Abstract
Memories can have different strengths, largely dependent on the intensity of reinforcers encountered. The relationship between reinforcement and memory strength is evident in asymptotic memory curves, with the level of the asymptote related to the intensity of the reinforcer. Although this is likely a fundamental property of memory formation, relatively little is known of how memory strength is determined. Memory performance at different levels in Drosophila can be measured in an operant heat-box conditioning paradigm. In this spatial learning paradigm, flies learn and remember to avoid one-half of a dark chamber associated with a temperature outside of the preferred range. The reinforcement temperature has a strong effect on the level of learning in wild-type flies, with higher temperatures inducing stronger memories. Additionally, two mutations alter memory-acquisition curves, either changing acquisition rate or asymptotic memory level. The rutabaga mutation, affecting a type-1 adenylyl cyclase, decreases the acquisition rate. In contrast, the white mutation, modifying an ABC transporter, limits asymptotic memory. The white mutation does not negatively affect classical olfactory conditioning but actually improves performance at low reinforcement levels. Thus, memory acquisition/memory strength and classical olfactory/operant spatial memories can be genetically dissociated. A conceptual model of operant conditioning and the levels at which rutabaga and white influence conditioning is proposed.
Memories are formed from unexpected experiences. Eventually, a sensory cue or behavior predicts positive or negative consequences. Importantly, memory strength depends on the intensity of those reinforcing stimuli such that weak reinforcers support memories of limited magnitude. This relationship is evident in asymptotic acquisition curves where the asymptote level is related to the intensity of the reinforcer. This has been found in operant and classical conditioning paradigms with both positive and negative reinforcers in many species (Herrnstein 1997), including several species of insects (e.g., in rewarded classical and operant conditioning in the honeybee and negatively associated olfactory classical conditioning in Drosophila) (Menzel and Erber 1972; Bitterman et al. 1983; Tully and Quinn 1985; Loo and Bitterman 1992). The repeated finding of the positive relationship between reinforcement intensity and memory strength indicates that this is a fundamental property of learning.
The biogenic amines (e.g., serotonin, dopamine, and octopamine) can function as teaching signals. These are the molecules that, together with sensory-based depolarization, feed into the cAMP/PKA and NMDA-receptor pathways. The biochemical changes in this pathway support synaptic plasticity and memory formation. In the Aplysia model of heterosynaptic plasticity, serotonin mediates the tail shock and is a sufficient teaching signal with in vitro synaptic plasticity tests (Martin et al. 1997; Kandel 2001). Dopamine is also critical in memory formation. For example, reducing dopamine levels in parts of the brain in rats trained to run down an alley to receive a food reward leads to decreased running speed toward that food goal (Ikemoto and Panksepp 1996). As the treated rats eat as much food in the goal box as nontreated animals, however, it is unlikely that the dopaminergic system is involved in the valuation of that food reward. Perhaps dopamine coordinates the reinforcement value of the food with behavior. Clues for how this reward signal might be encoded comes from recordings in nonhuman primates (Hollerman and Schultz 1998; Waelti et al. 2001; Montague et al. 2004; Schultz 2004). Generally speaking, in trained monkeys, dopaminergic neuron firing rate increases when a reward is more than expected and decreases when a reward is less than expected. This phasic dopaminergic signal is providing at least a component of the value code that is used in coordinating the level of expression of a learned behavior (Montague et al. 2004). There is an analogous role of the biogenic amines in reinforcement in insects. In the honeybee and Drosophila, octopamine is important for positive, and dopamine (so far only shown in Drosophila) for negative associations (Hammer and Menzel 1998; Menzel et al. 1999; Schwaerzel et al. 2003). Despite the well-accepted notion that the biogenic amines are involved in reinforcement, little is known of the circuits that feed into or out of this system or how the teaching neurons regulate the graded release of neurotransmitter.
A fast operant learning paradigm, the heat-box, lends itself to a genetic dissection of the reinforcement intensity/memory strength relationship in Drosophila (Wustmann and Heisenberg 1997; Putz and Heisenberg 2002). In this paradigm, single flies are allowed to run freely in a dark chamber that is lined top and bottom with heating elements (Fig. 1). When a fly runs to one-half of the chamber, the whole chamber heats to an elevated temperature, and when it runs back, the chamber cools down again. Flies can be trained within minutes to avoid one-half of the chamber. Trained flies continue to avoid that chamber half even in the absence of the elevated temperature contingency. Flies presumably use idiothetic information for orientation and avoidance of the chamber half associated with high-temperature reinforcement. Both the duration of training and the temperature used as reinforcement can be readily manipulated in this paradigm, providing ideal conditions for investigating the relation of reinforcement intensity and memory strength in a genetically tractable organism.
Figure 1.
Schematic of the heat-box learning assay. In the heat-box learning paradigm, single flies run inside a chamber that can be heated (A). During a pre-test, flies can freely run from side to side with no danger of increasing temperature. The situation changes in the training session such that when a fly runs into one of the chamber halves (the right half in the example provided here), the chamber temperature rises, and when the fly returns to the previous side, the chamber cools. A memory posttest (the chamber is now permanently cool) shows the persistence in avoidance of the chamber half associated with an elevated temperature. The change in time spent in one chamber half over the other is used as a quantitative measure of memory formation (Wustmann and Heisenberg 1997; Putz and Heisenberg 2002). On average, wild-type flies typically have little to no spontaneous preference, as indicated in the pre-test phase in the sample experiment (B). During training, flies spend more and more time on the side of the chamber not associated with high temperature, evident in higher PIs through the training period. In the memory test, flies continue to avoid the previously reinforcement-associated side of the chamber. This memory is typically measured for 3 min. The bars in B represent means, and the error bars are SEMs in this and following figures.
In the present study, it is shown that wild-type flies have memory strength that is influenced by the reinforcement temperature. Also, mutations in two genes that show little or no memory formation under screening conditions indicate that acquisition curves can be modified in two ways. A rutabaga mutation, defective in a type-1 adenylyl cyclase, alters the acquisition rate. A white mutation, however, affecting an ABC-transporter, limits asymptotic memory performance. Interestingly, the white mutation enhances olfactory memory performance at low reinforcer intensities. Therefore, the molecular bases of memory acquisition/memory strength and classical olfactory/operant spatial memories can be genetically dissociated.
Results
Reinforcer/memory level relationship
A critical requirement in addressing genetically the reinforcement intensity/memory strength relationship in Drosophila is a fast robust conditioning paradigm that shows this correlation. That Drosophila is capable of varying memory strength has been demonstrated in both classical olfactory conditioning and operant visual learning (Tully and Quinn 1985; Brembs 2000). These paradigms are not ideal, however, in screening for mutations, as they are labor intensive or require many hundreds of flies. The heat-box spatial learning paradigm, however, was developed at least in part to screen for learning mutations (Wustmann et al. 1996). It is fast and requires relatively few flies, and robust memories are formed that last minutes to hours (Putz and Heisenberg 2002). To determine whether wild-type Canton-S (CS) flies would vary their place memory strength based on reinforcing temperature, they were trained with different temperature reinforcement for increasingly longer periods. With increased training time, wild-type CS flies reach what appears to be a plateau in memory performance, with the increase in performance between 10 and 20 min of training reduced compared with that found early in the training session (i.e., in the first 4 min) (Fig. 2A). To determine whether memory performance after 20 min of training is indeed asymptotic, wild-type flies were trained 15, 20, or 25 min using either 37° or 45°C as negative reinforcement (Fig. 2B). No significant differences between these different training sessions indicate that 20 min of training induces asymptotic memory, at least in the temperature range tested. Furthermore, memory performance displayed by flies is higher when higher temperature reinforcement is used. Thus, flies modulate their memory strength in the heat-box depending on the temperature of reinforcement. This provides a paradigm in which this phenomenon can be addressed genetically in Drosophila.
