Abstract
Using the biocide triclosan as a selective agent, several triclosan-resistant mutants of a susceptible Pseudomonas aeruginosa strain were isolated. Cloning and characterization of a DNA fragment conferring triclosan resistance from one of these mutants revealed a hitherto uncharacterized efflux system of the resistance nodulation cell division (RND) family, which was named MexJK and which is encoded by the mexJK operon. Expression of this operon is negatively regulated by the product of mexL, a gene located upstream of and transcribed divergently from mexJK. The triclosan-resistant mutant contained a single nucleotide change in mexL, which caused an amino acid change in the putative helix-turn-helix domain of MexL. The MexL protein belongs to the TetR family of repressor proteins. The MexJK system effluxed tetracycline and erythromycin but only in the presence of the outer membrane protein channel OprM; OprJ and OprN did not function with MexJK. Triclosan efflux required neither of the outer membrane protein channels tested but necessitated the MexJ membrane fusion protein and the MexK inner membrane RND transporter. The results presented in this study suggest that MexJK may function as a two-component RND pump for triclosan efflux but must associate with OprM to form a tripartite antibiotic efflux system. Furthermore, the results confirm that triclosan is an excellent tool for the study of RND multidrug efflux systems and that this popular biocide therefore readily selects mutants which are cross-resistant with antibiotics.
Pseudomonas aeruginosa is intrinsically resistant to many antimicrobial agents, including antibiotics, biocides, and, to some extent, heavy metals. This intrinsic resistance can be attributed to synergy between an outer membrane with low permeability (12) and other contributing mechanisms, most notably drug efflux (32, 57).
Genome analyses revealed that P. aeruginosa encodes as many as twelve possible efflux systems of the resistance nodulation cell division (RND) family alone (50). Of these, only four, MexAB-OprM (34), MexCD-OprJ (33), MexEF-OprN (21), and MexXY (1, 52), have been characterized. It is now generally assumed that these efflux systems function as tripartite systems; i.e., they require an inner membrane RND family transporter (MexB, MexD, MexF, or MexY), a periplasmic membrane fusion protein (MFP) (MexA, MexC, MexE, or MexX), and an outer membrane translocase channel (OprM, OprJ, or OprN). The MexXY system, whose operon does not encode an outer membrane protein (Opr) component, requires OprM for function (25). In contrast to OprJ and OprN, whose expression is tightly regulated along with that of their associated Mex proteins, OprM is always expressed at low levels, and there is evidence that its structural gene may be transcribed from a promoter independent of mexAB-oprM transcription (58). Unlike MexAB-OprM, which is always expressed at low but detectable levels, the expression of the other hitherto-characterized efflux systems is tightly regulated. They are expressed only in mutants obtained by antibiotic exposure in vitro or in vivo (19, 21, 33, 38, 48, 59). Many of these mutants contain mutations in adjacent regulatory genes, e.g., mexR, nfxB, or mexZ. In contrast, the mexEF-oprN operon is positively regulated by the mexT product, encoding a transcriptional activator of the LysR family (21), although the involvement of a putative negative regulator, MexS, has also been proposed (23). Since many patient isolates of animal and human origin constitutively express these efflux systems without displaying mutations in known regulators or regulatory regions, other hitherto-unidentified regulatory mechanisms governing efflux operon expression must exist (3, 36, 59). It has recently been shown that expression of at least one RND efflux system, PA4206-PA4207-PA4208 or qsc, is regulated by quorum sensing (53). Expression of the Czr system, a divalent cation efflux pump, is regulated by a two-component sensor kinase regulatory system (13). Furthermore, a ribosomal mutation has been implicated in MexXY expression in a tobramycin-resistant clinical P. aeruginosa isolate (52).
Although antibiotics have clearly been documented in laboratory and clinical settings as selective agents leading to upregulated efflux operon expression, our recent findings showed that triclosan, a broad-spectrum biocide, can do the same (5, 43). Prior to identification of enoyl (acyl carrier protein) reductase (FabI) as the intracellular triclosan target (16, 18, 27), it was thought that its antibacterial action resulted from nonspecific disruption of cellular membranes. Triclosan is now used in a wide range of consumer products, including cosmetics, toothpastes, lotions, soaps, cutting boards, mattress pads, and many other products (4). Other antibacterial compounds with structures similar to triclosan include hexachlorophene and dichlorophene. Both compounds contain the hydroxyphenyl moiety that is essential for the activity of triclosan and related hydroxydiphenylethers (15, 16). Hexachlorophene is used in disinfectants, surgical scrubs (20), and other consumer materials (47). It has a more limited spectrum of biological activity than triclosan, being more active against gram-positive bacteria but less effective against gram-negative organisms (11). Although hexachlorophene inhibits FabI activity, this inhibition is not a component of its biocidal activity (14). Dichlorophene is also used as a broad-spectrum biocide, including as an agricultural fungicide and an antimicrobial in soaps and shampoos. Its cellular target has not yet been defined.
