Abstract
1. The membrane potential of isolated muscle fibres in solutions containing tetrodotoxin (TTX) was controlled with a two-electrode voltage clamp. The striation pattern in the region of the electrodes was observed microscopically.
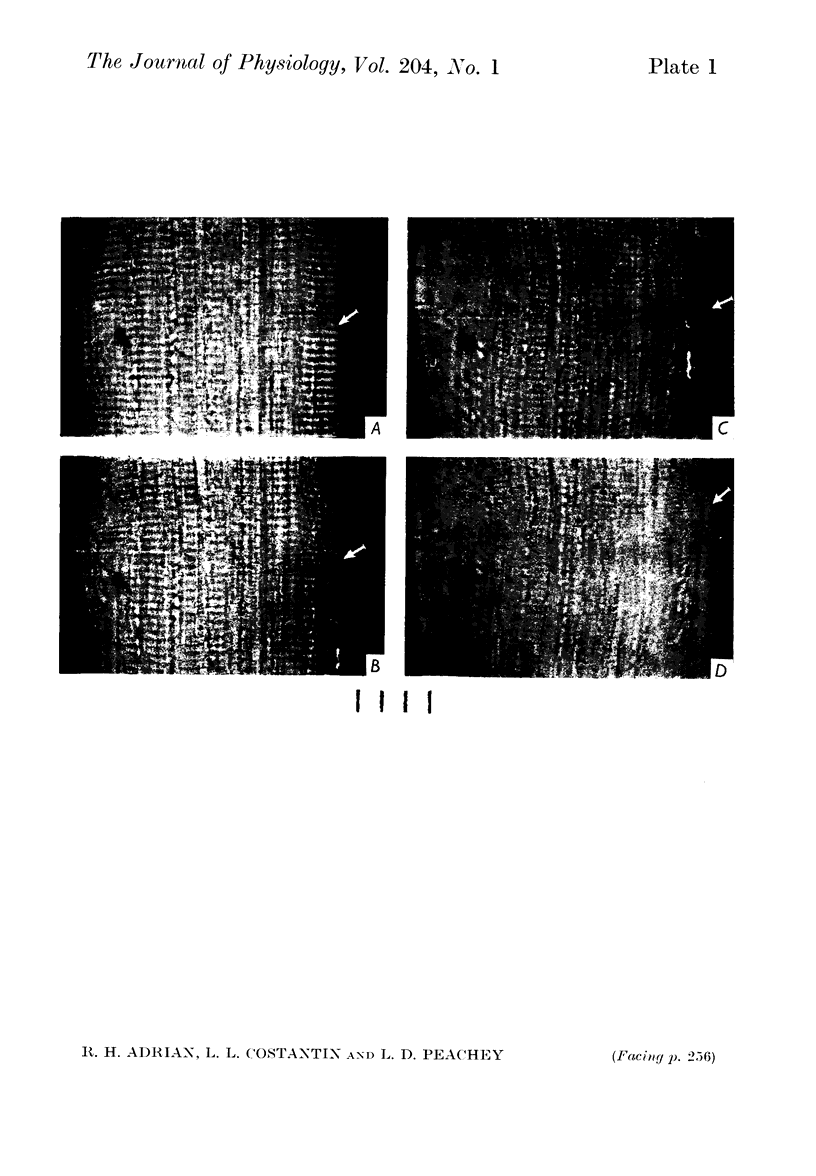
2. With square steps of depolarization of increasing magnitude, contraction occurs first in the myofibrils just beneath the surface membrane, and then spreads inwards towards the axis of the fibre as the depolarization is increased.