Figure 2.
Higher reinforcing temperature increases memory strength in the heat-box. (A) Wild-type CS flies were trained for increasingly longer periods at different reinforcing temperatures (33°, 37°, 41°, and 45°C). The 3-min memory was measured after training for 2, 4, 6, 10, or 20 min. Higher reinforcement temperature increases memory levels. Comparing memory performance with different temperatures and training duration showed significant differences (Kruskal-Wallis Test: 2 min, H = 40.9, P = 0.000; 4 min, H = 27.9, P = 0.000; 6 min, H = 43.5, P = 0.000; 10 min, H = 58.3, P = 0.000; 20 min, H = 78.6, P = 0.000). Significance values after multiple comparisons are shown comparing 33 with 41 and 37 with 45°C reinforcement (P-values represented in this and following figures and tables are: P < 0.05 = *; P < 0.01 = **, P < 0.001 = ***). At no time point was the performance significantly different between flies trained at 41° and at 45°C. (B) Wild-type CS flies were trained for 15, 20, and 25 min to determine whether the 20-min training schedule induced asymptotic memory levels. Comparing values within a temperature regime indicates a significant difference only with the 37°C reinforcer (Kruskal-Wallis Test: 37°C, H = 7.41, P = 0.02; 45°C, H = 5.17, P = N.S.), although with multiple comparison in the 37°C reinforcer group there were no significant differences between 15 and 20 or 25 min of training (P = N.S.). The number of flies tested for each condition was >100. The exceptions are with the 37°C reinforcer in which between 200 and 300 flies were tested in each training protocol and the 33°C reinforcer at 4 (n = 48) and 20 (n = 72) min of training.
The white-ABC transporter affects spatial conditioning
Mutations in the white (w) and rutabaga (rut) genes affect avoidance behavior during training and display memory performance deficits in the heat-box learning paradigm. The white mutation was found to be defective in the heat-box spatial learning paradigm as part of a mutant screen (a nonvisual task) (Fig. 3). A pilot screen of ∼100 extant lines used 4 min of training with a 37°C reinforcer. These conditions were chosen as they quickly induce a submaximal yet robust memory. The rut allele rut2080 (Levin et al. 1992) was used as a control learning mutant, as it has proven defective in all learning paradigms in which it has been tested. In the heat-box, the rut2080 mutant flies have lower avoidance behavior during training and a short-lived avoidance memory (Fig. 3B), consistent with previous findings (Wustmann et al. 1996; Zars et al. 2000b). This poor performance is the specific consequence of a decrease in rut function, as transgenic expression of the wild-type form of rut can rescue this phenotype (Zars et al. 2000b). A more severe avoidance behavior phenotype and low-memory performance was found in white mutant flies (Fig. 3C), a strain initially considered a control for many of the screened lines. Low-avoidance behavior and poor memory performance could be due to a low-memory acquisition rate or altered high-temperature reinforcement processing. Extended training experiments can differentiate between these possibilities (see below).
Figure 3.
The white and rut-AC mutations affect learning and memory in the heat-box spatial learning paradigm. The data are the avoidance behaviors during 4 min of training (minutes 0-4) and persistence in avoidance in the 3 min following training (minutes 5-7) using 24°C as the reference temperature and 37°C as the reinforcer. Wild-type CS flies (A) have significantly higher avoidance behavior during the training and test periods than rut2080 (B) and wCS13 (C) mutant flies (Kruskal-Wallis Tests: first 2 min of training, H = 83.9, P = 0.000; second 2 min of training, H = 118.9, P = 0.000; 3-min post-test period, H = 56.0, P = 0.000). Significance levels with multiple comparisons are shown in the figure compared with wild-type CS flies. The white mutant phenotype was rescued by the small X-chromosome duplication containing the white gene on the Y chromosome (D) (P < 0.001 for each training session and the memory post-test). The number of flies tested was between 100 and 112 for each genotype. See legend of Figure 2 for levels of significance denoted by the asterisks.
The white gene is implicated as critical for place conditioning. The white mutant flies identified in this screen have the w1118 null mutant allele. They are called wCS13, as they have been out-crossed to the wild-type strain CS for 13 generations. As each out-crossing generation involved more than 20 individuals, and this was done 13 times, it is likely that the white mutation causes the conditioning phenotype. Further mapping of the mutant phenotype took advantage of an X-chromosome duplication containing a wild-type form of the white gene attached to the Y-chromosome. This was out-crossed for six generations to the poor learning wCS13 strain. The wCS13/Dp(1:y)w+ flies show wild-type-like avoidance performance and memory (Fig. 3D), making it even more likely that it is a mutation in the white gene that is responsible for the poor conditioning. As the wCS13 flies provide the strongest behavioral phenotype in the heat-box to date and the rut2080 mutation affects a classic learning gene (a type-1 adenylyl cyclase) (Han et al. 1992; Levin et al. 1992), they were the focus of continued genetic and phenotypic characterization.
For flies to learn the association of chamber position and high-temperature reinforcement, they must be able to sense and avoid elevated temperatures. This can be tested with a thermosensitivity assay (Zars 2001), where flies are allowed to choose between chamber halves that have different temperatures. To do this, the same chambers are used, but the temperature inside is altered independent of the flies' behavior. The term “reference” temperature corresponds to 24°C, a temperature that wild-type flies prefer over lower and higher temperatures (Sayeed and Benzer 1996). The “probe” temperature is a temperature that is different from the 24°C reference temperature. Individual flies are presented with a chamber that initially is at 24°C on both sides, but then on one side increases to a probe temperature of 27°C and further to 30°, 33°, 37°, 41°, and 45°C, while the other chamber half is kept at 24°C. The chamber half with the lower temperature switches every minute. Flies are tested a total of 7 min. When flies show wild-type-like avoidance behavior, it implies their ability to sense a given temperature and, importantly, the locomotor ability to walk away from a high-temperature source. Interestingly, the white mutant flies are not “normal” at any temperature tested except 41° and 45°C (Fig. 4A). Although the avoidance behavior of wCS13 flies of 41°C was not significantly reduced, it is somewhat lower than wild-type CS flies' avoidance behavior. A second experiment was done to test the ability of these flies to sense and avoid a 41°C probe temperature (Fig. 4B). In this test, a probe temperature of 41°C was paired with 24°C in minutes 2 and 3. The chamber half with the high temperature switched between minutes 2 and 3. Consistently, white mutant flies were not significantly different from wild-type CS flies in avoiding the 41°C chamber half. The reduced avoidance of the second 41°C probe in both genotypes is probably a consequence of all flies being on the former 24°C side when the next test phase began, rather than being distributed randomly as in the first pretest minute. The rut mutant flies show high-temperature avoidance behavior similar to wild-type flies at all temperatures tested. A defect in temperature sensation may describe part of the wCS13 mutant flies' poor memory tested with screening conditions. However, it also suggests temperatures at which wCS13 flies could be tested to determine whether their conditioning deficit uniformly depends on lowered thermosensitivity (i.e., >41°C). Thermosensitivity defects cannot explain the rut2080 mutant conditioning phenotype. Additionally, as both mutant genotypes show normal avoidance of a chamber half at the highest temperatures, it indicates that the mutant flies have a sensory-motor system sufficient to avoid high temperatures.
