Abstract
1. Single barnacle muscle fibres from Megabalanus psittacus (Darwin) were internally perfused and the effects of various internal and external solutions on voltage clamp currents were examined.
2. The usual internal solution was 180 mM-K+ aspartate (osmotic pressure adjusted to 1000 m-osmole by adding sucrose). Fibres perfused with this solution gave an average resting potential of -55 ± 5 mV (all potentials are referred to the external solutions as ground). Further increase in internal K concentration depolarized the fibres.
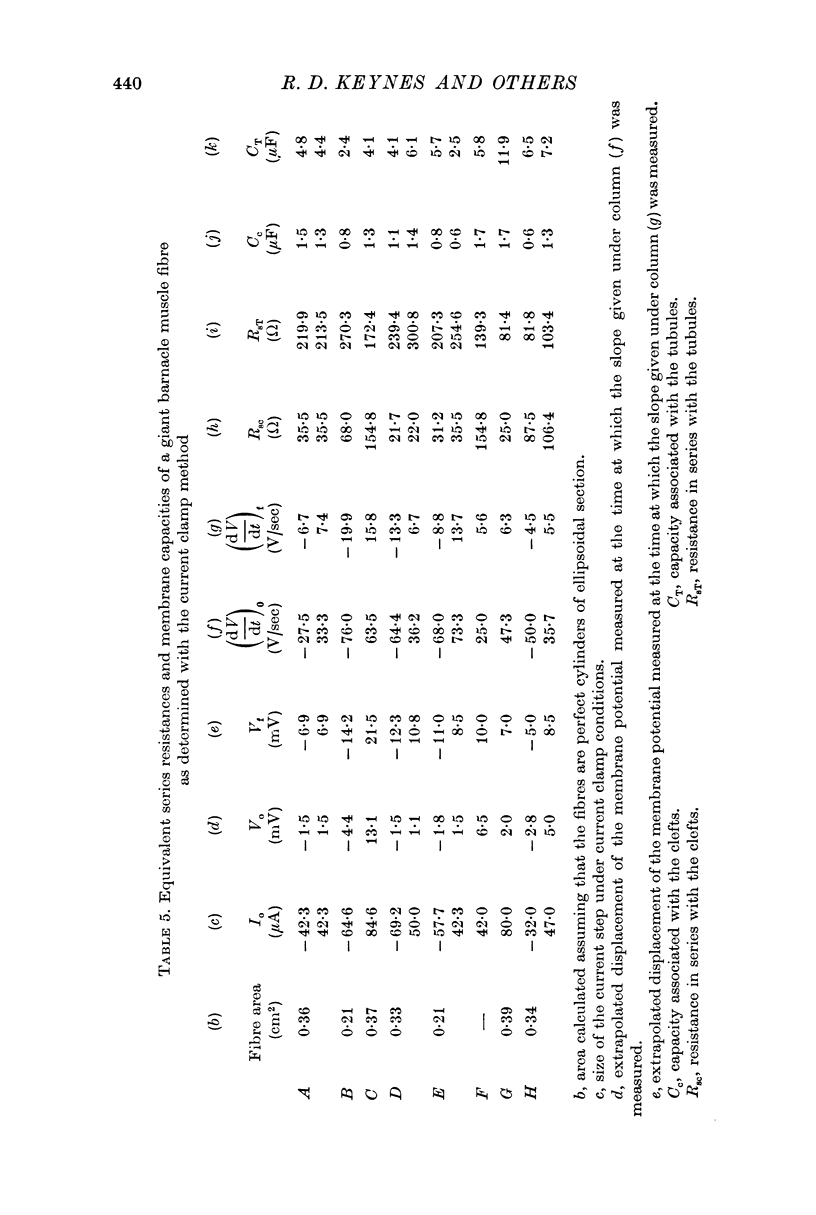
3. With membrane current control the total capacitance, referred to apparent membrane surface area, was 21·2 ± 2·4 μF/cm2.
4. Under normal conditions, with demonstrably good longitudinal space clamp control, voltage clamp currents associated with certain depolarizing pulses showed oscillations. These oscillations were reduced in frequency and magnitude by lowering the temperature from 20 to 10° C, by eliminating the inward currents with external Ca-free saline or by reducing the outward currents with internal tetraethylammonium (TEA) or replacement of internal K by Cs.