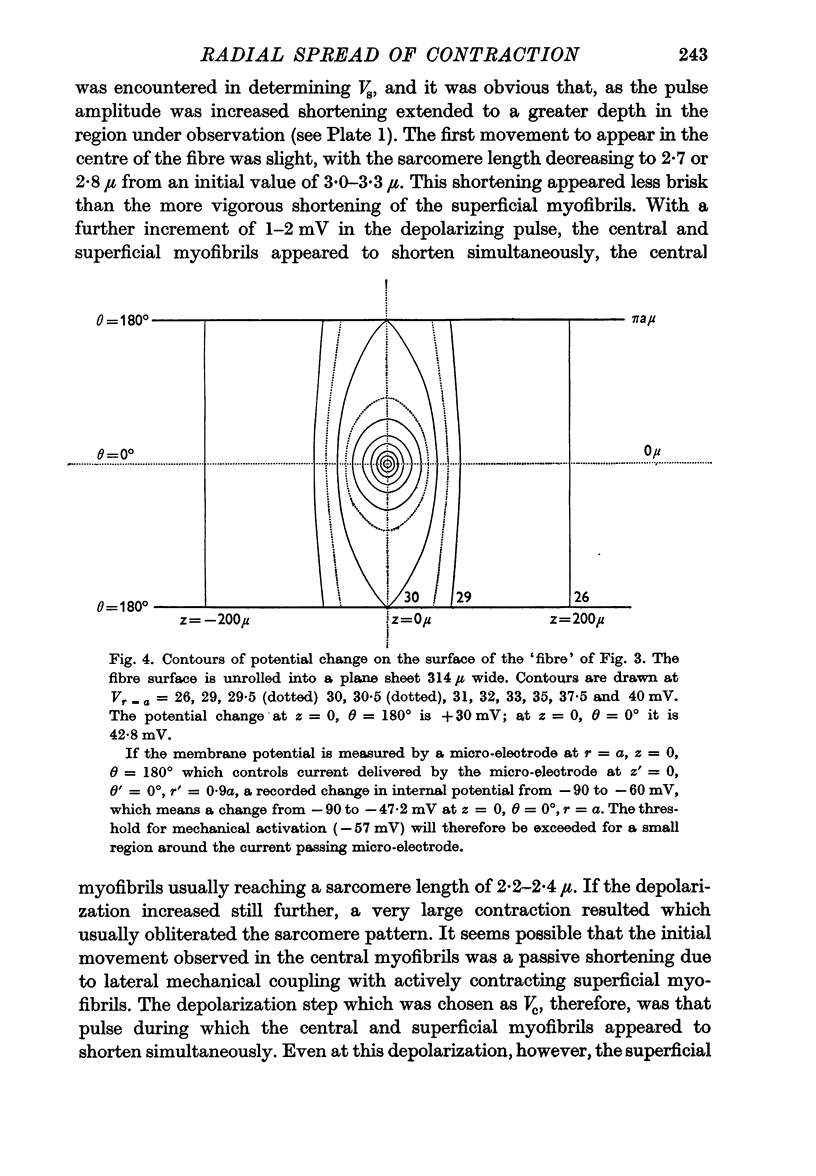
3. From the depolarizations which make the superficial and axial myofibrils contract it is possible to estimate a space constant (λT) for electrotonic spread in a transverse tubular network.