Figure 4.
Thermosensitivity in wild-type and mutant flies. (A) There are significant differences between wild-type CS, wCS13, and rut2080 mutant flies (Kruskal-Wallis tests, probe temperatures: 24°C, H = 0.37, P = N.S.; 27°C, H = 34.6, P = 0.000; 30°C, H = 46.2, P = 0.000; 33°C, H = 67.6, P = 0.000; 37°C, H = 49.7, P = 0.000; 41°C, H = 9.7, P < 0.01; 45°C, H = 5.04, P = N.S.). The white mutant flies showed temperature avoidance behavior significantly lower than wild-type CS flies at most temperatures tested, the exception being when tested at 41° and 45°C. Significance levels with multiple comparisons are shown compared with wild-type CS flies. Wild-type CS flies, measured in parallel to both wCS13 and rut2080, were not significantly different from each other (data not shown) and were therefore combined. The number of rut2080, wCS13, and CS flies tested was 71, 87, and 174, respectively. (B) A test for avoidance of high temperature was repeated with two choices between 24° and 41°C; wild-type CS and wCS13 flies were not significantly different with either of the choices (Mann-Whitney U-tests: probe temperature 24°C, Z = -1.36, P = N.S.; 41°C, 1st test, Z = 1.74, P = N.S.; 41°C, 2nd test, Z = 0.08, P = N.S.). The number of flies tested was 156 (CS) and 155 (wCS13). See legend of Figure 2 for levels of significance denoted by the asterisks.
To gain confidence that the white-ABC transporter is critical for place conditioning in the heat-box, flies mutant for a second, temperature-sensitive, white allele (wblood [wbl]) were raised at permissive and restrictive temperatures and tested. Using the mutant screening conditions of 4 min of training and 3 min of memory test with a 37°C reinforcer, wbl mutant flies showed impaired performance during training and memory test when raised at the nonpermissive temperatures of 25° and 29°C compared with wild-type CS flies raised at the same temperatures (Fig. 5). These rearing temperatures led to a lightening of the eye color, from ruby to light yellow (Ephrussi and Herold 1945; Bingham and Chapman 1986). This performance deficit was not evident when wbl mutant flies were raised at the permissive temperature of 18°C. As the primary change leading to reduced performance was the rearing temperature in wbl mutant flies, it is highly probable that the decreased White-ABC transporter expression (caused by a “blood” transposable element located in the second intron of the white gene) (Bingham and Chapman 1986) is the reason for the poor training and memory-test phase performance.
Figure 5.
The wbl temperature-sensitive white allele affects place memory when raised at restrictive temperatures. Both wild-type and mutant wbl flies were raised at 18°, 25°, and 29°C, the higher two temperatures being restrictive for the wbl mutation based on lightening of eye color. Two training trials (Tr1 and Tr2) of 2 min each (using 24°C as the reference temperature and 37°C as the reinforcer) were followed directly by a 3-min memory test (Te). Mann-Whitney U-tests showed significant differences between wild-type CS flies and wbl mutant flies only when raised at 25° and 29°C (U-tests: 2-min training periods 1 and 2, followed by the memory post-test, respectively; 18°C, Z = 0.84, 1.38, 0.54; 25°C, Z = 1.69, 3.89, 2.54; 29°C, Z = 1.01, 3.21, 3.76). Significance levels are shown. The number of flies tested in each manipulation was between 40 and 60, except for flies raised at 25°C, where n = 103 (wbl) and 116 (CS). See legend of Figure 2 for levels of significance denoted by the asterisks.
Both wbl mutant and wild-type CS flies were reared at 29°C and tested in the thermosensitivity assay. Comparing these two genotypes showed significantly reduced avoidance of 27° and 30°C in wbl mutant flies compared with wild-type CS flies, similar to the cantonized white null allele (wCS13) (Figs. 4, 6). However, avoidance of all other temperatures were at levels not significantly different from wild-type levels, including avoidance of 37°C, the temperature used for reinforcement in the conditioning experiments. Thus, the poor performance in the conditioning paradigm in wbl mutant flies raised at the restrictive temperatures is not a consequence of a poor sensorimotor process.
Figure 6.
The wbl mutation at the restrictive temperature of 29°C decreases avoidance behavior of lower temperatures (i.e., 27° and 30°C) but not temperatures >33°C. Wild-type CS and wbl mutant flies raised at 29°C were tested in the thermosensitivity assay to determine whether they have altered avoidance behavior to temperatures used for reinforcement. Mann-Whitney U-tests showed significant differences between wbl and CS flies when probed with 27° and 30°C but not temperatures that were higher (U-tests: probe temperature 24°C, Z = 0.72; 27°C, Z = 2.04; 30°C, Z = 1.99; 33°C, Z = 1.38; 37°C, Z = 1.68; 41°C, Z = -1.22; 45°C, Z = 0.60). Significance levels are shown. The number of wild-type and wbl flies tested was 114 and 113. See legend of Figure 2 for levels of significance denoted by the asterisks.
To further test the relationship between mutations at the white locus and performance in the heat-box, five additional mutant alleles were tested. These alleles were chosen as they span the range of eye-color changes caused by mutations in the white gene. These include eye colors of white (w1 and wec3), yellow-orange to orange (wa and wa2), and brownish-orange (wa3). In tests of thermosensitivity, flies of all but the wec3 allele had lower avoidance of the 37°C probe temperatures than wild-type CS flies (Table 1). All five white mutant alleles had wild-type-like avoidance behavior of 41°C. Similar to the white null mutant flies, four of the additional mutant alleles showed significantly lower conditioned memory performance than wild-type flies after training using both 37°C and 41°C reinforcement. Although the wec3 allele showed lower memory scores than wild-type flies after conditioning, it did not reach significance in these tests. Curiously, there does not appear to be a correlation between the severity of eye-color change in the white mutant alleles and performance in the heat-box. This likely indicates the systems requiring white-ABC transporter function for conditioning are not similarly sensitive to the mutations as eye coloration. A detailed understanding of the relationship between gene structure and heat-box conditioning must await a high-resolution analysis of the molecular changes in these mutant lines. (The w1 and wa mutations have transposable elements in regulatory regions of the gene [Zachar and Bingham 1982; O'Hare et al. 1991]).
Table 1.
