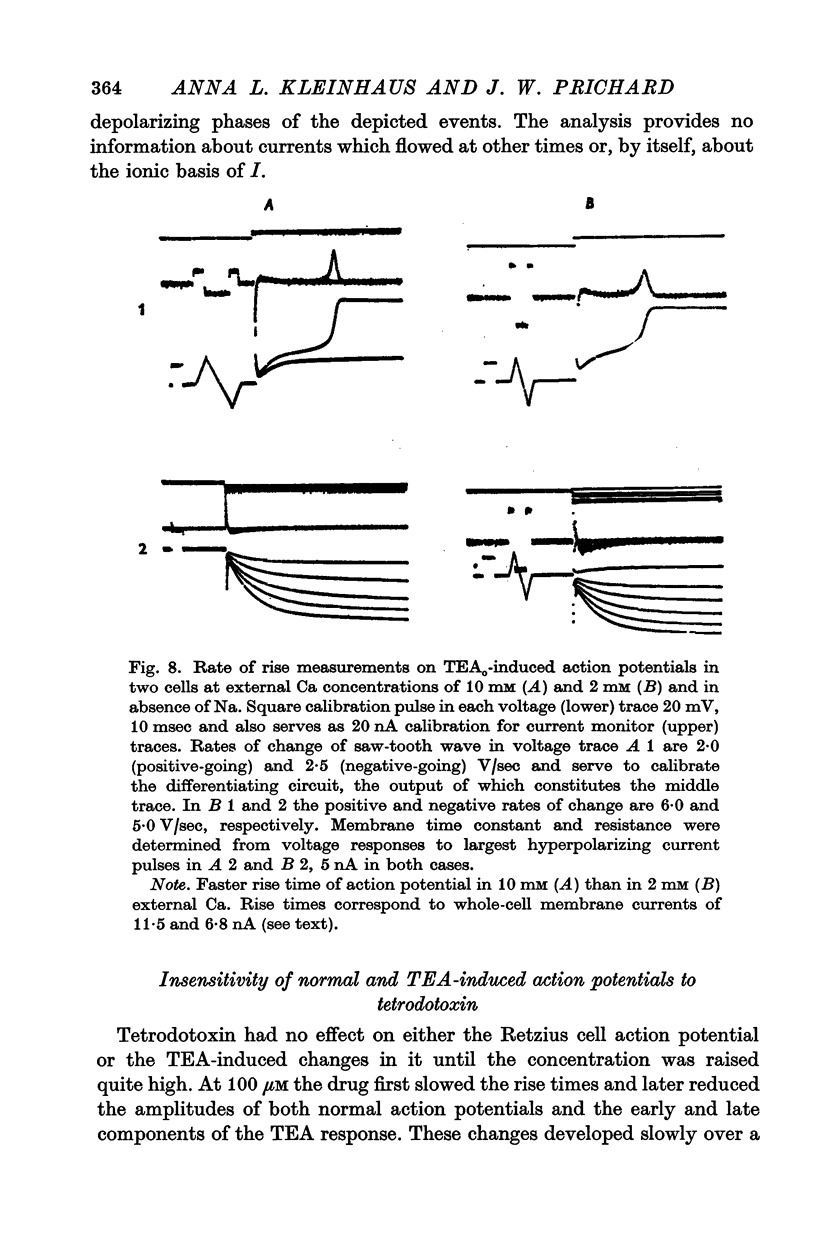
1. Retzius cells of leech segmental ganglia were exposed to tetraethylammonium chloride (TEA) presented both extracellularly, dissolved in the perfusing fluid, and intracellulary, by iontophoresis from a microelectrode. 2. Extracellular TEA, 10 and 25 mM, greatly prolonged the cells' action potentials, and the higher concentration increased their amplitude as well. At 10 mM the characteristic changes developed gradually over a period of about half an hour, while at 25 mM they appeared much more rapidly. However, at both concentrations the changes were reversible within minutes, even after long soaks in drug-containing solution. It is therefore probable that the drug acted at the outer surface of the membrane. 3. Intracellular TEA also prolonged the action potentials but there were several differences from the response produced by extracellular application. The changes developed gradually, and for a time, each firing of the cell was a complex event consisting of several early, brief depolarizations followed by a single much larger and more prolonged one. The large, late depolarization eventually obliterated the early ones; its gradual development suggested that it was produced only after TEA diffused to some extrasomatic portion of the cell. Intracellular TEA always caused progressive depolarization; this and the changes in the action potential were both irreversible, suggesting that the site of action was on the inner surface of the membrane. 4. Manipulations of external Na and Ca provided evidence that (a) in the absence of TEA, Retzius cell action potentials were exclusively Na-dependent, (b) that the early depolarizations in the complex action potentials produced by intracellular TEA were Na-dependent, while the later, large depolarization was Ca-dependent and (c) that the prolonged action potentials produced by extracellular TEA contained a large Ca-dependent component. 5. We conclude that TEA, acting from either side of the membrane, caused a voltage-sensitive, slowly activated Ca current to become a major contributor to the inward current of the action potential, probably by blocking the outward K current which ordinarily counteracts it. However, we cannot rule out the possibility that TEA enabled a Ca current by some means independent of its presumed action on K conductance. 6. Data resembling ours in some respects have been obtained from studies of the action of TEA on frog dorsal root ganglion cells, frog neuromuscular junction, and squid stellate ganglion. No clear counterpart of our findings has been reported form experiments on squid and amphibian axons, molluscan neurones, or frog skeletal muscle fibres.