Abstract
Micropipette measurements of isotropic tension vs. area expansion in pre-swollen single human red cells gave a value of 288 +/- 50 SD dyn/cm for the elastic, area compressibility modulus of the total membrane at 25 degrees C. This elastic constant, characterizing the resistance to area expansion or compression, is about 4 X 10(4) times greater than the elastic modulus for shear rigidity; therefore, in situations where deformation of the membrane does not require large isotropic tensions (e.g., in passage through normal capillaries), the membrane can be treated by a simple constitutive relation for a two-dimensionally, incompressible material (i.e. fixed area). The tension was found to be linear and reversible for the range of area changes observed (within the experimental system resolution of 10%). The maximum fractional area expansion required to produce lysis was uniformly distributed between 2 and 4% with 3% average and 0.7% SD. By heating the cells to 50 degrees C, it appears that the structural matrix (responsible for the shear rigidity and most of the strength in isotropic tension) is disrupted and primarily the lipid bilayer resists lysis. Therefore, the relative contributions of the structural matrix and lipid bilayer to the elastic, area compressibility could be estimated. The maximum isotropic tension at 25 degrees C is 10-12 dyn/cm and at 50 degrees C is between 3 and 4 dyn/cm. From this data, the respective compressibilities are estimated at 193 dyn/cm and 95 dyn/cm for structural network and bilayer. The latter value correlates well with data on in vitro, monolayer surface pressure versus area curves at oil-water interfaces.
Full text
PDF

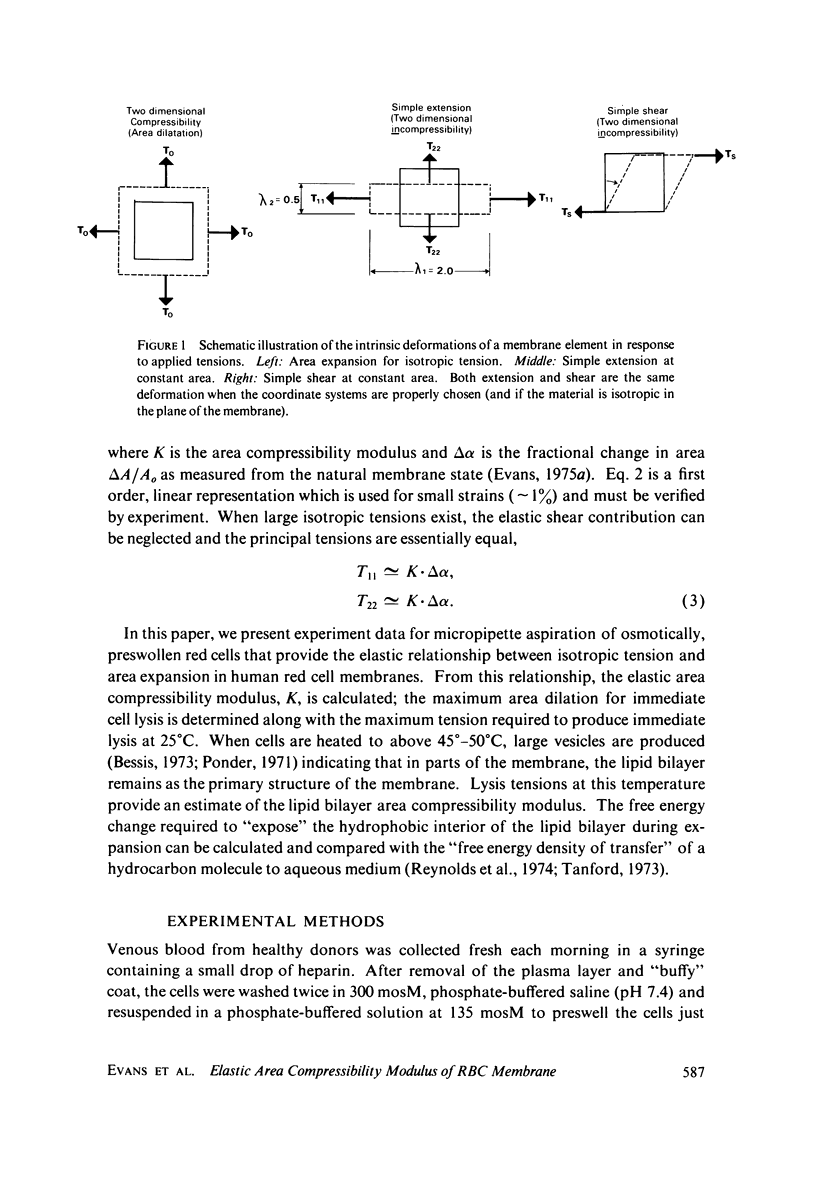








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans E. A. A new material concept for the red cell membrane. Biophys J. 1973 Sep;13(9):926–940. doi: 10.1016/S0006-3495(73)86035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoelasticity. Biophys J. 1976 Jan;16(1):1–11. doi: 10.1016/S0006-3495(76)85658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoplastic flow. Biophys J. 1976 Jan;16(1):13–26. doi: 10.1016/S0006-3495(76)85659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., La Celle P. L. Intrinsic material properties of the erythrocyte membrane indicated by mechanical analysis of deformation. Blood. 1975 Jan;45(1):29–43. [PubMed] [Google Scholar]
- Evans E. A., Leblond P. F. Geometric properties of individual red blood cell discocyte-spherocyte transformations. Biorheology. 1973 Sep;10(3):393–404. doi: 10.3233/bir-1973-10313. [DOI] [PubMed] [Google Scholar]
- Evans E. A. New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophys J. 1973 Sep;13(9):941–954. doi: 10.1016/S0006-3495(73)86036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Fung Y. C. Improved measurements of the erythrocyte geometry. Microvasc Res. 1972 Oct;4(4):335–347. doi: 10.1016/0026-2862(72)90069-6. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Mohandas N. Uniaxial loading of the red-cell membrane. J Biomech. 1972 Sep;5(5):501–509. doi: 10.1016/0021-9290(72)90007-3. [DOI] [PubMed] [Google Scholar]
- Marchesi S. L., Steers E., Marchesi V. T., Tillack T. W. Physical and chemical properties of a protein isolated from red cell membranes. Biochemistry. 1970 Jan 6;9(1):50–57. doi: 10.1021/bi00803a007. [DOI] [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. Area and volume changes in hemolysis of single erythrocytes. J Cell Comp Physiol. 1963 Jun;61:245–253. doi: 10.1002/jcp.1030610306. [DOI] [PubMed] [Google Scholar]
- RAND R. P. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. II. VISCOELASTIC BREAKDOWN OF THE MEMBRANE. Biophys J. 1964 Jul;4:303–316. doi: 10.1016/s0006-3495(64)86784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Gilbert D. B., Tanford C. Empirical correlation between hydrophobic free energy and aqueous cavity surface area. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2925–2927. doi: 10.1073/pnas.71.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalak R., Tozeren A., Zarda R. P., Chien S. Strain energy function of red blood cell membranes. Biophys J. 1973 Mar;13(3):245–264. doi: 10.1016/S0006-3495(73)85983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]



