Abstract
The ability to comprehend narratives constitutes an important component of human development and experience. The neural correlates of auditory narrative comprehension in children were investigated in a large-scale functional magnetic resonance imaging (fMRI) study involving 313 subjects ages 5–18. Using group Independent Component Analysis (ICA), bilateral task-related components were found comprising the primary auditory cortex, the mid-superior temporal gyrus, the hippocampus, the angular gyrus and medial aspect of the parietal lobule (precuneus/posterior cingulate). In addition, a right-lateralized component was found involving the most posterior aspect of the superior temporal gyrus, and a left-lateralized component was found comprising the inferior frontal gyrus (including Broca’s area), the inferior parietal lobule, and the medial temporal gyrus. Using a novel data-driven analysis technique, increased task-related activity related to age was found in the components comprising the mid-superior temporal gyrus (Wernicke’s area) and the posterior aspect of the superior temporal gyrus, while decreased activity related to age was found in the component comprising the angular gyrus. The results are discussed in light of recent hypotheses involving the functional segregation of Wernicke’s area and the specific role of the mid-superior temporal gyrus in speech comprehension.
Introduction
During the early school years, school performance is significantly impacted by story comprehension skills (Lorch et al., 1998). Children’s ability to comprehend fictional narratives is related to three key aspects of the story: the causal relationships in stories, the goals and internal states of the characters in the stories, and the integration of the different parts of the stories (Trabasso and Stein, 1997; Van Den Broek, 1997). The listener (or reader) has the expectation of logical coherence (cause and effect) between events (Barthes, 1981; Graesser et al., 1980). However, often the inference of specific intentions, goals, or mental/emotional states for a specific character in the narrative is necessary for the listener to properly construct causal relationships (Frith and Frith, 1999; Gernsbacher et al., 1992; Oatley, 1992; Peterson, 1999). Therefore, comprehension of narratives involves more than comprehension of the individual sentences that comprise the story.
Previous neuroimaging studies of simple narrative comprehension have yielded conflicting results. A functional magnetic resonance imaging (fMRI) study (Vogeley et al., 2001) yielded no increase in neuronal activation during reading of “physical” stories without an element of “theory-of-mind” (predicting the behavior of others by means of their mental states) (Frith et al., 1991; Happe et al., 1996)) versus a baseline condition of the reading of unlinked sentences. However, an earlier positron emission tomography (PET) study (Fletcher et al., 1995) did show activation increases in the temporal poles bilaterally, the left superior temporal gyrus and the posterior cingulate cortex for the same functional contrast (reading physical stories versus unlinked sentences). Activation increases in similar regions were observed in a separate PET study (Mazoyer et al., 1993) investigating auditory narrative comprehension (listening to stories in native language versus an unknown foreign language). It has been suggested (Mar, 2004) that the discrepancy in results may be due to methodological differences (imaging method and threshold set for statistical significance) as well as a different baseline task: Vogeley et al. used unlinked sentences as a control task, which may mask some activation, as the subjects will likely attempt to make sense of the incoherent presentation.
To investigate the neural correlates of auditory sentence processing and narrative comprehension in children, we performed a large-scale study using fMRI involving subjects ages 5–18 years old. The stories presented were simple stories, without an element of “theory-of-mind” and were quite short, obviating the necessity of higher-order integrative processes (e.g. between groups of events), in order make the difficulty level suitable for young children. In addition to activation in primary auditory areas, we predict, based on previous results (Fletcher et al., 1995; Gallagher et al., 2000; Mazoyer et al., 1993) that the task of auditory narrative processing will recruit the superior temporal gyrus, posterior cingulate gyrus, and inferior parietal lobules, as well as areas recruited for semantic and syntactic elements of language processing such as the angular gyrus and inferior frontal gyrus (Fiebach et al., 2005; Holland et al., 2001).
Since we wished to investigate the neural correlates of macro-level (e.g. recognition of causal relationships in the stories) processes as well as micro-level (e.g. words and sentences) language processes, we selected group Independent Component Analysis (ICA) (Calhoun et al., 2001; Mckeown et al., 1998; Schmithorst and Holland, 2004) as our data analysis technique. Group ICA has the advantage that the design matrix or hemodynamic response functions (HRFs) need not be specified a priori and are allowed to vary across subjects. This is important for the investigation of macro-level processes as the HRF may have considerable variance across subjects as well as not correlate well with the typical regressor used in the General Linear Model (GLM) (Worsley and Friston, 1995) approach (e.g. a square wave convolved with an impulse HRF). Group ICA analysis has been previously used for a variety of fMRI studies including those investigating math processing (Schmithorst and Brown, 2004), alcohol intoxication effects on simulated driving (Calhoun et al., 2004b), and music perception (Schmithorst, 2005).
Materials and Methods
313 children (152 boys, 161 girls) were successfully scanned as part of this study following Institutional Review Board approval and informed consent by the child’s parent or guardian (assent was also obtained from subjects eight years and older). Exclusion criteria were: previous neurological illness; learning disability; head trauma with loss of consciousness; current or past use of psychostimulant medication; pregnancy; birth at 37 weeks gestational age or earlier; or abnormal findings at a routine neurological examination performed by an experienced pediatric neurologist. All subjects were native monolingual English speakers. A complete age and gender breakdown of the subjects is detailed in Table 1. 287 of the subjects were right-handed, 23 were left-handed, and 3 were ambidextrous according to the Edinburgh (Oldfield, 1971) test for handedness. The racial/ethnic background of the subjects was: 279 Caucasian, 22 African-American, 2 Asian, 2 Hispanic, 1 Native American, 7 Multi-Ethnic. All subjects were pre-screened for any conditions (such as the presence of orthodontic braces) which would prevent an MRI scan from being acquired. All subjects received the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) or the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) and the Oral and Written Language Scales (OWLS) (Carrow-Woolfolk, 1996). Mean age = 11.9 ± 3.75 yrs. (range = 4.9 – 18.9 yrs.); Mean Wechsler Full-Scale IQ = 111.3 ± 14.1 (range = 70 – 147); Mean OWLS = 107.5 ± 14.1 (range = 66 – 151). Four subjects had a Full-Scale IQ < 80 and six subjects had an OWLS score < 80; they were not excluded from the study population as there was no documented history of learning disability, and the frequency of such findings was not greater than expected given the total sample size.
Table 1.
Age and gender breakdown of the study population (* = includes one girl 4 years 11 months).
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 7 | 8 | 10 | 15 | 10 | 10 | 18 | 16 | 16 | 9 | 10 | 9 | 11 | 3 |
| F | 7* | 11 | 16 | 11 | 13 | 10 | 9 | 10 | 21 | 10 | 11 | 10 | 11 | 11 |
MRI scans were obtained using a Bruker 3T Medspec (Bruker Medizintechnik, Karlsruhe, Germany) imaging system. An MRI-compatible audiovisual system was used for presentation of the stimuli as well as a movie during the preparation (e.g. shimming) and acquisition of the whole-brain anatomical scans. Details of the techniques used to obtain fMRI data from younger children, as well as the success rates, are given in (Byars et al., 2002). EPI-fMRI scan parameters were: TR/TE = 3000/38 ms; BW = 125 kHz; FOV = 25.6 X 25.6 cm; matrix = 64 X 64; slice thickness = 5 mm. Twenty-four slices were acquired, covering the entire cerebrum. 110 scans were acquired (the first 10 were discarded to allow the spins to reach relaxation equilibrium) for a total scan time of 5 min. 30 sec. Techniques detailed elsewhere (Byars et al., 2002) were used to acclimatize the subjects to the MRI procedure and render them comfortable inside the scanner. An elastic strap was attached to either side of the head coil apparatus by means of Velcro strips and stretched over the subjects’ foreheads in order to minimize head motion. In addition to the fMRI scans, whole-brain T1-weighted MP-RAGE scans were acquired for anatomical coregistration.