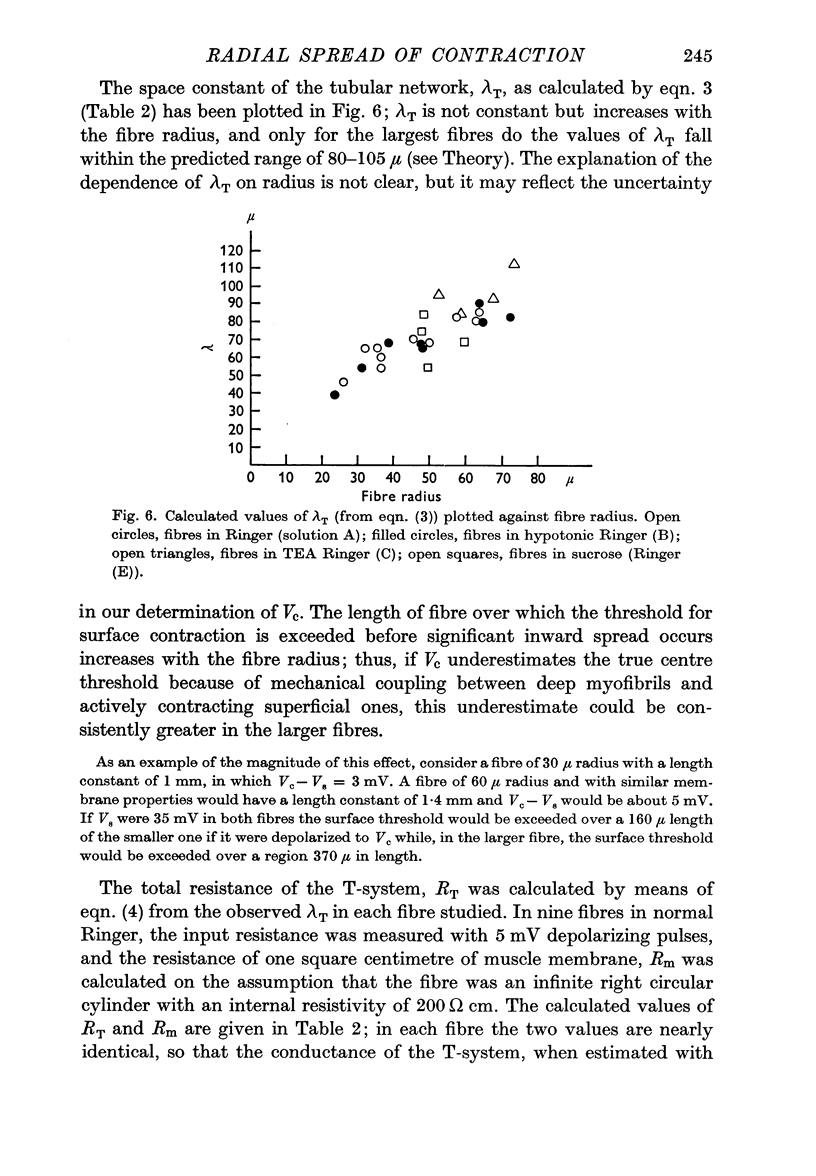
4. λT was found to vary with fibre radius; for a 50 μ fibre it was about 60 μ. λT was not greatly affected by tetraethylammonium (TEA) chloride (111 mM), or by sucrose substitution of most of the sodium chloride in the Ringer solution.
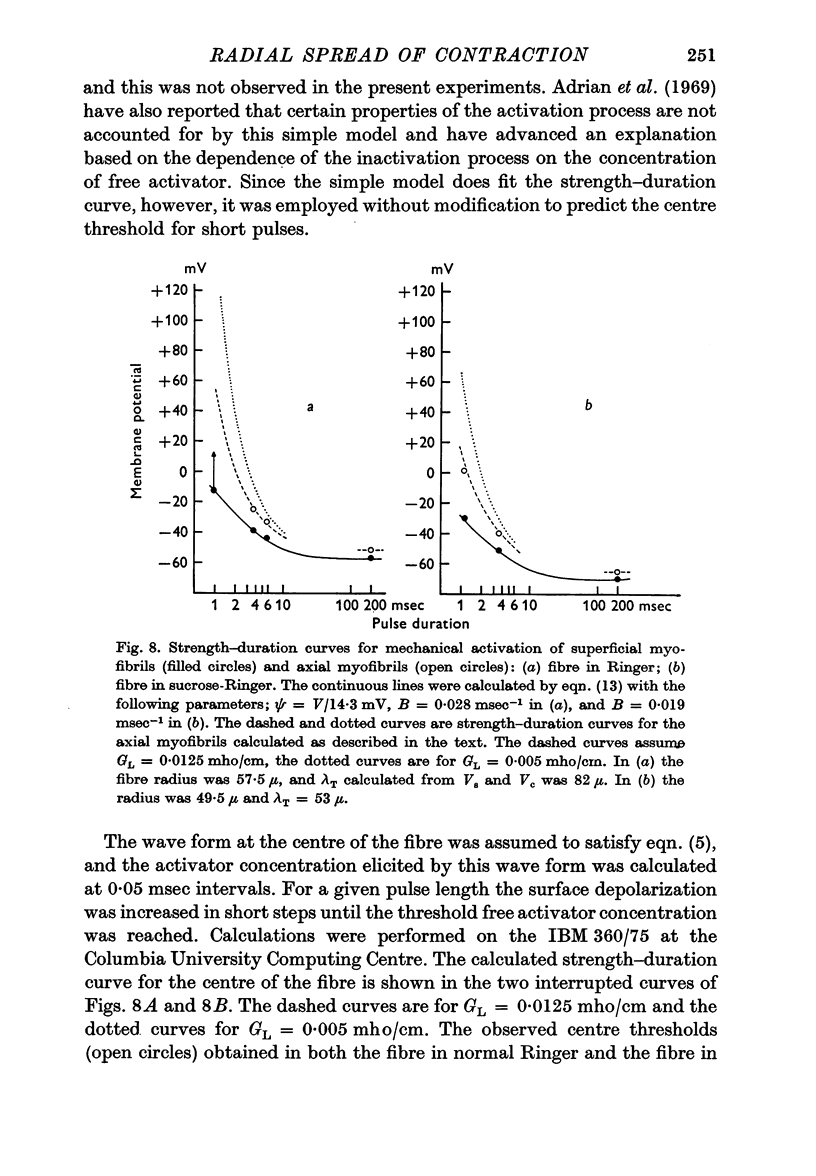
5. The ratio of the depolarization threshold for contraction of surface myofibrils and of central myofibrils was smaller for short (3 msec) than for long depolarization.
6. Action potentials, recorded from a sartorius fibre, were used as the command signal for the voltage-clamped fibre in tetrodotoxin. The central myofibrils of this fibre did not appear to contract unless the imposed `action potentials' were of normal size.
7. The passive electrical characteristics of the transverse tubular system will just allow an action potential, at room temperature, to activate the myofibrils at the centre of a frog muscle fibre. An active potential change would be required to achieve a safety factor appreciably greater than one for this process.
Full text
PDF
























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J. Depolarization of the internal membrane system in the activation of frog skeletal muscle. J Gen Physiol. 1967 May;50(5):1101–1124. doi: 10.1085/jgp.50.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J Physiol. 