Even though P. aeruginosa expresses a triclosan-sensitive FabI (18), wild-type strains are triclosan resistant (Trir) due to expression of the MexAB-OprM system (42). Deletion of this efflux system generates triclosan-susceptible mutants, and exposure of these to this biocide selected MexCD-OprJ-hyperexpressing mutants due to mutations in its negative regulatory gene, nfxB (5). Since the amphiphilic triclosan seems to be a substrate for most RND efflux systems, we reasoned that it might be a useful tool for the study of efflux systems whose substrate profiles and regulatory mechanisms have not yet been identified. In this report we describe a new efflux system, MexJK, which is constitutively expressed in mexL mutants obtained through exposure of a susceptible mutant strain to triclosan and describe the properties of this efflux pump.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains and plasmids used in this study are shown in Table 1. Luria-Bertani (LB) medium from Gibco (Gaithersburg, Md.) was routinely used as the rich medium for all bacterial strains. The minimal medium used was M9 (28) supplemented with 0.2% Casamino Acids (Difco, Detroit, Mich.). Antibiotics used in selection medium for Escherichia coli were as follows: ampicillin, 100 μg/ml; spectinomycin, 150 μg/ml; and tetracycline, 15 μg/ml. Those used for P. aeruginosa were as follows: carbenicillin, 200 μg/ml; spectinomycin, 400 μg/ml; tetracycline, 25 μg/ml; and triclosan, 25 to 50 μg/ml. Spontaneous Trir mutants were selected by plating dilutions of PAO238 cells on Pseudomonas isolation agar (Difco) (this formulation contains 25 μg of triclosan per ml), as previously described (5). Antibiotics were from commercial sources. Triclosan was a gift from KIC Chemicals (Armonk, N.Y.); hexachlorophene and dichlorophene [2,2′-methylenebis(4-chlorphenol)] were purchased form Sigma (St. Louis, Mo.) and TCI America (Portland, Oreg.), respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or characteristic | Source or reference |
|---|---|---|
| Strains | ||
| PAO1 | Wild type; produces MexAB-OprM | 51 |
| PAO238 | PAO1 derivative with Δ(mexAB-oprM) nfxB Δ(mexCD-oprJ)a | 5 |
| PAO238-1 | Spontaneous Trir derivative of PAO238 | This study |
| PAO298 | PAO238-1 with mexJ::Tn<TET-1> | This study |
| PAO314 | PAO238-1 with ΔmexJKL::FRT | This study |
| PAO315 | PAO314 with chromosomal mexJ′-′lacZ transcriptional fusion integrated at attB | This study |
| PAO318 | PAO238 with ΔmexL::FRT | This study |
| PAO325 | PAO314 with Δ(mexXY::FRT) | This study |
| PAO327 | PAO238-1 with Δ(mexXY::FRT) | This study |
| Plasmids | ||
| pADD948 | Cbr; broad-host-range in vivo cloning vector | 6 |
| pAK1900 | Cbr; broad-host-range cloning vector | 35 |
| pBluescript SK(−) | Apr; cloning vector | Stratagene, La Jolla, Calif. |
| pBSP II SK(−) | Apr; broad-host-range cloning vector | 45 |
| pUC18 | Apr; cloning vector | 54 |
| pPS856 | Cbr Gmr; source of FRT-Gmr cassette | 17 |
| pKMM128 | Cbr; pAK1900 expressing oprM from Placb | 25 |
| pTZ110 | Cbr; broad-host-range lacZ fusion vector | 44 |
| pUCP20T | Cbr; broad-host-range cloning vector | 46 |
| pVLT35 | Smr Spr; broad-host-range expression vector | 7 |
| pJ22 | Cbr; pADD948 carrying the triclosan resistance determinant from PAO238-1 on an ∼32-kb chromosomal DNA fragment | This study |
| pJ22::Tn<TET-1> | Cbr Tcr; pJ22 with mexJ::Tn<TET-1> | This study |
| pJZ110 | Cbr; pTZ110 with 655-bp BamHI-XhoI fragment from pPS1176 containing the mexJK operon regulatory region | This study |
| pPS1150 | Cbr; pBSP II SK(−) carrying the mexL-mexJK genes on a 6,945-bp NotI fragment from pJ22 | This study |
| pPS1151 | Cbr Tcr; pBSP II SK(−) with a NotI fragment from pJ22::Tn<TET-1> carrying mexJ::Tn<TET-1> | This study |
| pPS1152 | Apr Tcr; pBluescript SK(−) with a NotI fragment from pJ22::Tn<TET-1> carrying mexJ::Tn<TET-1> | This study |
| pPS1153 | Cbr; pUCP20T with 816-bp mexL fragment | This study |
| pPS1162 | Cbr; pAK1900 expressing oprN from Plac | This study |
| pPS1163 | Cbr; pAK1900 expressing oprJ from Plac | This study |
| pPS1168 | Cbr; pUC18 with a 2, 145-bp BglII-HindIII fragment from pPS1150 carrying the 5′ end of mexL, all of mexJ, and the 5′ end of mexK in pUC18 | This study |
| pPS1169 | Cbr; pPS1168 with 1,414-bp NcoI-XhoI deletion removing the 5′ end of mexL, all of mexJ and the 5′ end of mexK replaced with FRT-Gmr fragment from pPS856 | This study |
| pPS1175 | Cbr; pBluescript SK(−) with 1,342-bp EcoRI-ClaI fragment from pPS1150 carrying all of mexL and the 5′ end of mexJ | This study |
| pPS1176 | Cbr; deletion of a 725-bp XhoI fragment internal to mexL from pPS1175 | This study |
| pPS1177 | Gmr; mini-CTX3-lacZ with 643-bp Xhol-PstI fragment from pPS1176 containing the mexJK regulatory region | This study |
| pPS1180 | Smr Spr; pVLT35 expressing oprM from Ptacc | This study |
| pPS1234 | Cbr; pUCP20T with 1,231-bp KpnI-PstI PCR fragment expressing mexJ from Plac | This study |
| pPS1235 | Smr Spr; pVLT35 with 3,759-bp EcoRI-SalI fragment from pPS1150 expressing mexK from Ptac | This study |
This strain and its derivatives contain a 1.58-Mb chromosomal inversion between the mexAB-oprM and mexCD-oprJ operons (2). This inversion has no effect on efflux pump function or any other phenotype since there is no net loss or gain of genetic information.
Plac, E. coli lac operon promoter.
Ptac, E. coli lac operon and trp operon hybrid promoter.
Antimicrobial susceptibility testing.
MICs were determined by the twofold broth microdilution technique according to National Committee for Clinical Laboratory Standards guidelines (30) or by the E-test method (AB Biodisk, Piscataway, N.J.) (ciprofloxacin and tetracycline only).
General DNA and mutagenesis procedures.
All routine DNA procedures were performed by previously described methods (17, 39). For disruption of the mexJ gene, pPS1152 was electroporated (8, 9) into strain PAO238-1, and tetracycline-resistant (Tcr) colonies were selected. The mexJ::Tn〈TET-1〉 mutants were identified as Tcr and carbenicillin susceptible (Cbs). Gene replacement at the mexJ locus was confirmed by using the inserts from pPS1150 and a 627-bp HindIII-SalI fragment from pBR322ΔAP (41), respectively, as mexJK- and tet-specific probes. A chromosomal ΔmexJKL deletion was isolated by deleting a 1,414-bp XhoI-NcoI fragment from pPS1168 and replacing it with a gentamicin resistance (Gmr)-FRT (Flp recombinase target site) cassette from pPS856 (17). The resulting pPS1169 was electroporated into PAO238-1, and Gmr colonies were selected. The ΔmexJKL colonies possessed a Gmr Cbs phenotype. Similarly, a chromosomal ΔmexL deletion strain was isolated by deleting a 371-bp XhoI fragment from pPS1175 and replacing it with a Gmr-FRT cassette from pPS856, followed by return of the deletion into the PAO238 chromosome via conjugation using the gene replacement vector pEX18Ap and sucrose counterselection (17). Flp recombinase-mediated excision was achieved by utilizing a previously described procedure (17). The mutant genotypes were confirmed by PCR and Southern analysis with the inserts from pPS1168 (mexJK′L), pPS1175 (mexL), and pPS856 (Gmr-FRT) as the probes. Genomic Southern blotting with biotin-labeled probes was performed as previously described (17). Mutants containing unmarked chromosomal Δ(mexXY) deletions were isolated by using previously described plasmid constructs and procedures (5) and were verified by PCR analysis.