The fMRI scan paradigm consisted of a 30 second on-off block design. One story, read by an adult female speaker, was presented during each 30 s task period. (A complete transcript of one of the stories is given in Table 2.) Each story contained 9, 10, or 11 sentences of contrasting syntactic constructions (e.g. conjoined sentences vs. center embeddings). The inclusion of complex syntactic structures was designed to increase the relative processing load for this aspect of language. During each 30 s control period, pure tones of 1 s duration were presented at unequal intervals of 1 to 3 s. The frequency of each tone was randomly selected from a choice of 150, 200, 250, 500, 700, 900, or 1000 Hz. The control condition was designed to control for sublexical auditory processing. The subject was instructed to listen to the stories so that he or she could answer questions about them after the scans. Performance data were obtained at the end of the scanning session by asking the subject to answer two multiple choice questions about each story (transcript of questions for one of the stories is also given in Table 2). Data was processed using in-house software written in IDL (Research Systems Inc., Boulder, CO). Nyquist ghosts and geometric distortion due to B0 field inhomogeneity were corrected for during reconstruction using a multi-echo reference scan (Schmithorst et al., 2001). Data was corrected for subject motion using a pyramid iterative algorithm (Thevenaz and Unser, 1998); all datasets met the criterion of median voxel displacement at the center of the brain < 2 mm. The fMRI data was subsequently transformed into stereotaxic space (Talairach and Tournoux, 1988) using a linear affine transformation, previously validated for the age range in our study (Muzik et al., 2000; Wilke et al., 2002).
Table 2.
Transcript of one of the stories and questions used to test post-scan recall.
| Story | A frog lived under a flower in the garden.
One day, a little boy picked the pretty flower. So the frog, who missed his flower, went inside. “Where could my flower be?” thought the puzzled frog. A cat sat in the chair above the frog. The cat blinked his eyes and wiggled his ears. The frog was scared and hopped in a cup. The boy saw the frog hiding inside the cup. He took it outside for the frog’s new home. |
| Question 1 | Where did the frog live?
|
| Question 2 | What was the frog afraid of?
|
The subject-wise concatenation approach (Calhoun et al., 2001), shown to provide superior performance to other proposed methods (Schmithorst and Holland, 2004) was used for the group ICA analysis. As a pre-processing step, each voxel time course was normalized to a percent change from the mean. Data from each subject was reduced to 40 time points using Principal Component Analysis (PCA). The data was then concatenated across subjects and again reduced to 50 sources using PCA. Due to the very large dimensionality (40 sources/subject X 313 subjects = greater than 12,000 sources) an EM algorithm (Roweis, 1997) was used for the PCA reduction. The model order selection for the second dimensionality reduction was an empirical choice, based on previous group ICA analyses (Schmithorst, 2005; Schmithorst and Brown, 2004); the extremely large dimensionality rendered other methods such as analyzing the distribution of eigenvalues (Minka, 2000) computationally intractable.
After the PCA reductions, the FastICA (Hyvarinen, 1999) algorithm was used for the ICA decomposition. FastICA is a stochastic algorithm, possibly yielding different results at every run of the algorithm, as the algorithm may find different local optima depending on the initial conditions (Himberg et al., 2004). Hence the FastICA algorithm was run 25 times, with hierarchical agglomerative clustering (Himberg et al., 2004) being used to estimate the most reliable components. Group average-link was chosen as the choice of agglomeration strategy, shown previously to yield superior results to other choices such as single- or complete-link (Himberg et al., 2004). Since in general there is no clear choice for a quantitative index to estimate the number of clusters (e.g. (Bandyopadhay and Maulik, 2001; Maulik and Bandyopadhay, 2002)) the following heuristic choice was used: the total number of clusters were successively reduced (as more clusters were joined together) until the greatest number of elements in a cluster was greater than the number of repetitions of FastICA (in this case, 25). Clusters with less than 6 elements were then discarded as “unreliable” as the given component appeared in very few of the FastICA runs. Since the preceding criteria in fact resulted in 52 retained clusters (and thus 52 components), greater than the dimensionality of the reduced dataset, prior to further analysis the components were quasi-decorrelated in a symmetric fashion using a procedure analogous to symmetric decorrelation (Hyvarinen et al., 2001). Assuming the sources are grouped into a two-dimensional matrix S, with the horizontal dimension being the voxel dimension and the vertical dimension being the component dimension, symmetric quasi-orthogonalization was performed via repeating S until convergence (normalizing the rows of S after each iteration; the pseudoinverse being necessary since SST is not of full rank). That the components were “quasi-decorrelated” or decorrelated to the maximum extent possible was verified via a check of the Welch lower boundary (Welch, 1974). (A slightly different algorithm for quasi-orthogonalization than the one suggested in (Hyvarinen et al., 2001) was employed.)
Using the previously proposed method (Calhoun et al., 2001), individual IC maps were estimated for each subject. Due to the overcomplete basis set, however, it was necessary to incorporate a slight modification to the manner in which the subject time courses were estimated. Assuming M is the data from an individual subject, the time courses are contained in the columns of A = MS (SST )+. To determine task-related or transiently task-related components of interest, analyses were performed on the Fourier transforms of the time courses, as previously proposed for single-subject block-design paradigms (Moritz et al., 2003). The technique was extended to multi-subject analyses in the manner described below. A significant difference, however, is that the goal of the analysis is not to rank the components as in the single-subject case, but to test them for a significant Fourier component at the on-off task frequency (1 cycle/60 sec in this case).
As a preprocessing step all time courses were corrected for low-frequency drift via quadratic drift correction and then normalized to unity standard deviation. The average (complex) Fourier component at the task frequency for each component was tested for a significant difference (i.e. two-dimensional distance) from zero using a one-sample T-test, under the assumptions of near-normal distributions and equal variances in the real and imaginary directions. A threshold of T = 3.59, corresponding to p = 0.01/52 (p = 0.01 Bonferroni-corrected for the 52 components) was used as a threshold for significance. Since we are only interested, however, in the components which have greater activation during the active, i.e. stories, rather than the control task, a further criterion was employed. Only those components with an average Fourier component at the task frequency with an absolute phase difference of less than 60 degrees from the phase of the on-off task reference function (shifted by 3 s relative to the task itself to account for the hemodynamic delay) in addition to a significant component at the task frequency were deemed task-related. It should be noted that the absolute value of the Fourier components (or, equivalently, the values of the power spectra) are not suitable parameters to use for low-frequency block-design paradigms since temporal autocorrelation in the data will bias those parameters upwards. As an additional test (and validation of the Fourier analysis) the time courses were tested for greater intensities during the active versus the control phases; all components deemed significant using the Fourier criteria were also significant (paired T-test; Bonferroni-corrected p < 0.01).
For the six components deemed to be “task-related” using the above criteria, a one-sample T-test was performed on the individual IC maps on a voxelwise basis in order to determine cortical regions active in those components. Due to the large sample size a stringent criterion of T > 15 was used, corresponding to p < 1e-10, Bonferroni-corrected for multiple voxel comparisons. For reference, a standard GLM analysis (Worsley and Friston, 1995) was also performed using the on-off task reference function as the regressor of interest; a random-effects analysis was performed, using the same threshold as used for the IC maps.
