Abstract
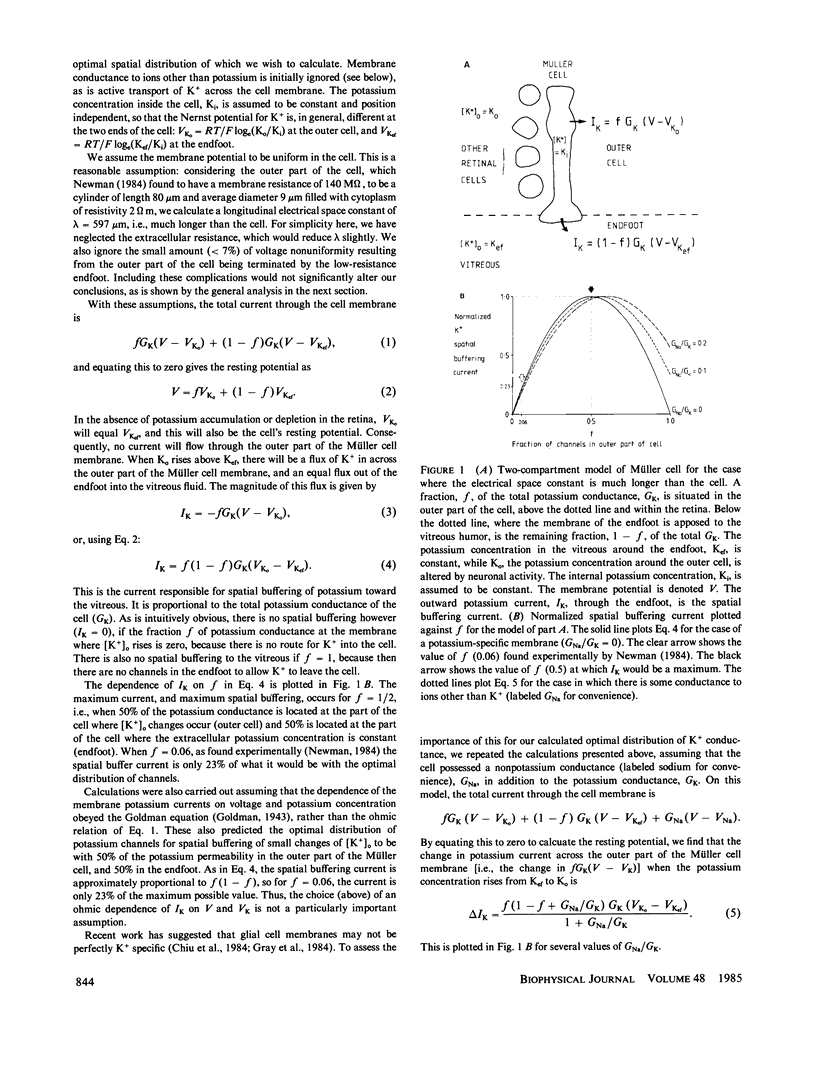
Glial cells in the nervous system are believed to reduce changes of extracellular potassium concentration ([K+]o), caused by neural activity, by carrying out spatial buffering of potassium. In the case of retinal glial cells (Müller cells), light-evoked increases of [K+]o within the retina are reduced by K ions flowing through the Müller cell to the vitreous fluid of the eye. We have calculated the optimal way to distribute the potassium conductance of the Müller cell to maximize spatial buffering to the vitreous fluid. The best distribution is with half the potassium conductance in the outer part of the cell, where K+ enters, and half the conductance in the vitreal endfoot, where K+ leaves the cell. This calculated distribution is very different from the actual distribution measured by Newman (1984, Nature [Lond.], 309: 155-157), where only 6% of the Müller cell conductance is in the outer cell and 94% is in the endfoot. The experimentally observed distribution gives less than a quarter of the spatial buffering that would be produced by the optimal distribution. The possible advantages of this arrangement are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiu S. Y., Schrager P., Ritchie J. M. Neuronal-type Na+ and K+ channels in rabbit cultured Schwann cells. Nature. 1984 Sep 13;311(5982):156–157. doi: 10.1038/311156a0. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Tomita T. Field potential induced by injection of potassium ion into the frog retina: a test of current interpretations of the electroretinographic (ERG) b-wave. Brain Res. 1981 Jan 5;204(1):51–64. doi: 10.1016/0006-8993(81)90651-x. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. Analysis of potassium dynamics in mammalian brain tissue. J Physiol. 1983 Feb;335:393–426. doi: 10.1113/jphysiol.1983.sp014541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. Possible roles of vertebrate neuroglia in potassium dynamics, spreading depression and migraine. J Exp Biol. 1981 Dec;95:111–127. doi: 10.1242/jeb.95.1.111. [DOI] [PubMed] [Google Scholar]
- Gold G. H., Dowling J. E. Photoreceptor coupling in retina of the toad, Bufo marinus. I. Anatomy. J Neurophysiol. 1979 Jan;42(1 Pt 1):292–310. doi: 10.1152/jn.1979.42.1.292. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. T., Bevan S., Ritchie J. M. High conductance anion-selective channels in rat cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):395–409. doi: 10.1098/rspb.1984.0041. [DOI] [PubMed] [Google Scholar]
- Kline R. P., Ripps H., Dowling J. E. Generation of b-wave currents in the skate retina. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5727–5731. doi: 10.1073/pnas.75.11.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Miller R. F., Dowling J. E. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970 May;33(3):323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Current source-density analysis of the b-wave of frog retina. J Neurophysiol. 1980 May;43(5):1355–1366. doi: 10.1152/jn.1980.43.5.1355. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Regional specialization of retinal glial cell membrane. Nature. 1984 May 10;309(5964):155–157. doi: 10.1038/309155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Flaming D. G., Brown K. T. Effects of the rod receptor potential upon retinal extracellular potassium concentration. J Gen Physiol. 1979 Dec;74(6):713–737. doi: 10.1085/jgp.74.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Oakley B., 2nd, Niemeyer G. Light-evoked changes in [K+]0 in retina of intact cat eye. J Neurophysiol. 1980 Nov;44(5):897–921. doi: 10.1152/jn.1980.44.5.897. [DOI] [PubMed] [Google Scholar]
- Trachtenberg M. C., Pollen D. A. Neuroglia: biophysical properties and physiologic function. Science. 1970 Feb 27;167(3922):1248–1252. doi: 10.1126/science.167.3922.1248. [DOI] [PubMed] [Google Scholar]