5. With a Na- and Ca-free, 60 mM-MgCl2 solution outside depolarizing voltage clamp pulses produced only outward currents. On repolarization the current tail reversed direction at about -70 mV for pulses of less than 50 msec duration. For longer pulses this reversal potential was less negative, suggesting an accumulation of external, or depletion of internal K.
6. Both the size of the outward currents and the rate at which they reached their maximum value increased with temperature. The activation energy for the rate constant was about 63 kJ/mole.
7. Fibres bathed in Na- and Mg-free, 60 mM-CaCl2 saline were excitable. After replacement of the internal K+ with Cs+ or adding 60 mM-TEA to the internal solution only sustained inward currents were recorded with depolarization.
8. Sustained inward currents could be reduced by external application of 5 mM-LaCl3. Tetrodotoxin was not effective even at a concentration of 1000 nM.
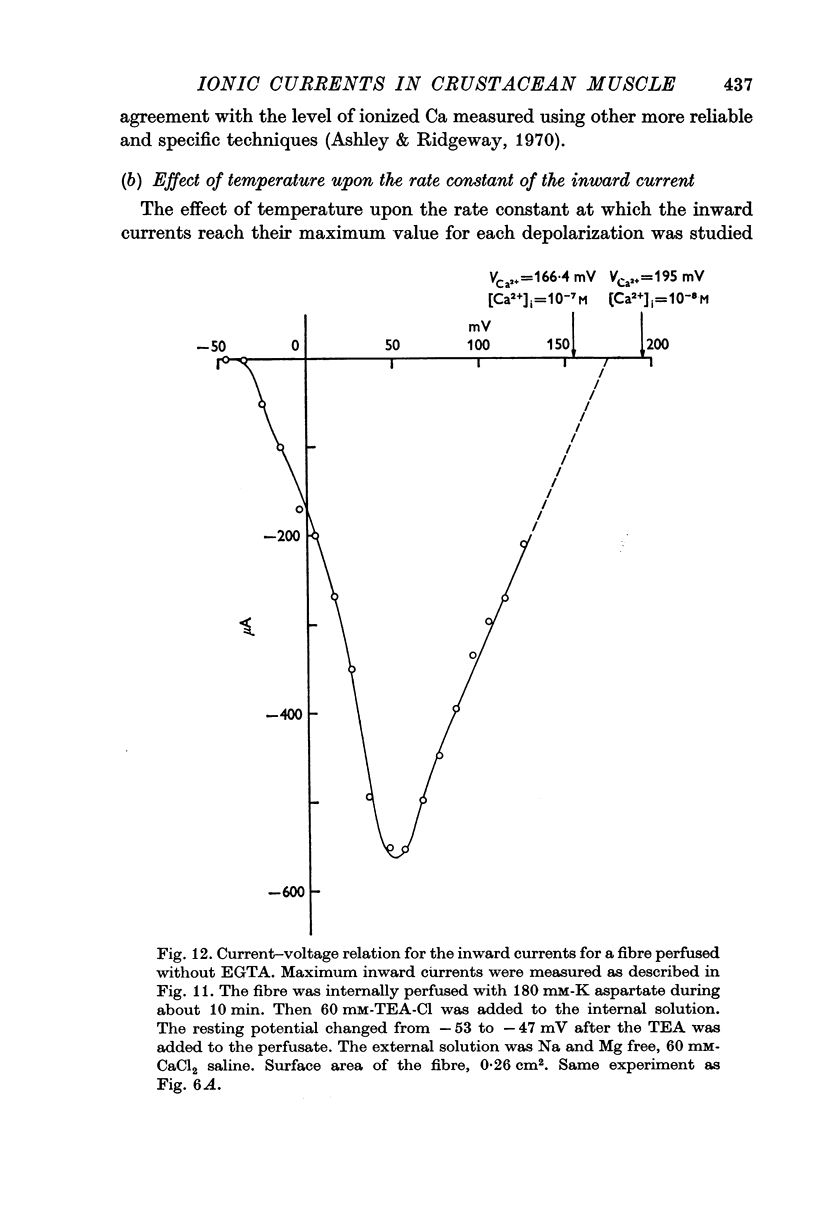
9. The rate at which these inward currents reached a maximum value increased with increase in temperature of the bathing solution with an activation energy of the order of 42 kJ/mole.