Cloning and analysis of the mexJK-containing region.
For the cloning of DNA fragments carrying the Trir-conferring chromosomal region from PAO238-1, the bacteriophage mini-D3112-based in vivo cloning method of Darzins and Casadaban (6) was used with the following modifications. Strain PAO238-1 was lysogenized with D3112cts (6). The resulting strain was transformed with pADD948, and a lysate was prepared by heat induction (6). Cells of recipient PAO238 were grown overnight and infected with the mixed lysate as previously described (40). The samples were spread on LB plates containing 30 μg of triclosan per ml and incubated at 30°C. Colonies growing on these plates after 24 to 36 h were purified on the same medium and analyzed for the presence of recombinant plasmids. Cbr- and Trir-conferring plasmids were confirmed by electroporation of PAO238. One plasmid was retained and named pJ22. The Trir-encoding region on this plasmid was localized by in vitro transposon mutagenesis with the EZ::TN〈TET-1〉 kit from Epicentre (Madison, Wis.) according to the manufacturer's protocol. An aliquot of the mutagenized plasmid pool was electroporated into PAO238, and Cbr Tcr transformants were selected and screened for loss of Trir. DNA templates were prepared by using the Qiagen miniprep kit, and the transposon insertion sites were determined by automated nucleotide sequencing. Sequencing reactions were primed by using the TET-1 FP-1 and TET-1 RP-1 primers from the EZ::TN 〈TET-1〉 mutagenesis kit. Homologous sequences were identified by using online BLAST searches of the National Library of Medicine databases.
Cloning and sequencing of mexL.
For PCR amplification of the mexL coding region from genomic and plasmid DNA templates, two primers were designed. Primer mexL-up (5′-CGTTCGAaTTCTTATACTGGGCGG) contained a single base mismatch (lowercase a) and introduced an EcoRI site (underlined) 70 bp upstream of the mexL start codon. Similarly, mexL-down (5′-ACTGGGTCGAcCACTGGGACATC) contained a single mismatch (lowercase c) and introduced a SalI site (underlined) 108 bp downstream of mexL. PCRs were performed with Pfu DNA polymerase (Stratagene). Reaction mixtures (50 μl) contained 1× Pfu buffer (Stratagene), 100 ng of each primer, 10 ng of plasmid DNA or 100 ng of chromosomal DNA, and 2.5 U of Pfu. The reaction mixture was subjected to the following thermal cycles: one cycle at 96°C for 5 min; 30 cycles (for plasmid DNA) or 35 cycles (for chromosomal DNA) of 96°C for 45 s, 60°C for 45 s, and 72°C for 1 min; and a final extension at 72°C for 10 min. Sequences of PCR fragments were determined by using the same primers employed for amplification. For complementation analyses, the PCR fragments were digested with EcoRI and SalI, gel purified, and then cloned between the same sites of pUCP20T (46) to yield pPS1153.
Construction of oprM, oprJ, and oprN expression vectors.
The oprM, oprJ, and oprN genes were PCR amplified from genomic DNA templates by using primers containing base mismatches (indicated by lowercase letters in each primer sequence) that introduced new restriction sites after PCR amplification. For oprM, the forward primer oprM-up (5′-CGAGGaaTtCAAGCAGCAGGCGTCCGT) introduced an EcoRI site (underlined) upstream of the oprM start codon and ribosome-binding site. The reverse primer oprM-down (5′-ACGCCAaGcTtAGGGTCGGCGTTCTTG) introduced a HindIII site (underlined) downstream of oprM. For oprJ, the forward primer oprJ-up (5′-GCAGCAAGCttGCACCCATCGAAC) introduced a HindIII site (underlined) upstream of the oprJ start codon and ribosome-binding site. The reverse primer oprJ-down (5′-CACCGgaTCCCACACGTTTACC) introduced a BamHI site (underlined) downstream of oprJ. The forward primer for oprN, primer oprN-up (5′-CGCGAAGCttGCCGCGCCGCCA), introduced a HindIII site (underlined) upstream of the oprN start codon and ribosome-binding site. The oprN reverse primer, oprN-down (5′-GAGTGGtCGAcTTCCATCGGCCG) introduced a SalI site (underlined) downstream of oprN. PCRs were performed with Taq DNA polymerase (Gibco). Reaction mixtures (50 μl) contained 1× Taq buffer (Gibco), 30 pmol of each primer, 100 ng of chromosomal DNA, and 2.5 U of Taq. For amplification of oprM, the reaction mixture was subjected to the following thermal cycles: one cycle at 96°C for 5 min; 35 cycles of 96°C for 45 s, 71°C for 45 s, and 72°C for 2 min; and a final extension at 72°C for 10 min. The oprJ and oprN genes were amplified using the same denaturation and extension conditions, except that the annealing conditions were changed to 60°C for 45 s and 70°C for 45 s for oprJ and oprN, respectively. The PCR fragments were digested with the appropriate enzymes, purified from an agarose gel, and ligated either to EcoRI-HindIII-digested pVLT35 for oprM, to HindIII-BamHI-digested pAK1900 for oprJ, or to HindIII-SalI-digested pAK1900 for oprN. This procedure generated pPS1180, pPS1162, and pPS1163, which express oprM, oprJ, and oprN, respectively, from Ptac (pVLT35) or Plac (pAK1900). Expression of OprM, OprJ, and OprN was verified in E. coli and P. aeruginosa by Western blot analysis with specific antibodies, as previously described (3, 5).
Measurements of fluorescence in cell suspensions.
Cells for fluorescence measurements were grown overnight in M9 medium (28) supplemented with 1% thiamine and 0.2% Casamino Acids. The cells were washed in 100 mM NaCl-50 mM sodium phosphate buffer (pH 7.0) as previously described (31). The washed cells were adjusted to an optical density at 540 nm of 0.1 in the wash buffer containing 0.05% of glycerol and used for fluorescence measurements within 2 h. The substrate used in fluorescence measurements was 2-(4-diethylaminostyryl)-1-methylpyridinium iodide (DMP), and it was added to cell suspensions to a final concentration of 25 μM. The fluorescence emission intensity at room temperature was measured with a Fluorolog-3 fluorometer (Instruments S.A., Inc., Edison, N.J.), and data were recorded and analyzed with DataMax and Igor Pro software. Excitation and emission wavelengths for DMP were, respectively, 467 and 557 nm. Slit widths were set at 5 nm for excitation and at 10 nm for emission.
Construction of a mexJ-lacZ fusion.
A plasmid-borne fusion was constructed by ligating a 655-bp BamHI-XhoI fragment from pPS1176 between the same sites of pTZ110 (44) to form pJZ110. The fused fragment contained 470 bp of mexJ, the 94-bp mexJ-mexL intergenic region, and 71 bp of mexL coding sequence. The fusion plasmid was transferred to P. aeruginosa by electroporation. β-Galactosidase activity was measured and activity units were calculated as described by Miller (28).