In order to test for developmental changes, the following procedure, analogous to a GLM approach, was used on the associated time courses. (The scaling ambiguity involved with standard ICA precludes testing for age-related effects on the individual IC maps, as they do not represent absolute BOLD signal intensities.) Each individual time course (for a given component) was fit as a function of the on-off task reference function. The T-scores from the fit (which are scale-invariant) were then incorporated into a second-level analysis, and correlated with subject age (in months). A significant second-level correlation coefficient will imply a significant effect of age on the task-relatedness of the component. Since, however, most of the average time courses associated with task-related components (except for the one shown in Figure 1b) did not correlate that strongly with the task reference, the following alternative and more flexible data-driven approach was used as an additional test for age effects. The reference time course was allowed to vary, and optimized to result in the maximum slope of T-score (of fit with each individual time course) as a function of subject age. The optimization was performed using the downhill simplex method (Press et al., 1992). To regularize the optimization (e.g. to prevent the algorithm from converging to an exact match to the time course from the subject with the largest age, resulting in a huge T-score for that subject), an upper bound was set of T = 25 for the fit with each individual time course. The regularization corresponds to setting a lower bound on the variance of each associated time course; or, equivalently, to incorporating a Bayesian prior (e.g. zero prior probability of zero variance). The null distribution for the slope was found via Monte Carlo simulation, repeatedly performing the algorithm on random Gaussian noise of the same dimensionality. A significant difference in the slope from the null distribution would indicate a significant age effect. The data-driven reference time course was also tested for task-relatedness by correlating with the on-off task reference function. The difference between the two approaches is that the first method tests for significant associations between subject age and shape of the time courses, as determined by an a priori criterion (e.g. correlation with the task reference), while the second method finds a maximum-likelihood descriptor of any age effect present, and then analyzes it a posteriori.
Figure 1.

Six task-related independent components found from group ICA analysis of 313 children ages 5–18 performing the task of auditory narrative comprehension. Slice range: Z = −25 to +50 mm (Talairach coordinates). All images in radiologic orientation.
Results
Performance data for post-scan recall was available from 307 out of the 313 subjects scanned. 267 out of the 307 subjects (or 87% of the study population) answered at least 6 out of the 10 questions correctly (corresponding to p < 0.02 for responding at a chance level). No significant performance difference was seen between boys and girls (p > 0.39, chi-squared contingency test). However, significant effects for subject age (Spearman’s R = 0.28, p < 0.001), Full-Scale IQ (Spearman’s R = 0.26, p < 0.001), and OWLS scores (Spearman’s R = 0.27, p < 0.001) on performance were found.
The six task-related ICA components were ordered (leading to lagging) according to the phase of the average Fourier component relative to the reference on-off time course and are displayed in Figure 1. The average time courses associated with each component are plotted in Figure 2. All components, with the exception of the one displayed in Figure 1c, were found in all 25 of the ICA runs (e.g. the cluster size was 25). The component in Figure 1c, however, was detected in a cluster size of only 12, indicating a weaker strength relative to the other components. The ICA analysis (Figure 3) resulted only in the detection of the regions shown in Figure 1b, in addition to left posterior superior temporal gyrus (shown in Figure 1e) and bilateral superior frontal gyrus; the lack of complete agreement between ICA and GLM results for group analyses has been discussed previously (Schmithorst and Brown, 2004).
Figure 2.
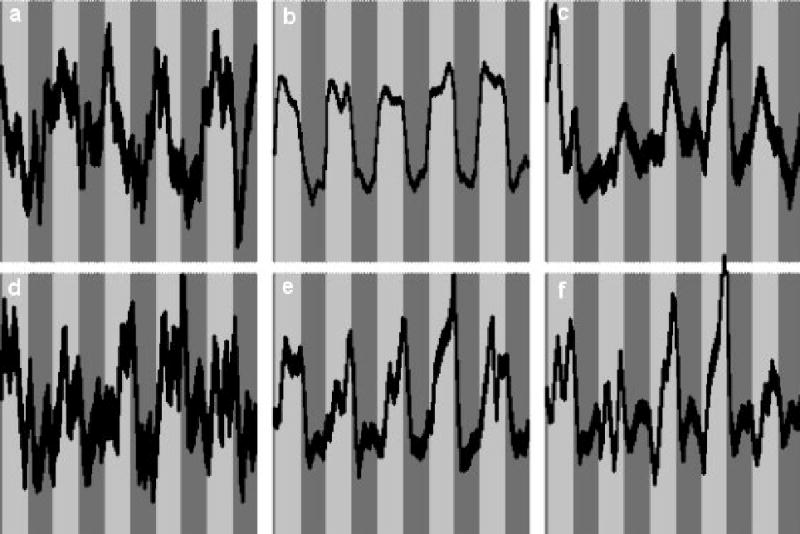
Associated time courses (bands ± 1 σ) for the independent components shown in Figure 1.
Figure 3.
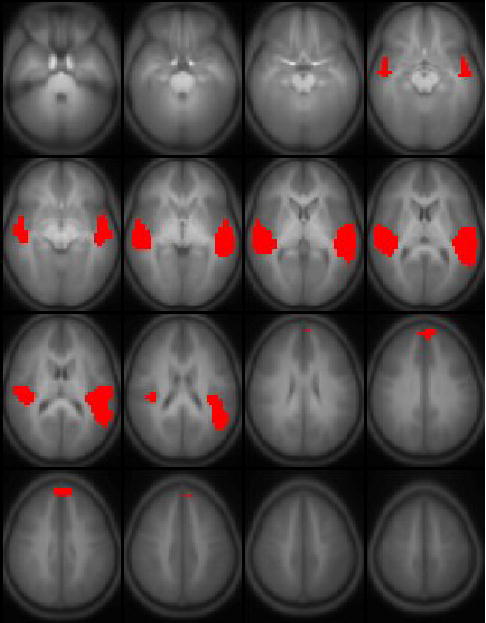
Results from a random-effects GLM analysis of 313 children ages 5–18 performing the task of auditory narrative comprehension. Slice range: Z = −25 to +50 mm (Talairach coordinates). All images in radiologic orientation.
From earliest to latest, relative to the task reference function, components were found with activation in primary auditory cortex bilaterally (Figure 1a), superior temporal gyrus bilaterally (Figure 1b), a left-lateralized network including the inferior parietal lobule and inferior frontal gyrus (Figure 1c), the hippocampus bilaterally (Figure 1d), the most posterior part of the superior temporal gyrus (Figure 1e), and a bilateral network involving the angular gyrus and the precuneus/posterior cingulate (Figure 1f). A summary of the activation foci for each component is listed in Table 3. The progression from leading to lagging the task reference function may be clearly visualized in the average associated time courses shown in Figure 2. All averaged time courses were correlated with the task on-off reference function with a correlation coefficient of R > 0.3 or greater (Table 4). The inter-subject variances of the ICA time courses were also computed and are listed in Table 5; the least variability is seen in the component in Figure 1b (superior temporal gyrus). Representative individual IC maps from a 5-year-old girl and a 14-year-old boy, for the components displayed in Figures 1b, 1e, and 1f, are displayed in Figure 4.
Table 3.
Activation foci (Talairach coordinates) for the ICA components displayed in Figure 1.
| Component | BA | Region | X, Y, Z |
|---|---|---|---|
| 1a | 42 | L. Superior Temporal Gyrus | −38, −21, 15 |
| 42 | R. Superior Temporal Gyrus | 46, −21, 20 | |
| 1b | 22 | L. Superior Temporal Gyrus | −54, −13, 5 |
| 22 | R. Superior Temporal Gyrus | 50, −17, 5 | |
| 1c | 21 | L. Medial Temporal Gyrus | −54, −33, 0 |
| 46 | L. Inferior Frontal Gyrus | −38, 43, 5 | |
| 44/45 | L. Inferior Frontal Gyrus | −42, 7, 30 | |
| 40 | L. Inferior Parietal Lobule | −50, −53, 30 | |
| 8 | L. Middle Frontal Gyrus | −6, 23, 45 | |
| 1d | L. Hippocampus | −26, −25, −5 | |
| R. Hippocampus | 22, −21, −5 | ||
| 1e | 22 | L. Superior Temporal Gyrus | −50, −49, 15 |
| 22 | R. Superior Temporal Gyrus | 46, −53, 10 | |
| 1f | 39 | L. Angular Gyrus | −46, −53, 25 |
| 39 | R. Angular Gyrus | 46, −49, 25 | |
| 7/31 | L. Precuneus/Posterior Cingulate | −10, −45, 30 |
Table 4.