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Hille B. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J Gen Physiol. 1972 Apr;59(4):388–400. doi: 10.1085/jgp.59.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu G., Frank G. B., Inoue F. Tetraethylammonium-induced contractions of frog's skeletal muscle. II. Effects on intramuscular nerve endings. Can J Physiol Pharmacol. 1967 Sep;45(5):833–844. doi: 10.1139/y67-098. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Restoration by barium of action potentials in sodium-deprived mammalian B and C fibres. J Physiol. 1959 Mar 12;145(3):562–569. doi: 10.1113/jphysiol.1959.sp006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Studies on tetrodotoxin resistant action potentials in denervated skeletal muscle. Acta Physiol Scand. 1971 Nov;83(3):382–388. doi: 10.1111/j.1748-1716.1971.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Satow Y. Sodium- and calcium-dependent spike potentials in the secretory neuron soma of the X-organ of the crayfish. J Gen Physiol. 1971 Feb;57(2):216–236. doi: 10.1085/jgp.57.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K., CERF J. A., NISHI S. Effect of quaternary ammonium ions on electrical activity of spinal ganglion cells in frogs. J Neurophysiol. 1959 Mar;22(2):177–194. doi: 10.1152/jn.1959.22.2.177. [DOI] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Electrophysiological properties of the giant neurons of the leech subesophageal ganglion. Brain Res. 1974 Jun 7;72(2):332–336. doi: 10.1016/0006-8993(74)90876-2. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Vogel W. Wirkung von Tetrodotoxin und Tetraäthylammoniumchlorid an der Innenseite der Schnürringsmembran von Xenopus laevis. Pflugers Arch. 1969;313(4):361–380. doi: 10.1007/BF00593959. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Differential action of TEA + on two K + -current componentss of a molluscan neurone. Pflugers Arch. 1972;336(2):87–100. doi: 10.1007/BF00592924. [DOI] [PubMed] [Google Scholar]
- Prichard J. W. Effect of phenobarbital on a leech neuron. Neuropharmacology. 1972 Jul;11(4):585–590. doi: 10.1016/0028-3908(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald F. Ionic differences between somatic and axonal action potentials in snail giant neurones. J Physiol. 1972 Jan;220(2):267–281. doi: 10.1113/jphysiol.1972.sp009706. [DOI] [PMC free article] [PubMed] [Google Scholar]