RESULTS
Isolation and characterization of triclosan-resistant mutants.
When triclosan-susceptible cells of the Δ(mexAB-oprM) Δ(mexCD-oprJ) strain PAO238 (Table 1) were exposed to 25 μg of triclosan per ml, Trir derivatives were obtained at a frequency of 10−8. Four randomly picked Trir derivatives, PAO238-1 to PAO238-4, were further analyzed, and all of them were highly Trir (MICs of at least 128 μg/ml). None of them was resistant to hexachlorophene (MIC, 8 μg/ml) or dichlorophene (MICs, 32 to 64 μg/ml), two other antimicrobials with structures similar to that of triclosan. For comparison, in PAO1 dichlorophene was found to be a good substrate for MexAB-OprM (MIC, 128 μg/ml), but hexachlorophene (MIC, 4 μg/ml) was not. In addition, none of the mutants were resistant to any of the antibiotics tested (see footnote a to Table 2), presumably due to lack of an Opr channel (see below). Analysis of total outer membrane proteins did not reveal overproduction of any novel proteins (data not shown). Although the MexEF-OprN system supports the efflux of triclosan, in Western blots none of the four mutants expressed detectable levels of OprN (data not shown). This finding was not surprising, since MexEF-OprN is not derepressible in PAO238 because this PAO background harbors an 8-bp insertion in the mexEF-oprN activator mexT (23). These preliminary results indicated that the system(s) responsible for the Trir observed in these mutants was novel and most likely did not involve overproduction of a new outer membrane protein. Since all four Trir PAO238 derivatives behaved similar in preliminary analyses, we decided to concentrate on one isolate, PAO238-1, and to decipher the mechanism(s) responsible for its Trir phenotype.
TABLE 2.
Antimicrobial susceptibililies of efflux pump mutant strains
| Strain | Mex efflux (outer membrane protein) expressed | MIC (μg/ml)a
|
|||
|---|---|---|---|---|---|
| TRI | TC | CIP | ERY | ||
| PAO1 | MexAB (OprM) MexXiYib | >128c | 16 | 0.064 | 512 |
| PAO238 | MexXiYi | 20 | 0.75 | 0.006 | 32 |
| PAO238-1 | MexJK MexXiYi | 128 | 1 | 0.004 | 16 |
| PAO298 | MexXiYi | 20 | NDd | 0.004 | 32 |
| PAO314 | MexXiYi | 20 | 0.75 | 0.006 | 32 |
| PAO238-1(pVLT35) | MexJK MexXiYi | >128 | 0.75 | 0.004 | 16 |
| PAO238-1(pPS1180) | MexJK MexXiYi (OprM) | >128 | 16 | 0.047 | 256 |
| PAO314(pVLT35) | MexXiYi | 32 | 0.38 | 0.006 | 16 |
| PAO314(pPS1180) | MexXiYi (OprM) | 32 | 10 | 0.016 | 128 |
| PAO327(pVLT35) | MexJK | 128 | 0.19 | 0.004 | 8 |
| PAO327(pPS1180) | MexJK (OprM) | 128 | 1.0 | 0.008 | 64 |
| PAO325/pVLT35 | None | 16 | 0.19 | 0.004 | 16 |
| PAO325(pPS1180) | (OprM) | 16 | 0.25 | 0.006 | 16 |
| PAO238-1(pUCP20T) | MexJK MexXiYi | >128 | 0.75 | 0.004 | 16 |
| PAO238-1(pPS1153) | MexJK MexXiYi | 20 | 0.75 | 0.006 | 16 |
| PAO318 | MexJK MexXiYi | >128 | 0.75 | 0.006 | 16 |
| PAO318(pUCP20T) | MexJK MexXiYi | >128 | 0.5 | 0.004 | 16 |
| PAO318(pPS1153) | MexJK MexXiYi | 20 | 0.5 | 0.004 | 16 |
Abbreviations: CIP, ciprofloxacin; ERY, erythromycin; TC, tetracycline; TRI, triclosan. All mutants were additionally tested for their susceptibilities to acriflavine, dichlorophene, carbenicillin, fusidic acid, gentamicin, hexachlorophene, and trimethoprim, most of which are substrates of other efflux systems but not MexJK.
MexXiYi, induced MexXY.
At concentrations of >128 μg/ml, triclosan is insoluble in aqueous solutions.
ND, not done.
Cloning of the triclosan resistance determinant from PAO238-1.
The phage D3112-based in vivo cloning technique was used to clone a ∼32-kb chromosomal DNA fragment from PAO238-1. The resulting plasmid, pJ22, conferred Trir when transferred to the susceptible parent strain PAO238. In vitro transposon mutagenesis was used to localize the Trir determinant in the chromosomal insert of pJ22. The relatively high frequency (∼13%) with which the transposon-mutagenized plasmid population lost their ability to confer Trir indicated that a relatively large region on pJ22 was required for the Trir phenotype. To further localize the Trir-encoding determinant to the mexJK region, the entire operon with its flanking DNA sequences was subcloned on a 6,945-bp NotI fragment (Fig. 1). As a control, the same fragment containing the 1,674-bp Tn〈TET-1〉 insertion in mexJ was also subcloned. When transformed into PAO238, the plasmid containing the unmutagenized NotI fragment conferred Trir, but the vector control and the plasmid containing mexJ::Tn〈TET-1〉 did not.
FIG. 1.
Organization of the mexJK operon and mexL, encoding a repressor of mexJK expression. The sizes of the genes and the corresponding PA annotations were taken from the published P. aeruginosa genome sequence (www.pseudomonas.com). The approximate extent of the mexJKL deletion in strain PAO314 is indicated by the black bar. The nucleotide and amino acid sequences of the predicted helix-turn-helix (HTH) DNA binding domain of MexL are also shown, including the single base change and resulting amino acid change in the mexL mutant PAO238-1. nt, nucleotide.
A novel efflux system is responsible for triclosan resistance in PAO238-1.
Nucleotide sequence analysis of one of the Tris transposon pJ22 insertion mutants revealed that it contained a single transposon insertion in a gene that was annotated as PA3677 in the published genome sequence (50). This gene (1,103 bp), which we named mexJ (Fig. 1), encodes the periplasmic MFP of an RND-type efflux system. The transposon in pJ22 is inserted between codons 110 and 111 of mexJ. Separated by 4 nucleotides from mexJ and in the same transcriptional orientation is another 3,077-bp gene, mexK, which encodes PA3676, a putative transmembrane protein sharing significant homology with other inner membrane transporter proteins of RND-type efflux systems of the AcrBF family. The proposed mexJK operon lies at 4.12 Mb on the PAO1 genome.