Correlation coefficients of the average time courses shown in Figure 2 with the task reference function (shifted by 3 s to account for the hemodynamic delay).
| Component | R | Component | R | Component | R |
|---|---|---|---|---|---|
| 1a | 0.43 | 1b | 0.92 | 1c | 0.37 |
| 1d | 0.30 | 1e | 0.49 | 1f | 0.31 |
Table 5.
Inter-subject variability for each of the ICA time courses shown in Figure 2.
| Component | Inter-Subject Variability |
|---|---|
| 1a | 0.97 |
| 1b | 0.58 |
| 1c | 0.96 |
| 1d | 0.98 |
| 1e | 0.93 |
Figure 4.

Individual IC maps from a 5-year-old girl (top) and a 14-year-old boy (bottom) for the components displayed in Figure 1b (left), Figure 1e (middle), and Figure 1f (right). Colored voxels range from T = 6 (blue) to T = 10 (red). T-scores for the IC maps computed according to the method of (Schmithorst and Holland, 2005). All images in radiologic orientation.
For each group ICA component, laterality indices (LIs) were computed in a similar manner as used for previous fMRI language studies (e.g. (Binder et al., 1995; Holland et al., 2001)). The formula used was where L = the number of voxels in the left hemisphere with Z > 3, and R = number voxels in the right hemisphere with Z > 3. (All IC maps were scaled to unity variance). Results are displayed in Table 6. Using a criterion of |LI| > 0.1 to define laterality the components in Figures 1a and 1d are bilateral; the components in Figures 1b and 1f are somewhat left-lateralized; the component in Figure 1c is strongly left-lateralized; and the component in Figure 1e is somewhat right-lateralized.
Table 6.
Lateralization indices (LIs) computed for each of the components in Figure 1.
| Component | LI | Component | LI | Component | LI |
|---|---|---|---|---|---|
| 1a | −0.02 | 1b | 0.16 | 1c | 1.0 |
| 1d | 0.04 | 1e | −0.13 | 1f | 0.15 |
For the analysis of developmental trends using the a priori criterion of fit to the on-off task reference function, the component in Figure 1b showed a highly significant increase (Bonferroni-correcting for the multiple comparisons across the six components) of task-relatedness with age (R = 0.33, corrected p < 1e-7) while the components in Figure 1c (R = 0.124, uncorrected p < 0.03), Figure 1e (R = 0.15, uncorrected p < 0.01) and Figure 1f (R = −0.13, uncorrected p < 0.03) showed only nominally significant age-related effects, which would not retain significance after correcting for the multiple comparisons across the six components. Using the second method of evaluating a maximum-likelihood reference time course, the null distribution (found via the Monte Carlo simulation of repeatedly performing the algorithm on Gaussian noise) yielded a result of 0.0126 ± 0.00086. All components demonstrated a significantly greater slope of T-score with subject age when compared with the null distribution (Figure 1a, slope = 0.016, corrected p < 0.001; Figure 1b, slope = 0.0476, corrected p < 1e-6; Figure 1c, slope = 0.0178, corrected p < 1e-6; Figure 1d, slope = 0.015, corrected p < 0.01; Figure 1e, slope = 0.016, corrected p < 0.001; Figure 1f, slope = 0.017, corrected p < 1e-6), approximating the T-distribution (due to the very large number of degrees of freedom) as Gaussian. Thus, a significant age effect was shown for each component. Correlating the optimal age-related task reference function with the on-off task reference function, the components in Figure 1b (R = 0.91, corrected p < 1e-10), Figure 1c (R = 0.35, corrected p < 0.01), Figure 1e (R = 0.52, corrected p < 1e-6), and Figure 1f (R = −0.5, corrected p < 1e-6) all showed significant task-related and age-related effects, agreeing with the results of the first method (with greater sensitivity). The results indicate increasing use of the networks in Figures 1b (bilateral superior temporal gyrus), 1c (left-lateralized frontal-temporoparietal), and 1e (posterior aspect of STG), and decreasing use of the network in Figure 1f (angular gyrus and precuneus/posterior cingulate) with subject age.
Discussion
Spatial ICA reveals “chronoarchitectonically identified areas” (Bartels and Zeki, 2004) or functionally connected regions. The presence of different cortical regions in the same ICA component implies that they are active at the same time (subject to the limitations of the temporal resolution of the fMRI data acquisition); otherwise, the ICA algorithm would have separated them out into different components. ICA provides different information than other techniques such as diffusion tensor imaging (DTI), or functional connectivity maps based on resting-state fMRI data (Lowe et al., 1998). The existence of strong anatomical connections between two cortical regions is a necessary but not sufficient condition for them to appear in the same task-related ICA component; if a given cognitive task recruits only one of those regions then there will be a component separated by ICA only containing that region.
In addition, under certain minimal assumptions (e.g. (Calhoun et al., 2004b; Duann et al., 2002)) the spatial independence of the ICA components may be equated with their modularity, linking each ICA component found to a specific cognitive task. This is subject to the caveat that, due to the finite number of voxels, spatial independence of the components found cannot be assumed with absolute certainty. This limitation is likely not significant in the current study, however, due to the excellent signal-to-noise ratio provided by the very large number of subjects. ICA is not, however, able to reveal the precise cognitive correlates of the components found and thus should be viewed as a tool for either corroboration of prior hypotheses or generation of new ones.
The regions detected in prior PET studies of narrative comprehension (Fletcher et al., 1995; Mazoyer et al., 1993), namely, left superior temporal gyrus and posterior cingulate cortex, were detected in the current study via ICA analyses (Figures 1b, 1e, and 1f). However, no component was detected with activation in the temporal poles (BA 38), also detected in the above-mentioned PET studies. This may be due to the fact that a gradient-echo EPI sequence was used for the fMRI acquisitions, which causes a severe artifact to due magnetic susceptibility, especially at 3 Tesla, in the area of the temporal poles (Ojemann et al., 1997). Future studies may incorporate recently-developed passive shimming techniques (e.g. (Cusack et al., 2005; Wilson and Jezzard, 2003)) or susceptibility-compensated pulse sequences (e.g. (Gu et al., 2002; Heberlein and Hu, 2004)) in order to investigate the role of BA 38 via fMRI. Our results are also in line with those from a recent fMRI study of word, sentence, and narrative comprehension (Xu et al., 2005). While no activation was found in primary auditory cortex or classical Wernicke’s area, the study found activation for processing at the narrative level in perisylvian language areas, as well as additional areas including the hippocampus, angular gyrus, posterior temporal sulcus, and posterior cingulate/precuneus, in agreement with the regions of activation detected in our study displayed in Figures 1c-f.
The results from the study add some insight into several current topics of debate in the neural bases of language processing and comprehension. With the exception of the left-lateralized network displayed in Figure 1c, all components recruited identical cortical regions in the left and right hemispheres. Several recent brain imaging studies, investigating a variety of language processes, have shown an important role for the right hemisphere in language. Syntactic transformations were found to recruit bilateral regions in the posterior superior temporal sulcus (Ben-Shachar et al., 2003). A sentence comprehension task (Just et al., 1996) recruited Broca’s and Wernicke’s areas in the left hemisphere but also the right hemisphere homologues, albeit to a significantly lesser extent. Right peri-sylvian areas were shown to be recruited when subjects were asked to repair sentences with incorrect grammar (Meyer et al., 2000). The right hemisphere homologues were also found to be recruited in a word fluency task performed by normal children (Holland et al., 2001).