Thermosensitivity and conditioning in eye-color mutant files
| Thermosensitivity (PI ± SEM)
|
Place memory (PI ± SEM)
|
||||||
|---|---|---|---|---|---|---|---|
| Genotype | N | 37°C | 41°C | N | 37°C | N | 41°C |
| CS | 140 | 0.49 ± 0.05 | 0.50 ± 0.05 | 163 | 0.27 ± 0.04 | 92 | 0.38 ± 0.06 |
| w1 | 138 | 0.32 ± 0.04*** | 0.50 ± 0.04 | 134 | 0.13 ± 0.04* | 92 | 0.20 ± 0.06* |
| wa | 140 | 0.37 ± 0.04* | 0.50 ± 0.04 | 127 | 0.04 ± 0.04** | 82 | 0.11 ± 0.06** |
| wa2 | 142 | 0.29 ± 0.05*** | 0.46 ± 0.05 | 129 | 0.07 ± 0.04* | 93 | 0.08 ± 0.06** |
| wa3 | 141 | 0.29 ± 0.04*** | 0.42 ± 0.04 | 124 | −0.01 ± 0.03*** | 83 | 0.19 ± 0.06* |
| wec3 | 122 | 0.41 ± 0.06 | 0.45 ± 0.05 | 107 | 0.17 ± 0.05 | 91 | 0.26 ± 0.06 |
| CS | 103 | 0.72 ± 0.03 | 0.73 ± 0.03 | 162 | 0.35 ± 0.04 | N.D. | |
| bw1 | 59 | 0.67 ± 0.03 | 0.81 ± 0.03 | 161 | 0.25 ± 0.04 | N.D. | |
| st1 | 63 | 0.63 ± 0.05 | 0.72 ± 0.04 | 192 | 0.11 ± 0.03*** | N.D. | |
Comparison of wild-type CS flies' performance to five white mutant alleles, brown (bw1), and scarlet (st1) mutant flies in a thermosensitivity test and the 3-min memory test with two reinforcing temperatures (37 and 41°C). In the thermosensitivity tests comparing wild-type CS flies with the white mutations, Kruskal-Wallis tests for each probe temperature are: 37°C, H = 38.87, P = 0.000; 41°C, H = 7.43, P = 0.19. Comparing wild-type CS flies with bw1 and st1 flies in the thermosensitivity tests found no significant differences with temperatures tested (37°C, H = 4.47, P = 0.10; 41°C, H = 4.50, P = 0.22). In tests for place memory using two different reinforcing temperatures, four of the five tested white alleles showed decreased performance with both temperatures (37°C, H = 25.60, P = 0.001; 41°C, H = 14.51, P = 0.013). The st1 but not bw1 mutation significantly reduced place memory using the 37°C reinforcer (H = 21.73, P = 0.000). Significance levels are shown for each genotype compared to wild-type CS flies performance tested in parallel. See legend of Figure 2 for levels of significance denoted by the asterisks.
Finally, transgenic copies of the white gene were tested for rescue of the conditioning deficit of null white mutant flies. In two cases, wCS13 mutant flies were completely restored to normal performance levels (Fig. 7; data not shown). However, in several other cases, performance was as poor as the null mutant levels, even though eye color was largely restored (data not shown). As the associated transgenes and insertion sites for the ectopic mini-white gene are different in these transgenic lines, it is presumed that the expression levels or spatial domains are not sufficient for rescue of the white mutant phenotype in those latter cases. Alternatively, insertion effects altering local gene expression or unregulated expression of effector genes in those transgenes could give rise to abnormal performance. Nevertheless, as all white mutations tested have similar performance deficits, and those deficits can be rescued by a chromosomal duplication and a mini-white transgene, it is concluded that the altered White-ABC transporter function is the cause of the abnormal performance of mutant flies in the heat-box conditioning paradigm.
Figure 7.
Rescue of the white mutant conditioning deficit in the heat-box with a mini-white transgene. Wild-type CS, wCS13, and wCS13; mini-w+ flies were trained for two 2-min training blocks, followed by a 3-min test of place preference using the 24°/37°C conditioning temperatures. wCS13 mutant flies had lower avoidance behavior during the training sessions (Tr1 and Tr2) and in the test phase (Te) than both wild-type and wCS13 flies with a mini-white transgene (Kruskal-Wallis tests, Tr 1, H = 33.6, P < 0.0000; Tr 2, H = 57.2, P < 0.000; Te, H = 14.5, P < 0.001). Significance levels of wCS13 performance to CS and wCS13; mini-w+ are shown. The number of flies tested was 43 (CS), 45 (wCS13), and 36 (wCS13; mini-w+). See legend of Figure 2 for levels of significance denoted by the asterisks.
Dwelling times indicate wild-type and wCS13 mutant flies typically receive full reinforcement
Even though wCS13 mutant flies can sense and avoid 41°C at wild-type levels, there remains a possibility that the poor performance of wCS13 mutant flies with the 41°C reinforcer is a consequence of poor avoidance of lower temperatures. This could be the case if wild-type flies use lower temperatures to determine which half of the chamber to avoid, but wCS13 mutant flies require the full measure of high-temperature reinforcement. The increased amount of time required for the wCS13 flies to receive the full reinforcement could lead to a decrease in the performance index during conditioning. To gain insights into the typical temperatures the flies are exposed to during training in the heat-box, the “dwelling times” during the 20-min training experiment with 41°C reinforcement were examined. A dwelling is delimited by the entry and exit from one chamber half. This is essentially the same measure used in determining the time in reinforced and nonreinforced quadrants of the visual surround in visual pattern conditioning (Dill et al. 1995).
Both wild-type CS and wCS13 flies are typically exposed to the predetermined maximum chamber temperature after they enter the chamber half associated with reinforcement. In both wild-type CS and wCS13 flies, there is a decrease in the number of dwellings per fly on the reinforcement-associated “hot” side of the chamber (Table 2). This reduction is not as pronounced in wCS13 as in wild-type CS flies and reflects the lower conditioning performance in these flies. The mean duration of a dwelling also tends to decrease in both wild-type CS and wCS13 mutant flies during the conditioning period, with wCS13 reducing their dwelling duration to ∼4.8 sec. Calculation of the average temperature of the chamber after either wild-type or mutant flies enter the reinforcement-associated side of the chamber indicates that both genotypes typically receive the full reinforcement temperature of 41°C. Based on this measure and the normal avoidance of 41°C in the thermosensitivity assay, the cause of the poor conditioning of wCS13 flies can be separated from a defective sensorimotor system.
Table 2.
Dwellings and dwelling times in CS and wCS13 flies during 20 min of training using 41°C reinforcement
| Genotype (N) | Training period | `Hot-side' dwellings/fly | `Hot-side' dwelling time(s) | Mean max. temp. (°C)/dwelling |
|---|---|---|---|---|
| CS (133) | 1 | 1.6 | 8.6 ± 1.3 | 41 |
| 2 | 1.0 | 7.9 ± 2.1 | 41 | |
| 3 | 0.8 | 15.5 ± 5.3 | 41 | |
| 4 | 0.5 | 7.2 ± 2.6 | 41 | |
| 5 | 0.9 | 8.7 ± 1.9 | 41 | |
| 6 | 0.5 | 10.7 ± 3.5 | 41 | |
| 7 | 0.4 | 13.9 ± 4.3 | 41 | |
| 8 | 0.4 | 11.7 ± 3.9 | 41 | |
| 9 | 0.6 | 10.6 ± 2.8 | 41 | |
| 10 | 0.3 | 8.0 ± 2.0 | 41 | |
| wCS13 (132) | 1 | 1.7 | 8.9 ± 1.1 | 41 |
| 2 | 1.5 | 8.0 ± 1.7 | 41 | |
| 3 | 1.3 | 6.9 ± 1.5 | 41 | |
| 4 | 1.3 | 7.9 ± 2.5 | 41 | |
| 5 | 2.2 | 5.2 ± 0.4 | 41 | |
| 6 | 1.0 | 5.4 ± 0.9 | 41 | |
| 7 | 1.0 | 5.0 ± 0.6 | 41 | |
| 8 | 1.0 | 5.3 ± 0.6 | 41 | |
| 9 | 1.8 | 4.9 ± 0.5 | 41 | |
| 10 | 0.8 | 4.8 ± 0.5 | 41 |
The 20 min of training was binned in 2-min periods. The average number of `hot-side' dwellings per fly was calculated based on the number of dwellings and number of flies. The average hot-side dwelling time was calculated and converted to the mean maximum chamber temperature per dwelling with an upper limit of 41°C using the formula: Δ-temp = (time - 0.05612)/0.28114. The formula was derived from data measuring temperature rise times. It fits the data (not shown) with R = 0.99971, P < 0.001.