MexJ is 37 to 51% similar to MFPs from other P. aeruginosa RND efflux systems and most similar (51 and 48%, respectively) to PA156 and PA157, two MFPs shared by the same efflux pump with divalent cation transporter homology (50). Like other MFPs of the RND efflux protein family, MexJ is most likely a lipoprotein, since it contains a signal peptidase II cleavage site (FLAACGNG) at the end of a 20-amino-acid NH2-terminal signal sequence. The role of acylation and membrane anchoring in MFP function remains unclear because many MFPs, including P. aeruginosa MexA (55) and E. coli AcrA (56), are functional as nonacylated proteins.
The inner membrane transporter protein MexK is 41 to 64% similar to transporters from other P. aeruginosa RND systems and most related (64% similar) to PA158, the transporter component of an efflux system with divalent cation transporter homology. Based on amino acid comparisons, MexJK is thus most related to PA156-PA157-PA158 than to the other known or proposed RND-type drug efflux systems in this bacterium. Although PA156-PA157-PA158 and the related CzrCBA divalent cation efflux pump were previously excluded from the list of putative RND-type drug efflux systems (50), our data suggest that they are even more related to MexJK than some of the known Mex proteins and therefore should be included in this list.
To verify that the mexJK operon was indeed responsible for triclosan efflux in PAO238-1, a chromosomal mexJ mutant was constructed by transferring the mexJ::Tn〈TET-1〉 to the PAO238-1 chromosome. The resulting mutant strain, PAO298, became Tris and behaved the same as the subsequently constructed ΔmexJKL mutant PAO314 (Table 2).
The MexJK efflux system requires OprM for efflux of antibiotics but not triclosan.
The molecular architecture of the MexJK operon is very similar to that of the MexXY system in that both systems do not contain their own Opr channel. Since it has been shown that MexXY requires OprM for antibiotic efflux (5, 25), we reasoned that PAO238-1 did not efflux antibiotics since it was lacking OprM. To test this hypothesis, PAO238-1 was electroporated with the OprM-expressing plasmid pPS1180 and its vector control, pVLT35. Only PAO238-1 containing pPS1180 effluxed tetracycline, erythromycin, and ciprofloxacin (Table 2), and to a lesser extent gentamicin and fusidic acid, but not carbenicillin, trimethoprim, dichlorophene, and hexachlorophene (data not shown).
Because the mexJK-overproducing strain PAO238-1 contains a wild-type mexXY operon, whose expression was recently shown to be inducible by several of its antibiotic substrates (25), we considered that inducible MexXY may have been at least partly responsible for the observed multidrug resistance phenotype observed in PAO238-1 expressing cloned OprM. To test this notion and to assess the true contribution of MexJK to multidrug resistance, we constructed three isogenic derivatives of PAO238-1: the Δ(mexJKL) strain PAO314, expressing only inducible MexXY; the Δ(mexXY) strain PAO327, which expresses only MexJK; and finally the Δ(mexJKL) Δ(mexXY) strain PAO325, expressing neither efflux system. These strains were then transformed with either the vector control pVLT35 or the OprM-expressing pPS1180, and MICs of various drugs were determined (Table 2). As expected, PAO314 effluxed the known MexXY-OprM substrates ciprofloxacin, erythromycin, tetracycline, and gentamicin (not shown) only when transformed with pPS1180 but did not efflux triclosan because it is not a MexXY-OprM substrate (5). In contrast, PAO327 expressing OprM from pPS1180 no longer effluxed ciprofloxacin and gentamicin (not shown) but still effluxed tetracycline and erythromycin, albeit more weakly than PAO314. Efflux of erythromycin and tetracycline in this strain was dependent on OprM. Levels of triclosan efflux were the same as those seen in PAO238-1 and were independent of OprM. Lastly, the Δ(mexJKL) Δ(mexXY) strain PAO325 effluxed neither triclosan nor any of the antibiotics tested. These results indicated that inducible MexXY, in concert with OprM, was mostly responsible for the multidrug resistance phenotype of PAO238-1, although MexJK contributed considerably to the triclosan, erythromycin, and tetracycline resistance of this strain. Neither the MexJK nor the MeXY system functioned with either OprJ or OprN, although the respective proteins were expressed in the cells used for MIC determinations, as indicated by Western blot analysis (data not shown).
The outer membrane channel requirement for MexJK was further assessed by using DMP. This fluorescent probe fluoresces intensely when present in nonpolar or hydrophobic environments but fluoresces weakly in aqueous environments (31). As previously shown (31), DMP is efficiently extruded from cells by the MexAB-OprM system expressed in PAO1 (Fig. 2). Even cells overexpressing OprM in the absence of other known pump proteins (PAO314/pPS1180) showed some DMP efflux. Efficient extrusion of DMP via MexJK in PAO238-1 required OprM, since a strain expressing MexJK alone (PAO238-1) behaved similarly to the Δ(mexJK) mutant PAO314. Although all of the cells studied for DMP efflux contained a functional MexXY system, its expression was not induced because the cells were not pregrown in inducing antibiotics. Therefore, DMP efflux measured in these cells was due to MexJK.
FIG. 2.
Efflux of the fluorophore DMP from various mutant strains. The indicated strains were pregrown in M9 medium containing 1% thiamine and 0.2% Casamino Acids and then were washed and adjusted to an optical density (600 nm) of 0.1. At 0 min, DMP was added to a final concentration of 25 μM, and fluorescence was immediately recorded. The strains used were as follows: PAO1, wild type expressing MexAB-OprM; PAO238-1, a mexL mutant expressing MexJK; and PAO314 Δ(mexJKL), expressing no efflux system. Strains containing pPS1180 express OprM.
MexJ and MexK are both required for a functional triclosan efflux system.
Because MexJK effluxed triclosan independently of the expression of a known Opr channel, we reasoned that MexK may function in triclosan export independently of a MFP, similarly to AcrD, an RND aminoglycoside pump of E. coli (37). To test this hypothesis, plasmids overproducing MexJ and MexK were independently and in combination transformed into strain PAO325, which expresses no efflux pump either because of deletion of the structural genes (MexAB-OprM, MexCD-OprJ, MexXY, and MexJK) or because of a regulatory mutation (MexEF-OprN). PAO325 cells expressing MexJ or MexK alone did not efflux triclosan (Table 3), and neither did uninduced cells containing both the MexJ and MexK expression plasmids. However, cells overproducing MexJ and MexK after induction with IPTG (isopropyl-[beta]-d-thiogalactopyranoside) effluxed triclosan at levels observed in the MexJK-overproducing mutants PAO238-1 and PAO318 (Table 2).
