Abstract
1. The average rate constant for loss of 45Ca from an unpoisoned squid axon was 1·8 × 10-3 min-1, corresponding to an efflux of 0·2 p-mole/cm2 sec.
2. The Ca efflux from unpoisoned axons was reduced if external calcium was replaced with magnesium, or external sodium with lithium, choline or dextrose. Replacing both sodium and calcium reduced the efflux to about 40%.
3. Cyanide caused little immediate change in Ca efflux but after 1½-2½ hr the efflux increased to 5-15 times its normal value. The effect was rapidly reversed when cyanide was removed.
4. The large Ca efflux into cyanide was reduced by a factor of three when external calcium was replaced with magnesium and by a further factor of about six when external sodium was replaced with lithium.
5. The Ca efflux from both poisoned and unpoisoned axons had a Q10 of 2-3, was not affected by ouabain and was greatly reduced by injecting ethyleneglycol bis (aminoethylether)-N,N′-tetra-acetic acid (EGTA).
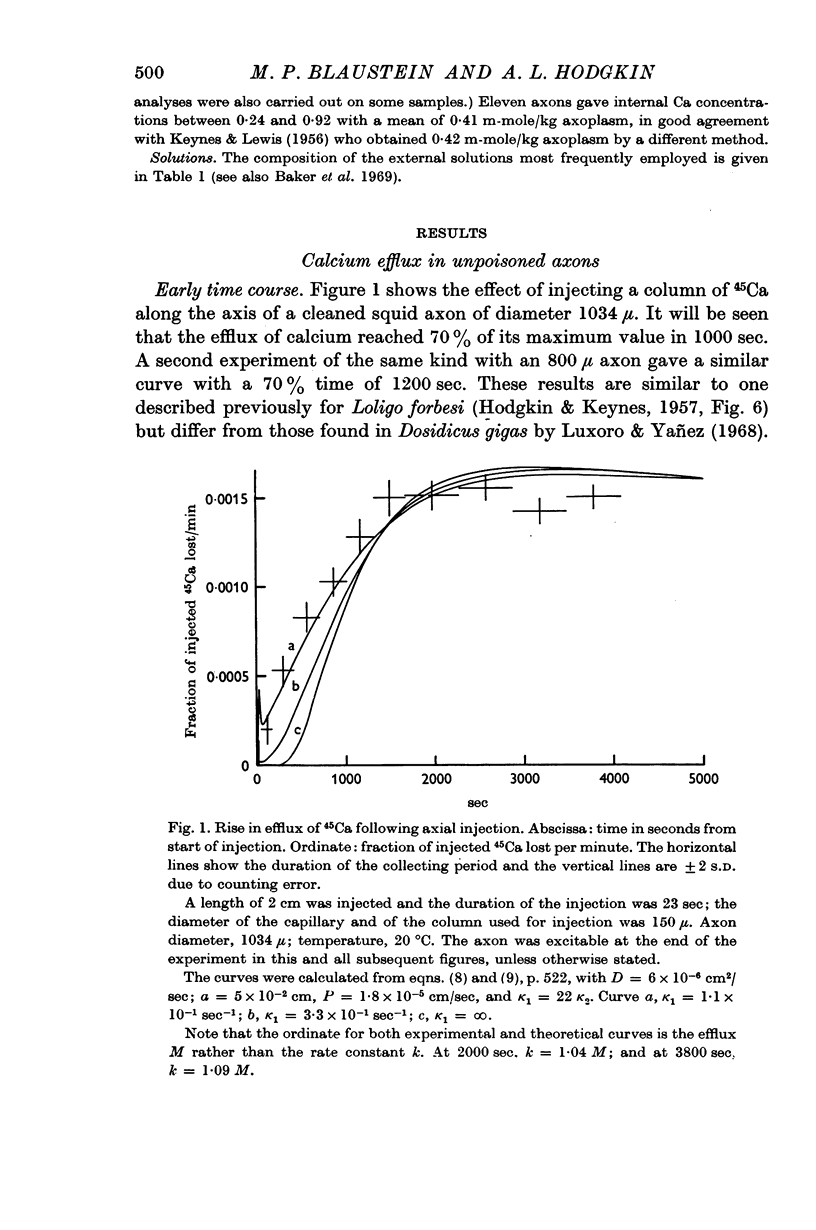
6. After injecting 45Ca along the axis, the efflux of calcium reached its maximum much more rapidly in a cyanide-treated axon than in an unpoisoned axon.
7. Pre-treatment with cyanide greatly increased the rate at which calcium was lost from axoplasm extruded into flattened dialysis bags. A similar effect was observed when cyanide was applied after extrusion.
8. Replacing external sodium glutamate with potassium glutamate greatly reduced the loss of 45Ca from intact axons poisoned with cyanide but had little effect on the loss from extruded axoplasm.
9. The rate constant for loss of the Ca EGTA complex was about 3 × 10-5 min-1 for intact axons and 2 × 10-2 min-1 for extruded axoplasm.
10. A possible explanation of the cyanide effect is that, after poisoning, calcium ions are released from a store and can then exchange at a higher rate with external sodium or calcium.
11. The experiments suggest that part of the calcium efflux may be coupled to sodium entry.
12. Theoretical equations for `diffusion and chemical reaction in a cylinder' are described in the Appendix.
Full text
PDF





























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Shaw T. I. A comparison of the phosphorus metabolism of intact squid nerve with that of the isolated axoplasm and sheath. J Physiol. 1965 Sep;180(2):424–438. doi: 10.1113/jphysiol.1965.sp007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The phosphorus metabolism of squid axons and its relationship to the active transport of sodium. J Physiol. 1960 Jul;152:545–560. doi: 10.1113/jphysiol.1960.sp006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. In vivo effect of uncoupling agents on the incorporation of calcium and strontium into mitochondria and other subcellular fractions of rat liver. J Gen Physiol. 1967 Aug;50(7):1849–1864. doi: 10.1085/jgp.50.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The intracellular calcium contents of some invertebrate nerves. J Physiol. 1956 Nov 28;134(2):399–407. doi: 10.1113/jphysiol.1956.sp005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxoro M., Yañez E. Permeability of the Giant Axon of Dosidicus gigas to Calcium Ions. J Gen Physiol. 1968 May 1;51(5):115–122. [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Some factors influencing sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2333–2355. doi: 10.1085/jgp.50.10.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Wins P., Schoffeniels E. ATP+ca++-linked contraction of red cell ghosts. Arch Int Physiol Biochim. 1966 Nov;74(5):812–820. doi: 10.3109/13813456609059954. [DOI] [PubMed] [Google Scholar]