scarlet, but not brown, alters spatial conditioning
Based on the pigmentation of the retina, the White-ABC transporter forms heterodimers with both the Brown and the Scarlet proteins. To test whether the known White-ABC transporter binding partners have a role in heat-box conditioning, genetically defined null alleles at the brown and scarlet loci (Dreesen et al. 1988; Tearle et al. 1989) were tested. Flies mutant for the brown gene did not significantly alter 3-min place memory performance compared with wild-type CS flies (Table 1). The scarlet mutant flies' performance, however, was significantly lower than wild-type levels. Indeed, memory tests after training for 20 min still found the scarlet mutant flies with significantly lower-place memory performance (CS = 0.59 ± 0.08, n = 67; st1 = 0.25 ± 0.07, n = 68; Mann-Whitney U-test, Z = 3.48, P < 0.001). Examination of brown and scarlet mutant flies shows that they can sense and avoid high temperatures in the thermosensitivity assay similar to wild-type flies (Table 1). Thus, poor performance in the conditioning paradigm by scarlet flies appears to be independent of a deficit in their ability to sense high temperatures or a failure to walk away from a high-temperature source.
Activity/spatial conditioning relationship
To explore the potential influence of general activity on the pre-disposition of flies of a genotype to perform well in heat-box conditioning, pre-test-phase walking speed (taken as a measure of activity uninfluenced by conditioning) was compared with performance levels in the memory-test phase. Walking speed can be used as a first measure for locomotor ability, as both step length and frequency are incorporated in this compound measure (Strauss and Heisenberg 1993). This analysis might reveal that low activity is associated with poor memory-phase performance, as flies are incapable of walking away from the side of the chamber associated with high temperature or that flies of some genotypes might be walking so quickly that they cannot draw the conclusion that one-half of the chamber is associated with high and the other half with low temperatures. This could be the case as the whole chamber heats when a fly walks to the high-temperature associated half and the whole chamber cools as it walks back. Table 3 lists the average walking speed in the pre-test phase in the genotypes of all tested flies. It is obvious from this table that there are significant differences between wild-type CS flies and nearly every mutant genotype, some with higher and some with lower walking speeds. Indeed, in different experiments, wild-type CS flies can have different activity levels. These are probably dependent on environmental differences between experiments. However, comparison of pre-test walking activity with memory-test phase performance shows no significant relationship (Fig. 8). Thus, at least with the 18 genotypes and experiments tested here, there is not a relationship between pre-test walking activity and ability to form a place memory in the heat-box. It seems that one cannot predict memory-phase performance in the heat-box based on activity levels. However, mutations with more extreme walking speeds might eventually be found that challenge this conclusion.
Table 3.
Walking speed in wild-type CS and mutant flies measured during the 30-sec pre-test phase
| Genotype | Rearing temperature (°C) | Average pre-test walking speed (mm/sec) |
|---|---|---|
| CS | 25 | 3.0 ± 0.2 |
| rut2080 | 25 | 4.3 ± 0.2*** |
| wCS13 | 25 | 4.9 ± 0.2*** |
| wCS13/Dp(1:y)w+ | 25 | 4.8 ± 0.3*** |
| bw1 | 25 | 4.1 ± 0.1*** |
| st1 | 25 | 5.1 ± 0.1*** |
| CS | 29 | 2.4 ± 0.2 |
| CS | 25 | 3.5 ± 0.2 |
| CS | 18 | 3.6 ± 0.2 |
| wbl | 29 | 1.5 ± 0.2** |
| wbl | 25 | 3.1 ± 0.2 |
| wbl | 18 | 2.7 ± 0.2** |
| CS | 25 | 4.6 ± 0.2 |
| w1 | 25 | 5.9 ± 0.2** |
| wa | 25 | 3.3 ± 0.1*** |
| wa2 | 25 | 5.2 ± 0.2 |
| wa3 | 25 | 3.6 ± 0.2*** |
| wec3 | 25 | 5.3 ± 0.2 |
Comparing pre-test walking speed of mutant and wild-type CS flies in the first section indicates significant differences (Kruskal-Wallis test: H = 127.9, P = 0.000). Comparing wild-type CS and wbl flies raised at different temperatures indicates significant differences for 18 and 29°C rearing conditions (U-tests: 18°C, Z = 2.68, P < 0.01; 25°C, Z = 1.39, P = N.S.; 29°C, Z = 2.78, P < 0.01). In the final set of experiments, there were significant differences in pre-test walking activity (Kruskal-Wallis test: H = 131.4, P = 0.000). Significance levels compared to CS in each section are shown. See legend of Figure 2 for levels of significance denoted by the asterisks.
Figure 8.
Relationship between pre-test walking activity and memory performance in wild-type and mutant flies. The pre-test walking speeds of 18 genotypes and experiments were compared to determine whether they could predict memory performance. The linear regression is shown (r = -0.20, r2 = 0.04, and P = 0.42). Thus, there is no significant relationship between pre-test activity and memory phase performance in the genotypes tested.
Genetic dissociation of memory acquisition and strength
The poor performance of mutant flies could be a consequence of poor memory formation or a defect in processing of the high-temperature reinforcing signal. Examining memory performance in wild-type and mutant flies with increased training duration could lead to one of two results. Either there is a decreased acquisition rate, but eventually the same memory level is reached, reflecting a defect in memory formation, or maximal memory levels for a given reinforcement intensity are lower, indicating a defect in reinforcement signal processing. To discriminate between these possibilities, a series of experiments increasing the training length was used to probe memory formation in wild-type CS, rutabaga, and white mutant flies. Different groups of wild-type and mutant flies were continuously trained with a 37°C reinforcer for 1-20 min and then tested for their avoidance memory. As expected, and consistent with the previous results (Fig. 2), wild-type CS flies showed increased memory performance with increased training time (Fig. 9). Interestingly, increased training of rut2080 mutant flies lead to wild-type levels of memory, being indistinguishable with 10 and 20 min of training. In contrast, wCS13 mutant flies showed severely reduced asymptotic memory scores using both the 37° and 41°C reinforcement temperatures. Because the wCS13 results are a hallmark of lower intensity reinforcement, i.e., a lower asymptote in memory performance, it indicates that the white mutant flies have a defect in the processing of reinforcement information.
Figure 9.