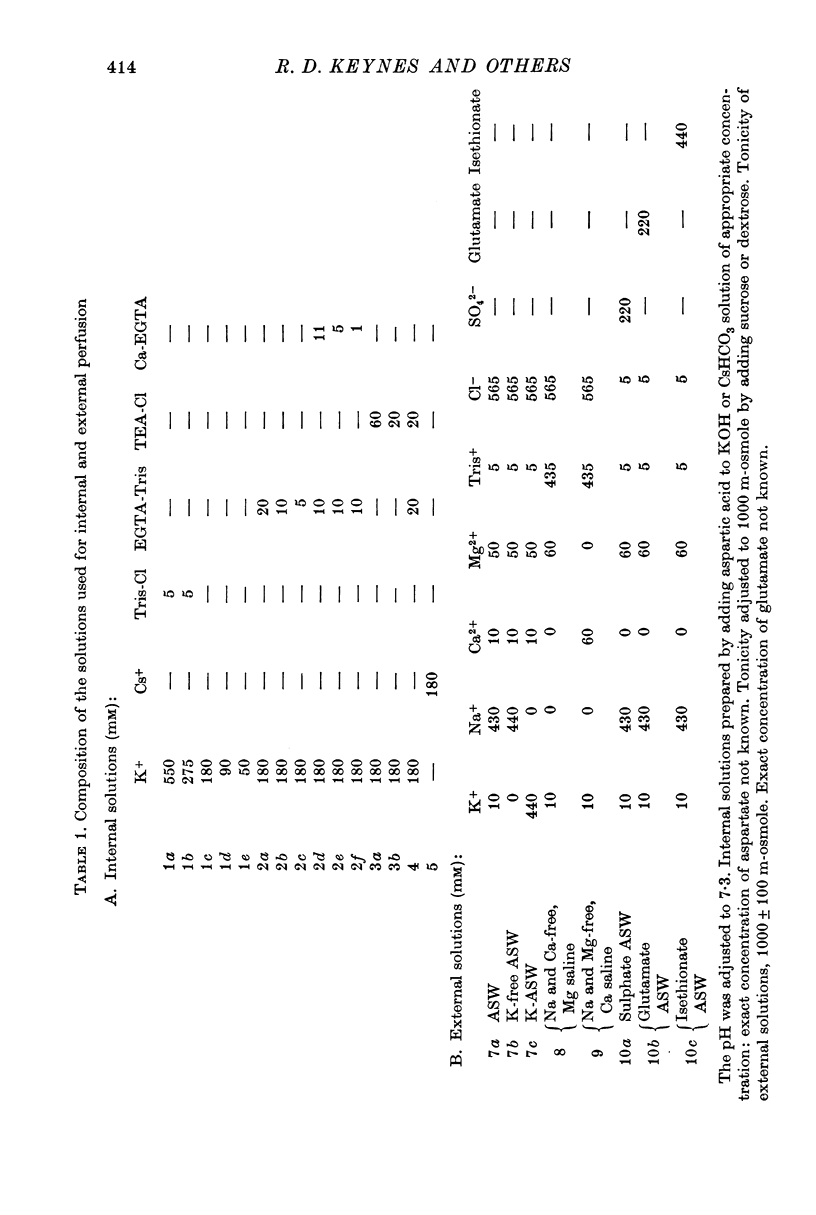
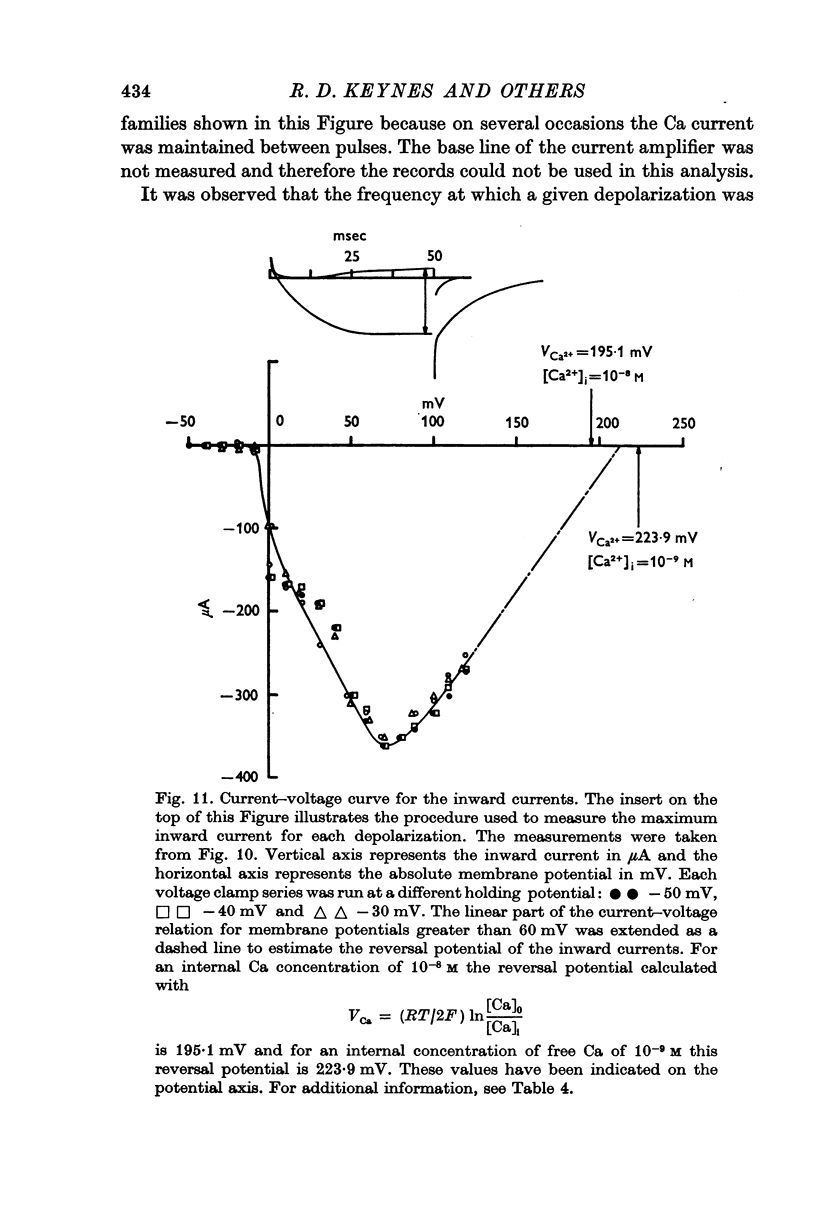
10. The reversal potential of the inward currents changed with the level of internal Ca ions. For a fibre perfused without ethyleneglycol-bis (β-aminoethyl ether) N,N′-tetraacetic acid (EGTA) this reversal potential was 175 mV (internal free Ca 5 × 10-7 M), and was 196 mV for a fibre perfused with 20 mM-Tris EGTA (internal free Ca 0·26 × 10-8 M).
11. We propose an electrical equivalent circuit to account for most of the observed electrical properties of barnacle muscle fibres. In this model the Ca and the K system are located at different anatomical places and they interact through a series resistance.
Full text
PDF










































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Potassium chloride movement and the membrane potential of frog muscle. J Physiol. 1960 Apr;151:154–185. [PMC free article] [PubMed] [Google Scholar]
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATWOOD H. L. DIFFERENCES IN MUSCLE FIBRE PROPERTIES AS A FACTOR IN "FAST" AND "SLOW" CONTRACTION IN CARCINUS. Comp Biochem Physiol. 1963 Sep;10:17–32. doi: 10.1016/0010-406x(63)90099-9. [DOI] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Senft J. P. Voltage clamp studies on the effect of internal cesium ion on sodium and potassium currents in the squid giant axon. J Gen Physiol. 1966 Nov;50(2):279–293. doi: 10.1085/jgp.50.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Costantin L. L., Peachey L. D. Radial spread of contraction in frog muscle fibres. J Physiol. 1969 Sep;204(1):231–257. doi: 10.1113/jphysiol.1969.sp008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Bezanilla F., Rojas E. Sodium influxes in internally perfused squid giant axon during voltage clamp. J Physiol. 1969 May;201(3):657–664. doi: 10.1113/jphysiol.1969.sp008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Phasic entry of calcium in response to depolarization of giant axons of Loligo forbesi. J Physiol. 1971 Jul;216(2):70P–71P. [PubMed] [Google Scholar]