1968 Mar;195(1):119–132. doi: 10.1113/jphysiol.1968.sp008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Entry of fluorescent dyes into the sarcotubular system of the frog muscle. J Physiol. 1966 Jul;185(1):224–238. doi: 10.1113/jphysiol.1966.sp007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FATT P. AN ANALYSIS OF THE TRANSVERSE ELECTRICAL IMPEDANCE OF STRIATED MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:606–651. doi: 10.1098/rspb.1964.0023. [DOI] [PubMed] [Google Scholar]
- Falk G. Predicted delays in the activation of the contractile system. Biophys J. 1968 May;8(5):608–625. doi: 10.1016/S0006-3495(68)86511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. EVIDENCE FOR CONTINUITY BETWEEN THE CENTRAL ELEMENTS OF THE TRIADS AND EXTRACELLULAR SPACE IN FROG SARTORIUS MUSCLE. Nature. 1964 Jun 13;202:1067–1071. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some anions on electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968 Sep;198(2):291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. A comparison of the fine structures of frog slow and twitch muscle fibers. J Cell Biol. 1965 Aug;26(2):477–497. doi: 10.1083/jcb.26.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the inwardly rectifying potassium channel of frog sartorius muscle. J Physiol. 1969 Jan;200(1):2P–3P. [PubMed] [Google Scholar]