The rut2080 mutation affects acquisition rates, while the wCS13 mutation influences asymptotic memory levels. Wild-type CS, rut2080, and wCS13 mutant flies were continuously trained from 1 to 20 min and tested directly afterward for continued side preference for 3 min. The 3-min memory score is presented. Wild-type flies show higher memory levels with increasing training duration (37°C and 41°C reinforcement), consistent with results in Figure 2. Interestingly, rut2080 mutant flies have indistinguishable memory scores from wild-type flies when trained for 10 or 20 min; all other training situations show deficits. The wCS13 mutant flies have a different phenotype, having a significantly lower asymptote than wild-type flies (Kruskal-Wallis tests for each training duration: 1 min, H = 8.4, P < 0.01; 2 min, H = 8.1, P < 0.05; 4 min, H = 22.8, P = 0.000; 6 min, H = 14.1, P < 0.001; 10 min, H = 24.4, P = 0.000; 20 min, H = 41.3, P = 0.000). Significance levels with multiple comparisons are shown comparing mutant with wild-type CS flies. As wCS13 mutant flies showed normal avoidance behavior for 41°C in the thermosensitivity assay, they were also tested for memory using this as a conditioning temperature. Under these conditions, wCS13 mutant flies showed significantly lower performance with all training situations except for 2 min of training (U-tests: 2 min, Z = 1.56; 4 min, Z = 3.73; 6 min, Z = 2.81; 10 min, Z = 3.74; 20 min, Z = 3.9). Significance levels are shown. The difference with wild-type flies, however, is less pronounced than when the reinforcing temperature was 37°C. In the 37°C experiments, the number of flies tested for each data point was typically between 50 and 80. The exceptions were with 10 and 20 min of training of CS and rut2080 flies, where between 110 and 180 flies were tested and for the 1-min training period for wCS13 mutant flies in which n = 24. The 41°C experiments had between 110 and 180 flies tested for each data point. See legend of Figure 2 for levels of significance denoted by the asterisks.
Olfactory conditioning
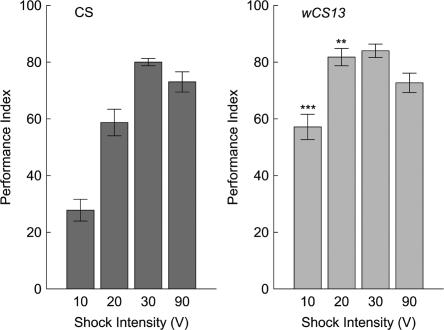
To determine whether the low memory levels found in white mutant flies was also evident in a second negatively reinforced conditioning paradigm, wild-type CS and white mutant flies were tested in the classical olfactory conditioning paradigm (Tully and Quinn 1985). Flies were trained under conditions previously shown to induce asymptotic memory (12 electric shocks evenly distributed within 1 min of odorant presentation with voltages ≥90 V). It was predicted that varying the intensity of the electric shock under these conditions might reveal differences between the genotypes. In contrast to the results in the heat-box, classical olfactory conditioning was not negatively affected by the wCS13 mutation (Fig. 10). Indeed, performance is significantly higher than wild-type CS flies at the lower voltages tested.
Figure 10.
Olfactory associative short-term memory in wild-type CS and wCS13 mutant flies. Flies were trained with different intensity electric shocks, from 10 to 90 V. Mutant wCS13 flies avoided shock-associated odorants at levels higher than wild-type flies at the lower intensity reinforcers of 10, 20, and 30 V (ANOVA: 10V, F = 31.76, P < 0.001; 20 V, F = 18.76, P < 0.001; 30 V, F = 6.4, P < 0.05; 90 V, F = 0.34, P = N.S.). n = 6 for each genotype and shock intensity. See legend of Figure 2 for levels of significance denoted by the asterisks.
The increased memory performance of white mutant flies could be influenced by the perceived value of the electric-shock reinforcer or odorant intensity. To address this, both wild-type and white mutant flies were tested for their ability to sense and avoid different electric-shock intensities and odorants. At the lowest shock intensities tested, white mutant flies avoid the shocked tube at higher levels than wild-type flies (Fig. 11). This likely indicates that the higher performance in the learning experiment is influenced by this increased shock sensitivity. Olfactory acuity of white mutant flies was also tested. There were no significant differences between wild-type CS and wCS13 mutant flies in avoidance of the odorants used in conditioning (CS: MCH 50.3 ± 1.8, OCT 31.6 ± 1.6; wCS13: MCH 55.2 ± 3.5, OCT 31.8 ± 1.2; F = 1.54, P = N.S. for MCH, F = 0.006, P = N.S. for OCT; n = 6 for each genotype/experiment). Based on this test, there are no effects of changed olfactory acuity on olfactory conditioning in wCS13 mutant flies.
Figure 11.
Electric shock avoidance in wild-type CS and wCS13 mutant flies. Flies were tested for their ability to sense and avoid different electric shock intensities (10, 20, 30, and 90 V). The wCS13 flies showed elevated avoidance of both 10 and 20 V compared with wild-type CS flies (ANOVA: 10 V, F = 24.99, P < 0.001; 20 V, F = 16.98, P < 0.01; 30 V, F = 2.24, P = N.S.; 90 V, F = 0.004, P = N.S.). n = 6 for each genotype and shock intensity. See legend of Figure 2 for levels of significance denoted by the asterisks.
Discussion
Although the relationship between reinforcement intensity and memory strength has been long known, relatively little is understood of the molecular mechanisms that support it. In current cellular models of memory formation, a “teaching” signal is critical for changing the synaptic strength between two neurons (Kandel 2001). Presumably, varying output from the neuron or neurons carrying the teaching signal alters the synaptic strength between the neurons in which a memory is stored.
Asymptotic memory strength, as measured in continued avoidance behavior after training, increases with increasing reinforcing temperature in the heat-box. This result is consistent with the principles of the matching law (Herrnstein 1997) and previous findings in insects (e.g., honeybees and Drosophila melanogaster) (Menzel and Erber 1972; Bitterman et al. 1983; Tully and Quinn 1985; Loo and Bitterman 1992). Addition of operant place learning in Drosophila as a learned behavior with similar properties allows for a genetic investigation of memory-strength determination. The examples of the rut-AC and white-ABC transporter indicate that acquisition and memory strength determination can be dissociated. In addition, the white mutation provides a molecular/genetic founder in determining the mechanisms that support memory formation of different strengths and evidence for a discrete comparator level in a model of operant conditioning (see below).
The rut2080 mutation affects memory acquisition. The rut2080 mutation alters the function of a type-1 adenylyl cyclase (AC). Current models of synaptic plasticity and memory formation posit a critical role for the type-1 AC as a potential molecular integrator of reinforcement and conditioned stimuli (Lechner and Byrne 1998; Antonov et al. 2003). Affecting the rate of learning, on the surface, makes sense for this mutation. But why do rutabaga mutant flies learn anything at all if a key molecule in synaptic plasticity is defective? There are two potential reasons why the rut2080 mutant flies learn and eventually reach wild-type memory levels with extended training. The first explanation is related to the rutabaga allele tested. The rut2080 allele has a P-element inserted ∼150 nt upstream of the first exon. This insertion leads to a strong reduction in transcript levels by influencing the rutabaga promoter (Han et al. 1992; Levin et al. 1992). In principle, as this P-element insertion lies upstream of the putative transcription start site, some residual expression could be present in this mutant line. This reduction could restrict the learning process but allow mutant flies to eventually learn as much as wild-type flies. The second possibility is that redundant molecular mechanisms underlie memory formation. This could be from a second source of cAMP or an independent signaling pathway (Levin et al. 1992; Isabel et al. 2004). In the latter proposal, the rut-AC-dependent mechanism is in parallel to a radish-dependent path. In support of redundant pathways, null mutations in the mouse type-1 AC show partial reduction deficits in the Morris water maze (Wu et al. 1995) and stronger phenotypes from type-I and type-VIII AC double knock-out mice (Wei et al. 2002; Wang et al. 2003). This redundant signal hypothesis indicates that something outside of the rut-AC pathway, functioning at a slower rate, allows rutabaga mutant flies to eventually learn as well as wild-type flies. The available genetic tools cannot dissociate these possibilities.