- Bezanilla F., Caputo C., Gonzalez-Serratos H., Venosa R. A. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. J Physiol. 1972 Jun;223(2):507–523. doi: 10.1113/jphysiol.1972.sp009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A. J., Woodbury J. W. The sodium-potassium hypothesis as the basis of electrical activity in frog ventricle. J Physiol. 1960 Dec;154(2):385–407. doi: 10.1113/jphysiol.1960.sp006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Sodium and potassium fluxes in isolated barnacle muscle fibers. J Gen Physiol. 1968 Apr;51(4):445–477. doi: 10.1085/jgp.51.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORABOEUF E., OTSUKA M. L'action des solutions hyposodiques sur les potentiels cellulaires de tissu cardiaque de mammifères. C R Hebd Seances Acad Sci. 1956 Jul 23;243(4):441–444. [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R., Latorre R. Effect of temperature on membrane potential and ionic fluxes in intact and dialysed barnacle muscle fibres. J Physiol. 1972 Sep;225(2):255–273. doi: 10.1113/jphysiol.1972.sp009939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., SASAOKA T., HOSOYA Y. Effects of tetrodotoxin on the neuromuscular junction. Jpn J Physiol. 1959 Jun 25;9(2):143–152. doi: 10.2170/jjphysiol.9.143. [DOI] [PubMed] [Google Scholar]
- Falk G. Predicted delays in the activation of the contractile system. Biophys J. 1968 May;8(5):608–625. doi: 10.1016/S0006-3495(68)86511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials, afterpotentials, and excitation-contraction coupling in frog sartorius fibers without transverse tubules. J Gen Physiol. 1969 Mar;53(3):298–310. doi: 10.1085/jgp.53.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Gruener R. Voltage clamp of the Aplysia giant neurone: early sodium and calcium currents. J Physiol. 1970 Nov;211(1):217–244. doi: 10.1113/jphysiol.1970.sp009276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Hayashi H., Takahashi K. Calcium and potassium currents of the membrane of a barnacle muscle fibre in relation to the calcium spike. J Physiol. 1969 Nov;205(1):115–129. doi: 10.1113/jphysiol.1969.sp008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Tetrodotoxin and manganese ion: effects on action potential of the frog heart. Science. 