TABLE 3.
Efflux component requirement for MexJK-mediated triclosan effluxa
| Strain | IPTG (mM) | Expressed efflux protein(s) | Triclosan MIC (μg/ml) |
|---|---|---|---|
| PAO325(pUCP20T) | 0 | None | 16 |
| PAO325(pVLT35) | 0 | None | 8 |
| PAO325(pPS1234) | 0b | MexJ | 16 |
| PAO325(pPS1235) | 0 | MexK | 16 |
| PAO325(pPS1235) | 1c | MexK | 16 |
| PAO325(pPS1234, pPS1235) | 0 | MexJ, MexK | 16 |
| PAO325(pPS1234, pPS1235) | 1 | MexJ, MexK | >128 |
The Δ(mexAB-oprM) Δ(mexCD-oprJ) Δ(mexJK) Δ(mexXY) and MexEF-OprN-uninducible strain PAO325 was transformed with the indicated plasmids, and MICs were determined by the microdilution method.
MexJ is constitutively expressed from Plac provided by pUCP20T.
MexK is expressed from the inducible Ptac provided by pVLT35.
The mexJK operon is negatively regulated by the mexL gene product.
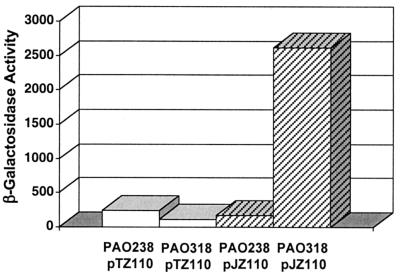
The mexJK operon is separated by 94 bp from an upstream open reading frame, PA3678 or mexL, which is transcribed divergently from mexJK (Fig. 1). PA3678 was annotated as a putative transcriptional regulator of the tetracycline repressor (TetR) family, since it contains the signature motif of this transcription regulator family. We therefore considered that mexL might encode a repressor of mexJK expression. If this were true, then mexL would most likely be mutated in PAO238-1 and on pJ22 but should be wild type in PAO238. The mexL gene was PCR amplified from PAO238, PAO238-1, and pJ22. Whereas the mexL sequence from PAO238 was identical to the one established for PAO1, the mexL genes from PAO238-1 and pJ22 contained a single base change. This missense mutation replaced an alanine with a glutamic acid residue in the first helix of the putative helix-turn-helix domain (Fig. 1). The cloned mexL gene from PAO1 carried on pPS1153 restored Tris on PAO238-1 (Table 2). Strain PAO318 containing a 371-bp chromosomal ΔmexL, which removes the helix-turn-helix coding sequence, constitutively expressed mexJK, as evidenced by high-level triclosan resistance (Table 2) and high-level expression of a mexJ-lacZ transcriptional fusion (Fig. 3). Cloned mexL restored triclosan susceptibility on PAO318 transformed with pPS1153 (Table 2).
FIG. 3.
Repression of a mexJ-lacZ transcriptional fusion by MexL. Cells of strains PAO238 (mexL wild type) or PAO318 (ΔmexL) containing a plasmid-encoded mexJ-lacZ transcriptional fusion (pJZ110) or the vector control (pTZ110) were grown to mid-log phase in LB medium supplemented with 100 μg of carbenicillin per ml. β-Galactosidase activity was measured in triplicate samples, and the t distribution was used to establish 95% confidence limits.
DISCUSSION
Although P. aeruginosa expresses FabI, the primary target for triclosan (16, 18, 27), mounting evidence now suggests that its primary defense mechanism against this biocide is efflux via multiple RND systems. We previously showed that MexAB-OprM (42) and MexCD-OprJ and MexEF-OprN, but not MexXY-OprM (5), efflux triclosan. By using triclosan as a selective tool, a novel efflux system, MexJK, was identified in mexL mutants in a Δ(mexAB-oprM) Δ(mexCD-oprJ) background. The frequency (10−8) with which triclosan resistant mutants were obtained was about two orders of magnitude lower than the frequency observed when a Δ(mexAB-oprM) strain was plated on the same medium (5). This observation is in agreement with results obtained by Lomovskaya et al. (22), who showed that the consequence of deletion of multiple pumps is a significant decrease in the frequency of spontaneously resistant mutants. In addition to MexCD-OprJ, MexJK is the second P. aeruginosa efflux system whose expression could be selected by exposure to triclosan, indicating that this biocide is a powerful tool for the study of efflux systems. In addition, this finding further underscores the previously raised concern that the widespread use of this antiseptic may compound antibiotic resistance. Although the originally isolated strain, PAO238-1, showed a multidrug resistance phenotype in the presence of OprM, a more careful analysis revealed that it was mostly due to inducible MexXY expression (25). This finding illustrates the importance of performing efflux pump characterization experiments in a MexXY mutant background.
Our results confirmed that mexJK transcription is governed by an upstream regulatory gene, mexL. This situation is similar to that for other negatively regulated P. aeruginosa efflux operons containing the repressor gene located upstream of the efflux operon structural genes (50). The missense mutation in strain PAO238-1 probably generated a nonfunctional MexL, since it replaces an alanine with a glutamic acid residue in the first helix of the putative helix-turn-helix domain (Fig. 1). Since this mutation could be complemented by wild-type mexL, MexL is a transcriptional regulator of mexJK expression. The 94-bp mexL-mexJ intergenic region is the smallest regulatory region identified thus far in any of the characterized negatively regulated efflux operons. By comparison, the mexR-mexA intergenic region is 273 bp long, the nfxB-mexC intergenic region is 159 bp, and the mexZ-mexX intergenic region is 236 bp.
Although the bisphenols analyzed in this study were similar in structure and size and both triclosan and dichlorophene were substrates of the MexAB-OprM system, only triclosan was a bisphenol substrate of the MexJK system. Since the substrate specificity of efflux systems seems to reside with the inner membrane transporters (24, 49), the differences found in the structures of the bisphenols, i.e., methane versus ether linkage of the rings and number and position of chlorines, may be indicative of the interaction, or lack thereof, of these substrates with the cytoplasmic membrane transporters.