The white-ABC transporter is necessary for asymptotic memory performance in the heat-box. Evidence that the white-ABC transporter is necessary for place learning in the heat-box is manifold. First, extensive outcrossing of the w1118 null allele with the wild-type CS line indicates a gene closely linked with the white locus is necessary for conditioning. The rescue of the behavioral deficit (and eye color) with a duplication that contains the white gene further restricts the region of the genome necessary for place conditioning. Second, the temperature-sensitive white allele, wbl, shows normal behavior when raised at the permissive (based on changes in eye color) temperature of 18°C but intermediate and severe deficits when raised at the restrictive temperatures of 25° and 29°C. As wild-type CS flies are not negatively affected by rearing at the same temperatures, it argues that changes in the white gene are responsible for deficits in conditioning. Third, five independent white mutant alleles give extraordinarily similar mutant phenotypes during conditioning. Finally, the rescue of wCS13 flies with a mini-white-labeled transgene argues in favor of the white-ABC transporter being necessary for place conditioning. This evidence leads to the conclusion that the white-ABC transporter is necessary for place conditioning in Drosophila.
The mechanisms determining memory strength in the classical olfactory conditioning paradigm must be different from those in heat-box place conditioning. The white null mutation does not negatively affect asymptotic memory levels in classical olfactory conditioning but increases performance with low-intensity shock reinforcement. Interestingly, and perhaps related, the timing of electric-shock and odor presentation can change conditioned behavior from negative to positive (Tanimoto et al. 2004). Perhaps these temporal manipulations selectively reveal positive and negative components in shock-associated memory. The white mutation could be altering the positive component, giving rise to higher levels of negative olfactory memory and more avoidance of the shock-associated odorants. That a positive or negative olfactory memory can be selectively manipulated gains support from the effects octopamine and dopamine signaling have on olfactory memories (Schwaerzel et al. 2003). The white mutation could be altering a component that functions in the low-voltage range that opposes the negatively reinforced memory. This is an example of a relatively rare event, a decrease in gene function increasing memory performance (e.g., Malleret et al. 2001). Nevertheless, it is clear that the white mutation affects classical olfactory and operant spatial memory differently.
A modified model of operant conditioning
With the addition of a comparator in which the difference between the preferred and actual stimulus state is calculated, the Wolf-Heisenberg model of operant conditioning can be used to conceptualize the place-conditioning results. In the Wolf-Heisenberg model (Wolf and Heisenberg 1991; Fig. 12), flies (1) have a desired state (or goal). To achieve this goal, they (2) initiate different motor programs. A coincidence detector compares the efference copy of a motor program with the difference between the goal and the current sensory input (3). Flies (4) modify the probability of performing a given motor program with significant coincidence between a decreasing difference between desired and actual state and the motor program efference copy. Finally, flies (5) can persist with a motor program if there is consistent control of a sensory stimulus with a given behavior. It is the initiation of one of several possible behaviors to decrease the difference between the goal and the actual state that differentiates this model from other models that indicate the motor program is a response to a stimulus (e.g., Skinner 1950; Killeen 1994; Dragoi and Staddon 1999). This describes the ability of flies (and other animals) to use arbitrary behaviors to solve conditioning tasks. That flies try different motor programs to reduce the difference between the desired and the actual state was reached when flies were shown capable of using totally artificial behaviors such as inverse coupling of yaw torque and lateral force generation from the legs with the angular position of visual land-marks (Heisenberg and Wolf 1984; Wolf and Heisenberg 1986, 1991; Wolf et al. 1992). Addition of a comparator for the desired and the actual stimulus state provides the substrate for the effects of differing reinforcing temperatures and the white mutation.
Figure 12.
The Wolf-Heisenberg model of operant conditioning and site of rut-AC and white mutation effects. The comparator receives information from the environment and is compared with a desired state. This difference is fed into the conditioning circuit, where behavioral motor programs are initiated. The initiated behaviors, some of which are mutually exclusive of other behaviors, can influence the sensory input. With successful reduction in the difference between the desired and current state, the conditioning circuit is reinforced and has a higher probabilityof being implemented. In this model, the white mutation affects the comparator and the rut-AC mutation affects the reinforcing circuit. (C.D.) is a coincidence detector. See the Discussion for a more detailed explanation of the model.
In the model, the effect of changing reinforcing temperatures on performance level in the heat-box is done initially at the level of the desired/actual state comparator. At least in naive flies, the desired state is presumably a temperature range that is non-deleterious. This inference is supported by temperature preferences on a linear temperature gradient in which flies strongly prefer 24°C over both higher and lower temperatures (Sayeed and Benzer 1996). The deviation from that desired state provides a graded input to the coincidence detector. That is, small differences from a preferred temperature centered at 24°C should lead to a small input into the coincidence detector, larger differences to a larger input. The positive feedback loop between the behavior initiator and the coincidence detector via the efferent and after-effect pathways leads to a graded change in the probability of initiating a given behavior.
Mutations in the white-ABC transporter affect the comparator. As with a change in the reinforcing temperature, the change evident in the wCS13 mutant flies indicates an effect on the comparator. The mutant flies could be altering the levels of the desired or actual state. The poor avoidance behavior of wCS13 flies of some elevated temperatures indicates a decrement in this avoidance behavior. However, as they still prefer the 24°C temperature over all other temperatures tested, their preferred temperature is still 24°C. The defect found in the wCS13 flies is, therefore, in the value of the term subtracted from the desired state.
Performance in the thermosensitivity assay can be dissociated from performance in place conditioning. The wCS13 mutant flies avoid 41°C at levels not significantly different from wild-type flies yet do not avoid a chamber half associated with that temperature to the same extent as wild-type flies. This dissociation was also seen in wbl flies at the restrictive temperature and four additional white alleles. That avoidance behavior is dissociated from memory forming behavior indicates that the mechanisms underlying these two behaviors are different, and that one function of the White-ABC transporter is to supply temperature value into the reinforcing circuit. Two other examples of this dissociation include the role of the VUMmx1 neuron mediating the reinforcing property of sugar reward but not eliciting the proboscis extension in the honeybee (Hammer 1993), and the conditioned avoidance of odors depending on the Kenyon cells of the mushroom bodies but not the spontaneous avoidance of noxious odorants (de Belle and Heisenberg 1994; Heimbeck et al. 2001). The different effects of the white mutation on avoidance and conditioned behavior might reflect differences in the neural structures mediating the two behaviors (as in the two other examples listed) or different sensitivities of the behaviors within the same neural circuit to mutations in the white gene, or both. Determining the neural structures that require White-ABC transporter function in the two behaviors will differentiate between these possibilities.
The rut2080 mutation, in contrast, affects the feedback loop of behavior initiation and coincidence detection. It is within this feedback loop that the effects on sensory input of an initiated behavior are recognized and maintained. The proposed role of the rut-AC in second messenger (i.e., Ca2+/calmodulin and G-protein) integration and synaptic plasticity are consistent with the feedback loop being the site of operant learning in this model. Whether the rut-AC function could be limited to a subset of the feedback loop is not clear.