1965 Sep 10;149(3689):1254–1255. doi: 10.1126/science.149.3689.1254. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K., Junge D. Excitation-contraction coupling in a barnacle muscle fiber as examined with voltage clamp technique. J Gen Physiol. 1968 Feb;51(2):157–175. doi: 10.1085/jgp.51.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Toyama K., Hayashi H. Mechanisms of anion and cation permeations in the resting membrane of a barnacle muscle fiber. J Gen Physiol. 1971 Apr;57(4):408–434. doi: 10.1085/jgp.57.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke J. A. Solvent water for electrolytes in the muscle fiber of the giant barnacle. J Gen Physiol. 1970 Oct;56(4):521–541. doi: 10.1085/jgp.56.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G. How is muscle turned on and off? Sci Am. 1970 Apr;222(4):84–93. doi: 10.1038/scientificamerican0470-84. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Hinke J. A. Sodium and water binding in single striated muscle fibers of the giant barnacle. Can J Physiol Pharmacol. 1966 Sep;44(5):837–848. doi: 10.1139/y66-102. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORKAND R. K., NIEDERGERKE R. HEART ACTION POTENTIAL: DEPENDENCE ON EXTERNAL CALCIUM AND SODIUM IONS. Science. 1964 Nov 27;146(3648):1176–1177. doi: 10.1126/science.146.3648.1176. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Page E., Mobley B. A., Johnson M., Upshaw J. E. Magnesium in single skeletal muscle cells of Balanus. J Gen Physiol. 1971 Feb;57(2):188–201. doi: 10.1085/jgp.57.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E., Atwater I., Bezanilla F. Analysis of the effects of calcium or magnesium on voltage-clamp currents in perfused squid axons bathed in solutions of high potassium. J Gen Physiol. 1969 Oct;54(4):532–552. doi: 10.1085/jgp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E. Calcium influxes in perfused squid giant axons during voltage clamp. J Physiol. 1970 Sep;210(2):135P–135P. [PubMed] [Google Scholar]
- STRICKHOLM A. Membrane current of crab muscle. Nature. 1963 Apr 27;198:393–394. doi: 10.1038/198393a0. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