Most lipophilic and amphiphilic drugs can cross the cytoplasmic membrane spontaneously, and their accumulation in the periplasm would accelerate their reentry into the cytoplasm. It has therefore been proposed that tripartite efflux systems, consisting of an inner membrane transporter, a periplasmic MFP, and an Opr channel, are crucial for their expulsion all the way into the medium (57). However, of the 12 potential RND-type efflux systems encoded by the chromosome, only seven operons encode their own Opr channel (50). Since the MexAB-OprM system is always expressed at low levels and since oprM can even be transcribed at low levels independently of mexAB from its own promoter (58), it has been speculated that OprM may serve as a universal Opr channel for efflux systems lacking their own Opr component. Indeed, it has been shown that OprM can functionally link with MexCD (10), MexEF (24), and MexXY (5, 25, 29). Moreover, since the mexXY operon does not encode its own Opr, it is dependent on OprM for function (5, 25, 29). In this study we show that MexJK, like MexXY, also requires OprM for efflux of its antibiotic substrates and the DMP fluorophore (Fig. 2). However, OprJ and OprN did not link functionally with the MexJK complex. It is presently not known why OprM can function with all inner membrane transporter-periplasmic MFP complexes studied to date, while other Oprs are more discriminative. Whereas OprJ and OprN did not function with MexJK (this study), and OprN failed to interact with MexAB (24), OprJ restored almost complete function to a MexAB system without OprM (49).
Interestingly, MexJK-mediated triclosan efflux did not require OprM although it required both the MexK transporter proteins and the MexJ MFP. There are two possible explanations to rationalize these observations. (i) For triclosan efflux, MexJK may interact with another, hitherto-unknown, Opr channel. This Opr channel would probably not function in antibiotic efflux because of the OprM requirement for this process. Besides OprM, the only known Opr channel to be expressed in wild-type cells is the quorum-sensing-regulated PA4208, which was shown to be expressed in stationary phase by using gene fusions (53) as well as by transcriptional profiling (S. Lory, personal communication). However, it is presently unknown whether this Opr channel functions with other efflux systems or what role, if any, it may play in the extrusion of antimicrobials from the cell. (ii) Alternatively, triclosan may be an exceptionally good substrate for MexJK and may therefore be efficiently removed from the cytoplasm independently of an Opr component. Thus, triclosan either may accumulate in the periplasm, similar to the case for tetracycline expelled from the cytoplasm by the action of tetracycline efflux pumps (26), or may be expelled into and/or through the outer membrane by an unknown mechanism. Because triclosan is an amphiphilic drug and its periplasmic accumulation would favor spontaneous reentry into the cytoplasm, one would have to favor the hypothesis that MexJK interacts with an Opr channel to expel triclosan completely from the cell, although it is puzzling why this channel would not function in the efflux of antibiotics. Alternatively, MexJ may play a more active role in depositing triclosan into or through the outer membrane, because MFPs are seemingly highly asymmetric proteins capable of spanning the periplasm, as previously shown for AcrA from E. coli (56). Thus, we cannot rule out the possibility that a two-component RND efflux system may be sufficient for efflux of selected amphiphilic drugs. If this was the case, then RND efflux pumps could possibly be divided into three categories: (i) single-component pumps consisting of an inner membrane transporter, as typified by AcrD of E. coli, for efflux of hydrophilic drugs; (ii) two-component pumps consisting minimally of an inner membrane transporter and an MFP for efflux of selected amphiphilic drugs; and (iii) three-component pumps consisting of an inner membrane transporter, an MFP, and an Opr channel for efflux of most amphiphilic and lipophilic drugs.
Acknowledgments
We thank N. Gotoh for providing monoclonal anti-OprM, anti-OprJ, and anti-OprN antibodies and KIC Chemicals for providing triclosan.
R.C. is the recipient of a Royal Predoctoral Fellowship from the Government of Thailand. C.T.N. was supported by the U.S. Air Force. This work was supported in part by NIH grant GM56685.
REFERENCES
- 1.Aires, J. R., T. Köhler, H. Nikaido, and P. Plesiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barekzi, N., K. Beinlich, T. T. Hoang, X. Q. Pham, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2000. High-frequency Flp recombinase-mediated inversions of the oriC-containing region of the Pseudomonas aeruginosa genome. J. Bacteriol. 182:7070-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beinlich, K. L., R. Chuanchuen, and H. P. Schweizer. 2001. Contribution of multidrug efflux pumps to multiple antibiotic resistance in veterinary clinical isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 198:129-134. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava, H. N., and P. A. Leonard. 1996. Triclosan: applications and safety. Am. J. Infect. Control 24:209-218. [DOI] [PubMed] [Google Scholar]
- 5.Chuanchuen, R., K. Beinlich, T. T. Hoang, A. Becher, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob. Agents Chemother. 45:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darzins, A., and M. J. Casadaban. 1989. In vivo cloning of Pseudomonas aeruginosa genes with mini-D3112 transposable bacteriophage. J. Bacteriol. 171:3917-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lorenzo, V., L. Eltis, B. Kessler, and K. N. Timmis. 1993. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 123:17-24. [DOI] [PubMed] [Google Scholar]
- 8.Dennis, J. J., and P. A. Sokol. 1995. Electrotransformation of Pseudomonas. Methods Mol. Biol. 47:125-133. [DOI] [PubMed] [Google Scholar]
- 9.Diver, J. M., L. E. Bryan, and P. A. Sokol. 1990. Transformation of Pseudomonas aeruginosa by electroporation. Anal. Biochem. 189:75-79. [DOI] [PubMed] [Google Scholar]
- 10.Gotoh, N., H. Tsujimoto, A. Nomura, K. Okamoto, M. Tsuda, and T. Nishino. 1998. Functional replacement of OprJ by OprM in the MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 165:21-27. [DOI] [PubMed] [Google Scholar]
- 11.Gump, W. S., and G. R. Walter. 1968. The bis-phenols, p. 257-277. In C. A. Lawrence and S. S. Block (ed.), Disinfection, sterilization and preservation. Lea and Febiger, Philadelphia, Pa.