It has been suggested that nonautomatic methods of comprehension during language processing may draw upon right hemispheric structures (Meyer et al., 2000). Relevant to this study, right frontal regions showed increased activation when listening to sentence sets that were more “narrative” like than almost equivalent sentences that seemed conceptually unrelated. Lesion and neuroimaging studies have identified a critical role for the right hemisphere in narrative comprehension (Robertson et al., 2000). Subjects with unilateral right-hemisphere damage have shown impairments in abstracting information from narrative passages (Benowitz et al., 1990; Moya et al., 1986). Right temporal and prefrontal regions were recruited when subjects were asked to appreciate the moral of an Aesop’s fable (Nichelli et al., 1995). Bilateral activation was also detected in an earlier study (Huettner et al., 1989) investigating the reading of controlled narrative text.
The ICA results also support the hypothesis of a critical role for the right hemisphere areas in story processing. The bilaterality of most of the components supports the existence of a bilateral language representation with right hemispheric networks being functionally and anatomically tied to their homologues on the left hemisphere, as proposed previously (Huettner et al., 1989). The ICA results suggest that the typical left-hemisphere dominance is mainly due to the left-hemisphere ICA component shown in Figure 1c alone. Only a weak degree of left-hemisphere laterality was detected in the components shown in Figures 1b and 1f. Furthermore, the existence of a component with right-hemisphere laterality (Figure 1e) supports the intriguing possibility that there may in fact exist a right-hemispheric dominant network for higher-order language processing involved with speech and/or narrative comprehension, as hypothesized previously (Meyer et al., 2000). Patients with right-hemisphere damage were found to recall and justify anomalous elements of narratives as accurately as normal information (Wapner et al., 1981), different from normal controls. Alternatively, bilateral activation components may suggest parallel right and left language networks that provide redundancy and reserve capacity in the event of failure of one network due to injury.
On the other hand, merely because the homologous cortical regions in the two hemispheres are “chronoarchitectonically identified” does not imply that their functionality must be absolutely identical. The idea of between- and across-hemisphere “parallel processing streams” (Scott and Johnsrude, 2003) has been previously proposed for sound processing, and current research in a variety of areas is focused on describing neural processing being performed in parallel by distributed groups of connected neurons (e.g. (Mcclelland and Rogers, 2003)). The specialization of the left hemisphere for language has been thought to result from the relative specialization of the left hemisphere for temporal and the right hemisphere for spectral analysis (Zatorre and Belin, 2001; Zatorre et al., 2002). Thus the possibility exists that different aspects of information are extracted from the stories and processed on the different hemispheres, being integrated at a final (or penultimate) step. To achieve the maximum processing efficiency, the brain would need to be “fine-tuned” in such a manner as to have the two processes proceed at identical speeds, thus accounting for asymmetries between the right and left arcuate fasciculi (Buchel et al., 2004) as shown via a recent DTI study.
The ICA results corroborate a recent hypothesis about functional segregation in classical (“Wernicke’s”) language areas. A recent DTI tractography study () proposed that there exist two parallel pathways connecting temporal and frontal regions in the left hemisphere: a direct segment between Wernicke’s and Broca’s areas, and a novel, indirect pathway connecting temporal with parietal, and parietal with frontal regions. The DTI results corroborated the results of a previous lesion study (Mccarthy and Warrington, 1984) in which the authors proposed a two-route model of speech production: automatic word production recruiting the direct pathway, and a two-step process of verbal comprehension and semantic/phonological transcoding recruiting the indirect pathway. The DTI study postulated the functional separation of the region typically referred to as “Wernicke’s area” (comprising BA 22, 37, 39, 40) into posterior temporal and inferior parietal areas (which the authors referred to as “Geschwind’s territory”). The ICA results, as shown in the different components seen in Figures 1b, 1e, and 1f, not only support the hypothesis of functional separation between posterior temporal and inferior parietal regions, but also between a medial and a posterior aspect of BA 22, agreeing with previous reports of functional heterogeneity in the planum temporale (Scott and Johnsrude, 2003), with the anterior aspects recruited for phonologic tasks and the posterior aspects recruited for semantic processing (Cannestra et al., 2000).
In reference to the cognitive correlates of the components found, we are able to hypothesize the following. The activation in Figure 1a is centered over the primary auditory cortex, suggesting this first component represents initial cortical processing of acoustic information. The bilateral STG activation (Figure 1b) displays the highest correlation with the on-off task reference function (Figure 2b). Activation of the STG has been previously reported in studies of processing of semantic anomalies (Friederici et al., 2003; Kuperberg et al., 2000; Ni et al., 2000). However, the idea that access to word meaning is contained in classical Wernicke’s area has been challenged (e.g. (Scott et al., 2000)) and the alternative hypothesis proposed that the STG is primarily involved with spectral and temporal processing of auditory input, with the information then projected to amodal higher-order association cortex. In support of this view, STG activation was not found for semantic violations when subjects read, rather than listened to, sentences with semantic anomalies (Newman et al., 2001). In addition, differential activation was not found in the STG when subjects listened to words vs. pseudowords, or words vs. reversed speech (Binder et al., 2000). A very strong age-related increase was seen for this component. Since the stories only used vocabulary at the level of very young children, our data seems to be more consistent with the hypothesis of the STG being involved with spectral and temporal processing (since children’s proficiency would be expected to increase with age), rather than access to word meaning.
With regard to the left-lateralized network including Broca’s area (Figure 1c), the regions at the origin and terminal ends of the arcuate fasciculus in the left hemisphere (Broca’s area and inferior parietal lobule) may be clearly visualized (Figure 3), together with an additional frontal region (BA 46). One possible interpretation is that this network is involved in covert speech generation (all subjects were instructed to remain silent during the complete duration of the scan). However, the only consistent similarity with activation patterns observed in other verbal fluency experiments conducted on children (e.g. (Gaillard et al., 2000; Holland et al., 2001)) is in Broca’s area. The left-hemisphere network may also be related to syntactic processing at the sentence level. Previous results (e.g. (Hashimoto and Sakai, 2002; Sakai et al., 2002)) have indicated a left-hemisphere specialization for sentence comprehension and processing, involving BA 44/45 and BA 8. However, a distinction has been made between explicit and implicit syntactic processing (Sakai et al., 2003), with BA 44/45 only recruited for explicit syntactic decisions. Moreover, judging by the relative weakness of this component (it was detected in only 12 of the ICA runs) it is likely present in only a small subset of the subjects, although the possible interpretation that the component is present in many subjects with much weaker intensity cannot be ruled out.
Hence we propose an alternative explanation for the functionality of this network. In contradiction to the classical Wernicke-Geschwind model, a recent evoked-potential study (Matsumoto et al., 2004) has shown bi-directional connectivity between Broca’s and Wernicke’s areas, and between Broca’s area and the inferior parietal lobule. A pathway from Broca’s area to the inferior parietal lobule has also been proposed using path analysis in a study of semantic decision and subvocal rehearsal (Bullmore et al., 2000). Thus, using the proposed indirect pathway () syntactic information is transmitted from Broca’s area to the inferior parietal lobule, and with BA 46 (also seen in this component) used for monitoring and manipulation in working memory (Braver et al., 2001). This hypothesis is in line with recent “connectionist” models of language processing (Mcclelland and Rogers, 2003; Nadeau, 2001), which argue against the conception of language processing as a one-way serial transfer of information between highly localized cortical centers specialized for a specific function; and also with a recently proposed dynamic model for interaction between semantic and syntactic processing (Sakai et al., 2003).
