Abstract
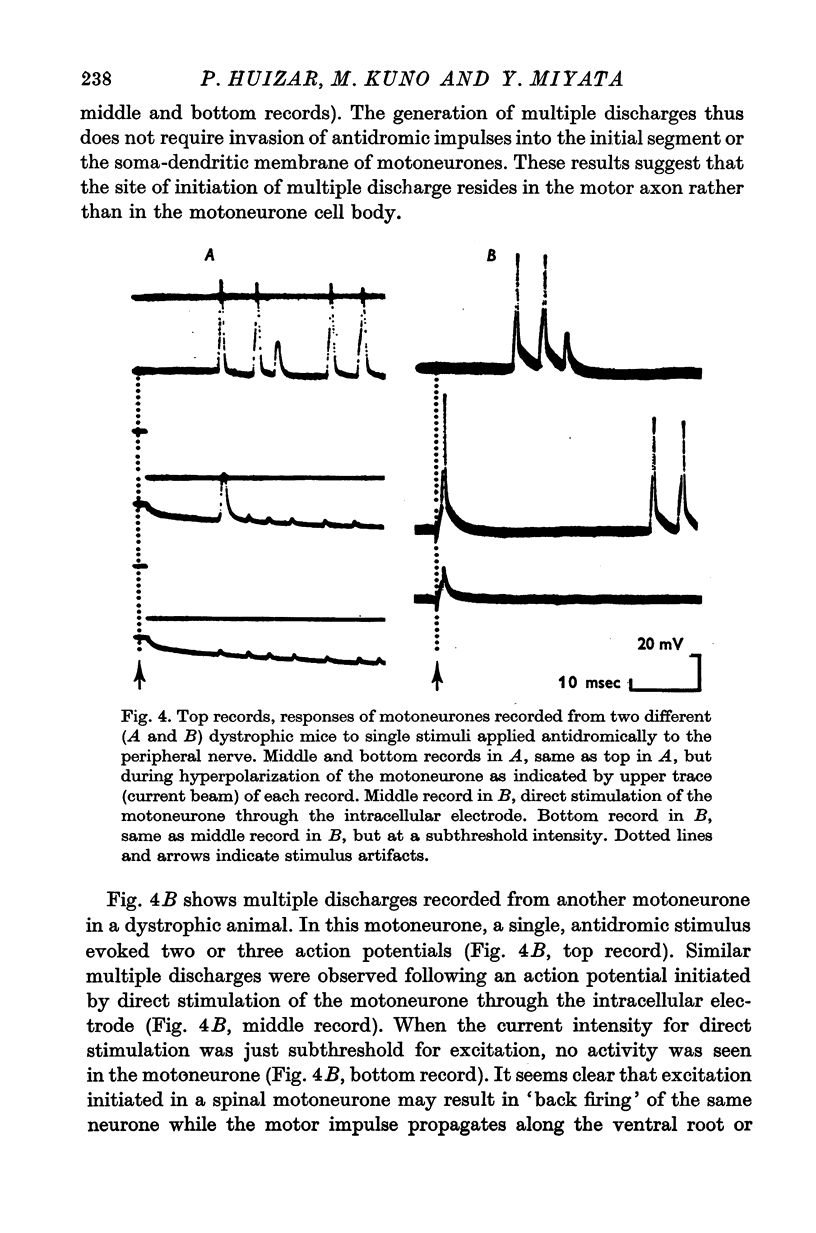
1. The properties of spinal motoneurones of normal and dystrophic mice (129/ReJ) were examined with intracellular electrodes. 2. The following parameters of spinal motoneurones showed no significant differences between normal and dystrophic mice: resting and action potentials, the amplitude and duration of after-hyperpolarization, rheobasic current for excitation, threshold for excitation of the somadendritic membrane (IS-SD inflexion) and input resistance. 3. The changes in motoneurone properties observed 13-16 days after section of the sciatic nerve (axotomy) were similar in both normal and dystrophic mice. 4. The axonal conduction velocity of motoneurones in dystrophic mice was about ten times slower than that in normal mice. The conduction velocity of the sciatic nerve was only about 25% slower in dystrophic mice than in the normal animal. The estimated ventral root conduction velocity as well as the observed dorsal root conduction velocity in dystrophic mice was at least twenty times slower than that in normal mice. 5. In dystrophic mice, spinal motoneurones often showed multiple discharges in response to single, antidromic stimuli. The site of initiation of multiple discharge was located in the motor axon rather than in the motoneurone cell body. 6. In dystrophic mice, nerve impulses were transmitted from fibre to fibre ('cross-talk'). The site of impulse transmission among nerve fibres was near the distal portion of the spinal roots. 7. Synaptic potentials and peripheral reflex discharges evoked by stimulation of the dorsal roots showed a longer latency and were more prolonged in dystrophic mice than in the control mice. 8. The motoneurone properties of dystrophic mice showed no tendency of progressive changes with age ranging from 63 to 148 days. 9. It is concluded that the properties of motoneurone cell bodies examined in dystrophic mice are indistinguishable from those in normal mice and that the only abnormality in motoneurones of the former residues in the motor axon. 10. It is suggested that integrity of the discharge pattern of spinal motoneurones in dystrophic mice is interfered by anomalous impluse transmission in the motor axons and that the motoneurones in dystrophic mice are a homogeneous group rather than a mixture of "normal" and "abnormal" neurones.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Caddy K. W., Pallot D. J., Pehrson U. M., Stirling C. A. The neurological lesion in the dystrophic mouse. Brain Res. 1974 Aug 23;76(3):534–536. doi: 10.1016/0006-8993(74)90830-0. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. J Neurol Sci. 1973 Feb;18(2):227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The interpretation of spike potentials of motoneurones. J Physiol. 1957 Dec 3;139(2):198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cosmos E. Muscle-nerve transplants. Experimental models to study influences on differentiation. Physiologist. 1973 May;16(2):167–177. [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S. Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol. 1956 Jan;19(1):75–98. doi: 10.1152/jn.1956.19.1.75. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Eccles R. M., Kozak W. Further investigations on the influence of motoneurones on the speed of muscle contraction. J Physiol. 1962 Sep;163(2):324–339. doi: 10.1113/jphysiol.1962.sp006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. J., Steinberg M. C., Banker B. Q. Ultrastructural alterations of the motor end plate in myotonic dystrophy of the mouse (dy2J dy2J). J Neuropathol Exp Neurol. 1973 Jul;32(3):345–364. doi: 10.1097/00005072-197307000-00001. [DOI] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Wallace C., Wing J. Myelinated nerve fibre counts in the nerves of normal and dystrophic mouse muscle. J Neurol Sci. 1972 Mar;15(3):245–249. doi: 10.1016/0022-510x(72)90067-6. [DOI] [PubMed] [Google Scholar]
- Hironaka T., Miyata Y. Muscle transplantation in the aetiological elucidation of murine muscular dystrophy. Nat New Biol. 1973 Aug 15;244(137):221–223. doi: 10.1038/newbio244221a0. [DOI] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird J. L., Timmer R. F. Homotransplantation of dystrophic and normal muscle. Arch Pathol. 1965 Nov;80(5):442–446. [PubMed] [Google Scholar]
- Laird J. L., Timmer R. F. Transplantation of skeletal muscle into a host with muscular dystrophy. Tex Rep Biol Med. 1966 Summer;24(2):169–179. [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARCE G. W., WALTON J. N. A histological study of muscle from the Bar Harbor strain of dystrophic mice. J Pathol Bacteriol. 1963 Jul;86:25–33. doi: 10.1002/path.1700860105. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Effects of temperature on conduction in single vagal and saphenous myelinated nerve fibres of the cat. J Physiol. 1965 Sep;180(1):20–49. [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos T. A., Bradley W. G. Spinal motor neurones in murine muscular dystrophy and spinal muscular atrophy. A quantitative histological study. J Neurol Neurosurg Psychiatry. 1972 Feb;35(1):60–65. doi: 10.1136/jnnp.35.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab A. H. Motor-end-plate changes in mouse muscular dystrophy. Lancet. 1971 Oct 9;2(7728):815–816. doi: 10.1016/s0140-6736(71)92764-4. [DOI] [PubMed] [Google Scholar]
- STANDAERT F. G. POST-TETANIC REPETITIVE ACTIVITY IN THE CAT SOLEUS NERVE. ITS ORIGIN, COURSE, AND MECHANISM OF GENERATION. J Gen Physiol. 1963 Sep;47:53–70. doi: 10.1085/jgp.47.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafsky B. Functional studies of reeenerated muscles from normal and dystrophic mice. Nature. 1971 Jan 22;229(5282):270–272. doi: 10.1038/229270a0. [DOI] [PubMed] [Google Scholar]
- Salafsky B., Stirling C. A. Altered neural protein in murine muscular dystrophy. Nat New Biol. 1973 Nov 28;246(152):126–128. doi: 10.1038/newbio246126a0. [DOI] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEST W. T., MURPHY E. D. Histopathology of hereditary, progressive muscular dystrophy in inbred strain 129 mice. Anat Rec. 1960 Jul;137:279–295. doi: 10.1002/ar.1091370306. [DOI] [PubMed] [Google Scholar]