- 12.Hancock, R. E. W. 1997. The bacterial outer membrane as a drug barrier. Trends Microbiol. 5:37-42. [DOI] [PubMed] [Google Scholar]
- 13.Hassan, M.-E.-T., D. van der Lelie, D. Springael, U. Römling, N. Ahmed, and M. Mergeay. 1999. Identification of a gene cluster, czr, involved in cadmium and zinc resistance in Pseudomonas aeruginosa. Gene 238:417-425. [DOI] [PubMed] [Google Scholar]
- 14.Heath, R. J., J. Li, G. E. Roland, and C. O. Rock. 2000. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 275:4654-4659. [DOI] [PubMed] [Google Scholar]
- 15.Heath, R. J., J. R. Rubin, D. R. Holland, E. Zhang, M. E. Snow, and C. O. Rock. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274:11110-11114. [DOI] [PubMed] [Google Scholar]
- 16.Heath, R. J., Y.-T. Yu, M. A. Shapiro, E. Olson, and C. O. Rock. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid biosynthesis. J. Biol. Chem. 273:30316-30320. [DOI] [PubMed] [Google Scholar]
- 17.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 18.Hoang, T. T., and H. P. Schweizer. 1999. Characterization of the Pseudomonas aeruginosa enoyl-acyl carrier protein reductase: a target for triclosan and its role in acylated homoserine lactone synthesis. J. Bacteriol. 181:5489-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Join-Lambert, O. F., M. Michea-Hamzehpour, T. Köhler, F. Chau, F. Faurisson, S. Dautrey, C. Vissuzaine, C. Carbon, and J.-C. Pechere. 2001. Differential selection of multidrug efflux mutants by trovafloxacin and ciprofloxacin in an experimental model of Pseudomonas aeruginosa acute pneumonia in rats. Antimicrob. Agents Chemother. 45:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jungermann, E. 1968. Soap bacteriostats. J. Am. Oil Chem. Soc. 45:345-350. [DOI] [PubMed] [Google Scholar]
- 21.Köhler, T., M. Michea-Hamzehpour, U. Henze, N. Gotoh, L. K. Curty, and J. C. Pechere. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol. Microbiol. 23:345-354. [DOI] [PubMed] [Google Scholar]
- 22.Lomovskaya, O., A. Lee, K. Hoshino, H. Ishida, A. Mistry, M. S. Warren, E. Boyer, S. Chamberland, and V. J. Lee. 1999. Use of a genetic approach to evaluate the consequence of inactivation of efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1340-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maseda, H., K. Saito, A. Nakajima, and T. Nakae. 2000. Variation of the mexT gene, a regulator of the MexEF-OprN efflux pump expression in wild-type strains of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 192:107-112. [DOI] [PubMed] [Google Scholar]
- 24.Maseda, H., H. Yoneyama, and T. Nakae. 2000. Assignment of the substrate-selective subunits of the MexEF-OprN multidrug efflux pump of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda, N., E. Sagagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:2242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurry, L., R. E. Petrucci, Jr., and S. B. Levy. 1980. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc. Natl. Acad. Sci. USA 77:3974-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurry, L. M., M. Oethinger, and S. B. Levy. 1998. Triclosan targets lipid synthesis. Nature 394:531-532. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1999. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:415-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. 1998. Performance standards for antimicrobial susceptibility testing. Eight informational supplement. Document M100-S8. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 31.Ocaktan, A., H. Yoneyama, and T. Nakae. 1997. Use of fluorescence probes to monitor function of the subunit proteins of the MexA-MexB-OprM drug extrusion machinery in Pseudomonas aeruginosa. J. Biol. Chem. 272:21964-21969. [DOI] [PubMed] [Google Scholar]
- 32.Poole, K. 2001. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 3:255-264. [PubMed] [Google Scholar]
- 33.Poole, K., N. Gotoh, H. Tsujimoto, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X. Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug resistant strains. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 34.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poole, K., K. Tetro, Q. Zhao, S. Neshat, D. Heinrichs, and N. Bianco. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 40:2021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pumbwe, L., and L. J. Piddock. 2000. Two efflux systems expressed simultaneously in multidrug-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:2861-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg, E. Y., D. Ma, and H. Nikaido. 2000. AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 182:1754-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito, K., H. Yoneyama, and T. Nakae. 1999. nalB-type mutations causing the overexpression of the MexAB-OprM efflux pump are located in the mexR gene of the Pseudomonas aeruginosa chromosome. FEMS Microbiol. Lett. 179:67-72. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Schweizer, H. P. 1991. The agmR gene, an environmentally responsive gene, complements defective glpR, which encodes the putative activator for glycerol metabolism in Pseudomonas aeruginosa. J. Bacteriol. 173:6798-6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweizer, H. P. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol. Microbiol. 6:1195-1204. [DOI] [PubMed] [Google Scholar]
- 42.Schweizer, H. P. 1998. Intrinsic resistance to inhibitors of fatty acid biosynthesis in Pseudomonas aeruginosa is due to efflux: application of a novel technique for generation of unmarked chromosomal mutations for the study of efflux systems. Antimicrob. Agents Chemother. 42:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schweizer, H. P. 2001. Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 202:1-7. [DOI] [PubMed] [Google Scholar]
- 44.Schweizer, H. P., and R. Chuanchuen. 2001. A small broad-host-range lacZ operon fusion vector with low background activity. BioTechniques 31:1258-1262. [DOI] [PubMed] [Google Scholar]
- 45.Schweizer, H. P., T. T. Hoang, K. L. Propst, H. R. Ornelas, and R. R. Karkhoff-Schweizer. 2001. Vector design and development of host systems for Pseudomonas, p. 69-81. In J. K. Setlow (ed.), Genetic engineering, vol. 23. Kluwer-Academic/Plenum, New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 46.Schweizer, H. P., T. R. Klassen, and T. Hoang. 1996. Improved methods for gene analysis and expression in Pseudomonas, p. 229-237. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. ASM Press, Washington, D.C.
- 47.Silvernale, J. N., H. L. Joswick, T. R. Corner, and P. Gerhardt. 1971. Antimicrobial actions of hexachlorophene: cytological manifestations. J. Bacteriol. 108:482-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srikumar, R., P. C.J., and K. Poole. 2000. Influence of mutations in the mexR repressor gene on expression of the MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 182:1410-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srikumar, R., X.-Z. Li, and K. Poole. 1997. Inner membrane efflux components are responsible for the β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J. Bacteriol. 179:7875-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stover, C. K., X.-Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, D. Spencer, G. K.-S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 51.Watson, J. M., and B. W. Holloway. 1978. Chromosome mapping in Pseudomonas aeruginosa. J. Bacteriol. 133:1113-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westbrock-Wadman, S., D. R. Sherman, M. J. Hickey, S. N. Coulter, Y. Q. Zhu, P. Warrener, L. Y. Nguyen, R. M. Shawar, K. R. Folger, and C. K. Stover. 1999. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside resistance. Antimicrob. Agents Chemother. 43:2975-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 55.Yoneyama, H., H. Maseda, H. Kamiguchi, and T. Nakae. 2000. Function of the membrane fusion protein, MexA, of the MexA, B-OprM efflux pump in Pseudomonas aeruginosa without an anchoring membrane. J. Biol. Chem. 275:4628-4634. [DOI] [PubMed] [Google Scholar]
- 56.Zgurskaya, H. I., and H. Nikaido. 1999. AcrA is a highly asymmetric protein capable of spanning the periplasm. J. Mol. Biol. 285:409-420. [DOI] [PubMed] [Google Scholar]
- 57.Zgurskaya, H. L., and H. Nikaido. 2000. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37:219-225. [DOI] [PubMed] [Google Scholar]
- 58.Zhao, Q., X. Z. Li, R. Srikumar, and K. Poole. 1998. Contribution of outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob. Agents Chemother. 42:1682-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziha-Zafiri, I., C. Llanes, T. Köhler, J.-C. Pechere, and P. Plesiat. 1999. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob. Agents Chemother. 43:287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]