As for the function of the IFG itself, while the left IFG has often been related to syntactic processing (e.g. (Caplan et al., 2000; Inui et al., 1998; Just et al., 1996; Stromswold et al., 1996)), more recent studies have postulated that this area may be related more to syntactic working memory rather than syntactic complexity (Fiebach et al., 2001; Fiebach et al., 2005); during the processing of a syntactically complex sentence the displaced element must be maintained in working memory over a prolonged distance (Friederici et al., 2003). While it has previously been proposed that dislocated elements in sentence processing are maintained as semantic representations (Cooke et al., 2002) without a phonologic element, that study displayed activation in BA 47, associated with semantic processing (Bookheimer, 2002), as opposed to the BA 44/45 seen in the current study. The region of Broca’s area (Figure 1c) recruited in the current study is the more posterior aspect, thought to be involved with phonological/syntactic, rather than semantic, processing (Cannestra et al., 2000). Thus, we hypothesize that this left-lateralized network may be related to maintenance in working memory of syntactic representations of the sentences as well as syntactic/semantic transcoding and interactions. This explanation would also be more consistent with the age-related increase seen in this component; the stories incorporated sentences with complex syntactic structures, for which older children would be expected to display more maturation in the necessary neural processing circuitry.
The posterior STG (Figure 1e) has been previously proposed (Friederici et al., 2003) as being recruited for non-domain-specific integrative processes, with increased effort being involved in incorporating anomalous structures into sentences. The delayed rise in the hemodynamic response (Figure 2e) supports the interpretation that the posterior STG may be involved in higher-order integrative processes, as the process of integrating the content of the sentences into the larger narrative will not begin until a certain period of time after the beginning of the story. It was also proposed (Friederici et al., 2003) that the posterior STG maps different types of information (semantic, syntactic, and pragmatic) onto each other in order to achieve a final interpretation. The age-related increase in this component is consistent with this hypothesis. A significant correlation was shown between the post-scan recall and subject age, and this is likely related to children’s increasing ability and proficiency with age to integrate various pieces of information into a coherent whole.
We propose that the bilateral angular gyrus (Figure 1f) is involved in higher-order semantic processing (Price, 2000). Various evidence suggests that the angular gyrus is involved in semantic processing, including lesion (e.g. (Hart and Gordon, 1990)) and neuroimaging studies (e.g. (Nakai et al., 1999; Newman et al., 2001)). The angular gyrus may also be of importance developmentally; decreased activation in the left angular gyrus was shown in a cohort of children with early lead exposure relative to normal controls in a verbal fluency task (Sohn et al., 2004). While some studies only implicate the left angular gyrus (e.g. in a study of dyslexia (Horwitz et al., 1998)), bilateral activation in the angular gyrus was seen in a study involving semantic violations in sentences (Newman et al., 2001). One possible explanation is that the left angular gyrus is recruited for semantic processing at the “word” level, while higher-order semantic processing recruits the angular gyri bilaterally, although further research will be necessary to confirm this hypothesis. Further investigation is also warranted of the hypothesis that the functional distinction between different parts of “Wernicke’s area”; namely, the posterior superior temporal gyrus (BA 22, in its most posterior aspect), and the inferior parietal lobule (BA 39/40) consists of integration vs. semantic processing. The bilateral network containing the angular gyri also recruits the anterior medial parietal (precuneus/posterior cingulate) cortex, which has been associated with linking new information with prior knowledge in a previous PET study of story processing (Maguire et al., 1999). Interestingly, our data showed an age-related decrease in the use of this component. One possible explanation is that this finding is related to practice effects; functional activation for higher-order motor tasks has been found to negatively correlate with sustained practice (e.g. (Meister et al., 2005)). The finding of an age-related decrease may also be related to effort; the relative complexity and difficulty of semantic processing for the simple stories used would be expected to decrease with subject age.
As for the activation seen bilaterally in the hippocampus (Figure 1d), while there is a clear correlation with the task reference function (Figure 2d), there is also a large amount of inter-subject variability. This is in line with the assumed role of the hippocampus in episodic memory (Deweer et al., 2001), as a substantial inter-subject variability would be expected. A key role for the left hippocampus in retention of story material has been previously reported (Frisk and Milner, 1990). The hippocampus has also been implicated in working memory (Cabeza et al., 2002; Ranganath and D’esposito, 2001), as well as a transitional function for long-term memory (Kato et al., 1998). A recent study (Zarahn et al., 2005) has challenged the role of the hippocampus for working memory maintenance of phonologically codable stimuli, in line with the hypothesized role of Broca’s area in syntactic working memory (Fiebach et al., 2005). Thus, the hippocampus is likely involved with active maintenance of the semantic content of the narratives, as well as a possible transitional role for long-term memory encoding. While a significant age effect was shown via the data-driven analysis, no statistically significant correlation of the maximum-likelihood age-related data-driven reference time course with the on-off task reference function was found. This is perhaps due to the fact that long-term memory encoding might be occurring during the control epochs as well as during the presentation of the stories, especially in the older subjects, eliminating much of the age-related effects from the data.
A novel aspect of the current study is the data-driven investigation of developmental changes in the IC components. (A previously developed technique for between-group ICA analyses (Calhoun et al., 2004a) does not provide a ready extension to regression analyses.) The advantage of ICA, a data-driven approach, lies in its ability to mine more information from the data than is possible via conventional hypothesis-driven analyses (e.g. (Schmithorst and Brown, 2004)). Thus, a natural extension is to also use a data-driven analysis on the associated time courses. With ICA, maximum-likelihood (ML) or maximum a posteriori (MAP) estimates of the sources and time courses are found and then analyzed post hoc for significance and task-relatedness. In a similar fashion, an ML descriptor of age effects was found from the associated time courses, which was also analyzed post hoc both for significance and for task-relatedness. The data-driven method resulted in much greater sensitivity then the hypothesis-driven method of correlating the associated time courses with the on-off task reference function, and illustrates the feasibility of the method for studies with much smaller sample sizes. Covariates may also be readily incorporated by maximizing the parameter estimate of a multivariate or stepwise regression instead of a simple linear regression. The analysis technique differs from a previously developed technique for correlations of ICA results with covariates of interest (Schmithorst and Holland, 2005) in that the task-relatedness of the components, rather than merely their spatial intensities (which may or may not be related to the active task) are analyzed.
Future analyses will provide additional information on the developmental trajectories of various elements of narrative comprehension, including possible gender differences and associations of task performance (e.g. post-scan recall) with the neuronal correlates of memory, syntactic processing, higher-order integrative processes, or other cognitive elements. Future large-scale DTI studies will provide additional insight regarding the development of the relevant neuronal connections and their relevance to language function; previous studies have found increased anisotropy with age in white matter association areas (Schmithorst et al., 2002) and a correlation between frontal white matter anisotropy and IQ (Schmithorst et al., 2005).
Newer, more sophisticated fMRI analysis techniques such as structural equation modeling (SEM) (Solodkin et al., 2004) or path analysis (Bullmore et al., 2000) may further help resolve unanswered questions regarding information flow in the brain. The ICA results may be used to generate a theoretical (predictive) model for path analysis, the validity of which may then be tested with the procedure. We also note that the control condition, designed to cancel out cortical regions related to primary auditory-related processing, is not strictly necessary for an ICA analysis, as the algorithm will separate out the cortical regions related to different cognitive components. However, failure to include the control period limits the interpretability of results obtained using hypothesis-driven techniques such as GLM analyses (Worsley and Friston, 1995), which may be desirable as a complementary analysis strategy to data-driven approaches such as ICA.
Conclusion
A large-scale fMRI study of auditory narrative comprehension was conducted on a group of over 300 children ages 5–18. Using group ICA analysis, separate task-related networks were detected, including the primary auditory cortex bilaterally, mid-superior temporal gyrus bilaterally, hippocampus bilaterally, a left-lateralized network including Broca’s area and the left inferior parietal lobule, a slightly right-lateralized network involving the posterior superior temporal gyri, and a bilateral network involving the angular gyri and the medial inferior parietal lobe (precuneus/posterior cingulate). Task-related activity was shown to increase with age in the networks involving the superior temporal gyri and the left-lateralized network involving Broca’s area, and shown to decrease with age in the network involving the angular gyri. The results support recent hypotheses regarding the functional parcellation of Wernicke’s area, and the key role of the right hemisphere in narrative comprehension. The results support the role of the posterior aspect of BA 22 in narrative comprehension, involving non-domain-specific integration in order to achieve a final interpretation, and its continued development throughout childhood and adolescence.
Figure 5.

Sagittal view of the IC map shown in Figure 1c. Slice range: X = −37 mm (Talairach coordinates).
Acknowledgments
This work was supported by a grant from the U.S. National Institute of Child Health and Human Development, #R01-HD38578. The authors acknowledge the assistance of Dr. Anna Byars, Ph.D., in the administration of the Wechsler Full-Scale IQ tests; and of Drs. Richard Strawsburg, M.D., and Mark Schapiro, M.D., for performing the neurological examinations.
References
- Bandyopadhay S, Maulik U. Nonparametric genetic clustering: comparison of validity indices. IEEE Trans Syst Man Cybern Part C Appl Rev. 2001;31:120–125. [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the human brain--natural viewing conditions reveal a time-based anatomy of the brain. Neuroimage. 2004;22:419–33. doi: 10.1016/j.neuroimage.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Barthes, R., 1981. Introduction to the structural analysis of narratives. In: Sontag, S. (Eds.), A barthes reader. Hill and Wang, New York, pp. 251–295.
- Benowitz LI, Moya KL, Levine DN. Impaired verbal reasoning and constructional apraxia in subjects with right hemisphere damage. Neuropsychologia. 1990;28:231–41. doi: 10.1016/0028-3932(90)90017-i. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations: evidence from fMRI. Psychological Science. 2003;14:433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–28. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, Jesmanowicz A, Hyde JS. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14:945–51. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T. How good is good enough in path analysis of fMRI data? Neuroimage. 2000;11:289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, Plante E. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17:885–90. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–30. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pekar JJ. A method for comparing group fMRI data using independent component analysis: application to visual, motor and visuomotor tasks. Magn Reson Imaging. 2004a;22:1181–91. doi: 10.1016/j.mri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, Pearlson GD. Alcohol intoxication effects on simulated driving: exploring alcohol-dose effects on brain activation using functional MRI. Neuropsychopharmacology. 2004b;29:2097–17. doi: 10.1038/sj.npp.1300543. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Bookheimer SY, Pouratian N, O’Farrell A, Sicotte N, Martin NA, Becker D, Rubino G, Toga AW. Temporal and topographical characterization of language cortices using intraoperative optical intrinsic signals. Neuroimage. 2000;12:41–54. doi: 10.1006/nimg.2000.0597. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G, Olivieri A. Activation of Broca’s area by syntactic processing under conditions of concurrent articulation. Hum Brain Mapp. 2000;9:65–71. doi: 10.1002/(SICI)1097-0193(200002)9:2<65::AID-HBM1>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow-Woolfolk, E., 1996. Oral and Written Language Scales. American Guidance Service, Circle Pines, MN.
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, Gee J, Pinango M, Balogh J, Grossman M. Neural basis for sentence comprehension: grammatical and short-term memory components. Hum Brain Mapp. 2002;15:80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack R, Russell B, Cox SM, De Panfilis C, Schwarzbauer C, Ansorge R. An evaluation of the use of passive shimming to improve frontal sensitivity in fMRI. Neuroimage. 2005;24:82–91. doi: 10.1016/j.neuroimage.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Deweer B, Pillon B, Pochon JB, Dubois B. Is the HM story only a “remote memory”? Some facts about hippocampus and memory in humans. Behav Brain Res. 2001;127:209–24. doi: 10.1016/s0166-4328(01)00366-7. [DOI] [PubMed] [Google Scholar]
- Duann JR, Jung TP, Kuo WJ, Yeh TC, Makeig S, Hsieh JC, Sejnowski TJ. Single-trial variability in event-related BOLD signals. Neuroimage. 2002;15:823–35. doi: 10.1006/nimg.2001.1049. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Friederici AD. Syntactic working memory and the establishment of filler-gap dependencies: insights from ERPs and fMRI. J Psycholinguist Res. 2001;30:321–38. doi: 10.1023/a:1010447102554. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–7. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Frisk V, Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia. 1990;28:349–59. doi: 10.1016/0028-3932(90)90061-r. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds - a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Morton J, Leslie AM. The cognitive basis of a biological disorder: autism. Trends Neurosci. 1991;14:433–8. doi: 10.1016/0166-2236(91)90041-r. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Goldsmith HH, Robertson RRW. Do readers mentally represent characters’ emotional states? Cognition and Emotion. 1992;6:89–111. doi: 10.1080/02699939208411061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graesser AC, Hauft-Smith K, Cohen AD, Pyles AD. Advanced outlines, familiarity, and text genre on retention of prose. Journal of Experimental Education. 1980;48:281–290. [Google Scholar]
- Gu H, Feng H, Zhan W, Xu S, Silbersweig DA, Stern E, Yang Y. Single-shot interleaved z-shim EPI with optimized compensation for signal losses due to susceptibility-induced field inhomogeneity at 3 T. Neuroimage. 2002;17:1358–64. doi: 10.1006/nimg.2002.1274. [DOI] [PubMed] [Google Scholar]
- Happe F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R, Frith C. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27:226–31. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. Specialization in the left prefrontal cortex for sentence comprehension. Neuron. 2002;35:589–97. doi: 10.1016/s0896-6273(02)00788-2. [DOI] [PubMed] [Google Scholar]
- Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magn Reson Med. 2004;51:212–6. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–22. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI Brain Activation Patterns in Children Performing a Verb Generation Task. Neuroimage. 2001;14:837–43. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci U S A. 1998;95:8939–44. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner MI, Rosenthal BL, Hynd GW. Regional cerebral blood flow (rCBF) in normal readers: bilateral activation with narrative text. Arch Clin Neuropsychol. 1989;4:71–8. [PubMed] [Google Scholar]
- Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Networks. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Hyvarinen, A., Karhunen, J., Oja, E., 2001. Independent Component Analysis. John Wiley & Sons, New York.
- Inui T, Otsu Y, Tanaka S, Okada T, Nishizawa S, Konishi J. A functional MRI analysis of comprehension processes of Japanese sentences. Neuroreport. 1998;9:3325–8. doi: 10.1097/00001756-199810050-00032. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–6. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kato T, Erhard P, Takayama Y, Strupp J, Le TH, Ogawa S, Ugurbil K. Human hippocampal long-term sustained response during word memory processing. Neuroreport. 1998;9:1041–7. doi: 10.1097/00001756-199804200-00016. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright IC, Lythgoe DJ, Williams SC, David AS. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci. 2000;12:321–41. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Lorch EP, Milich R, Sanchez RP. Story comprehension in children with ADHD. Clin Child Fam Psychol Rev. 1998;1:163–78. doi: 10.1023/a:1022602814828. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Morris RG. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122 ( Pt 10):1839–50. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neuropsychology of narrative: story comprehension, story production and their interrelation. Neuropsychologia. 2004;42:1414–34. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–30. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Maulik U, Bandyopadhay S. Performance evaluation on some clustering algorithms and validity indices. IEEE Trans on Pattern Analysis and Machine Intelligence. 2002;24:1650–1654. [Google Scholar]
- Mazoyer B, Tzourio N, Frak V, Syrota A, Muruyama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J. The cortical representation of speech. J Cogn Neurosci. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK. A two-route model of speech production. Evidence from aphasia Brain. 1984;107 ( Pt 2):463–85. doi: 10.1093/brain/107.2.463. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 2003;4:310–22. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–88. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister I, Krings T, Foltys H, Boroojerdi B, Muller M, Topper R, Thron A. Effects of long-term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks: Implications for cortical motor organization. Hum Brain Mapp. 2005;25:345–352. doi: 10.1002/hbm.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY. Neurocognition of auditory sentence comprehension: event related fMRI reveals sensitivity to syntactic violations and task demands. Brain Res Cogn Brain Res. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Minka TP. Automatic Choice of Dimensionality for PCA. NIPS. 2000;13:598–604. [Google Scholar]
- Moritz CH, Rogers BP, Meyerand ME. Power spectrum ranked independent component analysis of a periodic fMRI complex motor paradigm. Hum Brain Mapp. 2003;18:111–22. doi: 10.1002/hbm.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya KL, Benowitz LI, Levine DN, Finklestein S. Covariant defects in visuospatial abilities and recall of verbal narrative after right hemisphere stroke. Cortex. 1986;22:381–97. doi: 10.1016/s0010-9452(86)80003-x. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12:538–49. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Nadeau SE. Phonology: a review and proposals from a connectionist perspective. Brain Lang. 2001;79:511–79. doi: 10.1006/brln.2001.2566. [DOI] [PubMed] [Google Scholar]
- Nakai T, Matsuo K, Kato C, Matsuzawa M, Okada T, Glover GH, Moriya T, nui T. A functional magnetic resonance imaging study of listening comprehension of languages in human at 3 tesla-comprehension level and activation of the language areas. Neurosci Lett. 1999;263:33–6. doi: 10.1016/s0304-3940(99)00103-2. [DOI] [PubMed] [Google Scholar]
- Newman AJ, Pancheva R, Ozawa K, Neville HJ, Ullman MT. An event-related fMRI study of syntactic and semantic violations. J Psycholinguist Res. 2001;30:339–64. doi: 10.1023/a:1010499119393. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, Shaywitz BA, Gore JC, Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci. 2000;12:120–33. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R. Where the brain appreciates the moral of a story. Neuroreport. 1995;6:2309–13. doi: 10.1097/00001756-199511270-00010. [DOI] [PubMed] [Google Scholar]
- Oatley, K., 1992. Best laid plans: the psychology of emotion. Cambridge University Press, Cambridge.
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–67. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peterson, J. B., 1999. Maps of meaning: the architecture of belief. Routledge, New York.
- Press, W. H., Teukolsky, S. A., Vetterling, W. T., Flannery, B. P., 1992. Numerical Recipes in C: The Art of Scientific Computing. Cambridge University Press, Cambridge, England.
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat 197 Pt. 2000;3:335–59. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–73. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Robertson DA, Gernsbacher MA, Guidotti SJ, Robertson RRW, rwin W, Mock BJ, Campana ME. Functional neuroanatomy of the cognitive process of mapping during discourse comprehension. Psychological Science. 2000;11:255–260. doi: 10.1111/1467-9280.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roweis S. EM algorithms for PCA and SPCA. Neural Information Processing Systems. 1997;10:626–632. [Google Scholar]
- Sakai KL, Homae F, Hashimoto R. Sentence processing is uniquely human. Neurosci Res. 2003;46:273–9. doi: 10.1016/s0168-0102(03)00122-6. [DOI] [PubMed] [Google Scholar]
- Sakai KL, Noguchi Y, Takeuchi T, Watanabe E. Selective priming of syntactic processing by event-related transcranial magnetic stimulation of Broca’s area. Neuron. 2002;35:1177–82. doi: 10.1016/s0896-6273(02)00873-5. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ. Separate Cortical Networks Involved in Music Perception: Preliminary Functional MRI Evidence for Modularity of Music Processing. Neuroimage. 2005;25:444–451. doi: 10.1016/j.neuroimage.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Brown RD. Empirical Validation of the Triple-Code Model of Numerical Processing for Complex Math Operations Using Functional MRI and Group Independent Component Analysis of the Mental Addition and Subtraction of Fractions. Neuroimage. 2004;22:1414–1420. doi: 10.1016/j.neuroimage.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–9. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Comparison of Three Methods for Generating Group Statistical Inferences from Independent Component Analysis of fMRI Data. J Magn Reson Imaging. 2004;19:365–368. doi: 10.1002/jmri.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst, V. J., Holland, S. K., 2005. A method for generating voxelwise between-groups statistical inferences from fMRI data using Independent Component Analysis. In: Proceedings of the ISMRM 13th Scientific Meeting.
- Schmithorst, V. J., Wilke, M., Dardzinski, B. J., Holland, S. K., 2005. Cognitive Functions Correlate with White Matter Architecture in a Normal Pediatric Population: A Diffusion Tensor MR Imaging Study. Human Brain Mapping In Press. [DOI] [PMC free article] [PubMed]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of White Matter Diffusivity and Anisotropy Changes with Age During Childhood: A Cross-Sectional Diffusion Tensor Imaging Study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123 Pt. 2000;12:2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–7. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Sohn, J. H., Cecil, K. M., Dunn, R. S., Schmithorst, V. J., Holland, S. K., 2004. Functional loss of angular gyrus in adolescents with early exposure to lead performing a verb generation task: An fMRI study. In: Proceedings of the Human Brain Mapping Annual Meeting.
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14:1246–55. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S. Localization of syntactic comprehension by positron emission tomography. Brain Lang. 1996;52:452–73. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- Talairach, J., Tournoux, P., 1988. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, New York.
- Thevenaz P, Unser M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Transactions on Image Processing. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Trabasso, T., Stein, N. L., 1997. Narrating, representing, and remembering event sequences. In: van den Broek, P. W., Bauer, P. J., Bourg, T. (Eds.), Developmental spans in event comprehension and representation: Bridging fictional and actual events. Erlbaum, Mahwah, NJ, pp. 237–270.
- van den Broek, P. W., 1997. Discovering the cement of the universe: The development of event comprehension from childhood to adulthood. In: van den Broek, P. W., Bauer, P. J., Bourg, T. (Eds.), Developmental spans in event comprehension and representation: Bridging fictional and actual events. Erlbaum, Mahwah, NJ, pp. 321–342.
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–81. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Wapner W, Hamby S, Gardner H. The role of the right hemisphere in the apprehension of complex linguistic materials. Brain Lang. 1981;14:15–33. doi: 10.1016/0093-934x(81)90061-4. [DOI] [PubMed] [Google Scholar]
- Welch LR. Lower bounds on the maximum cross correlation of signals. IEEE Trans Inform Theory. 1974;20:397–399. [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Human Brain Mapping. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JL, Jezzard P. Utilization of an intra-oral diamagnetic passive shim in functional MRI of the inferior frontal cortex. Magn Reson Med. 2003;50:1089–94. doi: 10.1002/mrm.10626. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Positive Evidence against Human Hippocampal Involvement in Working Memory Maintenance of Familiar Stimuli. Cereb Cortex. 2005;15:303–316. doi: 10.1093/cercor/bhh132. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11:946–53. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]