Further implications of behavioral deficits in white mutant flies
The null white allele restricts maximal memory level, indicating a defect in reinforcement signal processing. Interestingly, the difference between wCS13 and wild-type maximal memory performance decreased as reinforcing temperatures increased. This suggests that the wCS13 mutant flies can learn, but that their subjective interpretation of the significance of the reinforcing temperature is altered. As the white allele used in these experiments is a molecular null mutation, the only interpretation for residual reinforcement evaluation is the presence of at least one additional processing mechanism.
The white gene encodes a member of the so-called half-size ABC transporter family. These proteins are critical for the transport of different substrates into cells. Consistent with the view that these proteins transport many different important substrates, mutations in this class of proteins leads to a large number of human diseases, including cystic fibrosis and Tangiers disease (Dean et al. 2001). The white-ABC transporter, together with the dimer binding partners Brown and Scarlet, is important in the translocation of guanine and tryptophan into cells of the retina (Dreesen et al. 1988; Tearle et al. 1989). Assuming the White-ABC transporter is interacting with Brown or Scarlet in the brain, where white transcripts have been detected (Campbell and Nash 2001), the tryptophan and guanine precursors could be used in the synthesis of many intra- and intercellular signaling molecules. One or some of these are presumably critical for normal heat-box learning. Finding that the scarlet mutation limits memory levels with 4 and 20 min of training restricts the candidates for this signal and centers on serotonin. A similar function for white has been suggested in overexpression and anesthesia studies (Zhang and Odenwald 1995; Hing and Carlson 1996; Nilsson et al. 2000; Campbell and Nash 2001). A better understanding of the neural structures in which these genes function will prove useful in determining the processes in which white and scarlet function in memory formation.
Beyond the specific study of memory performance, the white and scarlet mutations could have applications to human mental health. The human genes homologous to the white- and scarlet-ABC transporters (ABCG1 and ABCG4) have been detected in many tissues, including the brain (Chen et al. 1996; Savary et al. 1996; Croop et al. 1997; Annilo et al. 2001; Oldfield et al. 2002). Additionally, the region 21q22.3, the chromosomal location of ABCG1, has been linked to major depression in humans (Straub et al. 1994; Detera-Wadleigh et al. 1996, 1997; Smyth et al. 1997). Indeed, a mutation in the ABCG1 gene has been identified in male patients with depression (Nakamura et al. 1999). Finally, the commonality of function in tryptophan metabolism (e.g., Zhang et al. 2005) and a common low-asymptotic memory phenotype in human depression (Nestler et al. 2002) may indicate that these gene products have application in understanding at least some components of human depression.
Conclusion
The heat-box operant place-learning paradigm provides for the rapid determination of a mutant effect on reinforcement processing or memory acquisition. Mutations in the rut-AC and white-ABC transporter provide principle examples of altering acquisition rate and asymptotic performance in Drosophila.
Materials and Methods
Genetic manipulations and culture conditions
The wCS10 stock is a “cantonized” white null allele (w1118) (Hazelrigg et al. 1984; Dura et al. 1993). The wild-type Canton S (CS) stock was used in behavioral tests and for outcrossing. Flies were raised at 25° and 60% relative humidity in a 12:12 h light:dark cycle on our standard food medium (Guo et al. 1996). The additional out-crossing of the x-linked wCS10 mutation to the wild-type CS line involved three outcrossings of two generations each and was renamed wCS13. The Dp(1:Y)w+ chromosome was chosen for cantonization to attempt a rescue of the white mutant memory scores. It contains a duplication of the X chromosome from 2D1 to 3D4 attached to the male Y chromosome (Smith and Konopka 1981). This chromosome was outcrossed to the wCS13 stock for six generations to have ∼3% of the original genome remaining. The white gene is at 3B2-3C2 (Pirotta et al. 1983) and is complemented by the Dp(1:Y)w+ chromosome based on eye color. The mini-white gene in the cantonized tetanus toxin light chain GAL4-UAS effector line CYO34-1 (Scholz et al. 2000) was also tested for behavioral rescue of wCS13 mutant flies. Additionally, the bw1 and st1 mutations were outcrossed to wild-type flies for greater than six generations (Dreesen et al. 1988; Tearle et al. 1989). The w1, wa, wa2, wa3, wec3, and temperature-sensitive wbl flies were not cantonized (Bingham and Judd 1981; Zachar and Bingham 1982; Bingham and Chapman 1986; Birchler and Hiebert 1989). The outcrossing schemes used at least 20 males and females in each generation. Since the Dp(1:Y)w+ duplicates the white gene on the Y-chromosome, only males of this genotype were tested. Males and females of CS and wCS13 were tested separately, but no significant differences were seen, and their scores were pooled, similar to the findings of Putz (2002) on a large sample size of male and female wild-type CS flies.
Behavioral tests
Flies were tested in the heat-box spatial learning paradigm and in the thermosensitivity assays described in Zars et al. (2000b) and Zars (2001). In the learning paradigm, a fly is allowed to run in a small chamber that is heated to a defined temperature within seconds when it crosses an invisible midline; the chamber quickly cools to baseline (24°C) when it returns to the original side. The baseline temperature of 24°C was used, as flies have a strong preference for this temperature over both higher and lower temperatures when given a prolonged choice (Sayeed and Benzer 1996). A performance index is calculated by subtracting the time spent on the side associated with reinforcement from the time spent on the nonreinforced side and dividing this by the total time. Thus, a scale of -1 to 1 is generated with a total preference for the punished side giving a -1, and for the non-punished side, a 1. In all learning experiments, a 30-sec pre-test was followed by training of different lengths (0-20 min) and a 3-min post-test. Reinforcement temperatures were changed as indicated in the figure legends, including 33°, 37°, 41°, and 45°C as measured at the surface of the Peltier elements with a thermocouple. Flies that were exceedingly inactive in the pre-test (<0.15 mm/sec walking speed) or that failed to have at least one experience with the punished side of the chamber during training were discarded from the analysis. Determining the number of dwellings on the reinforcement-associated chamber half and the average dwelling times were calculated using custom software similar to that used in determining dwellings in the flight simulator (Dill et al. 1995; R. Wolf, pers. comm.). A dwelling was defined as the period between entry and exit from one side of the chamber. A dwelling period (in seconds) was assigned to the training session in which a fly exited the reinforcement-associated chamber half.
The thermosensitivity assay used the same chambers, but the temperature inside was altered independently of the flies' behavior. Individual flies were presented with a chamber that initially was at 24°C on both sides but then on one side increased to a probe temperature of 27°C and further to 30°, 33°, 37°, 41°, and 45°C, while the other chamber half was kept at 24°C. The chamber half with the lower temperature switched every minute. Flies were tested a total of 7 min. A performance index was calculated for this assay as in the learning assay. As tests for normal distribution of performance indices gave varying results, more conservative nonparametric tests for significance were used (Putz 2002). Genotypes were compared using a Kruskal-Wallis ANOVA or Mann-Whitney U-test, P values <0.05 were considered significant.
The olfactory conditioning paradigm followed previous protocols (Tully and Quinn 1985; Zars et al. 2000a). Flies were trained to avoid two odorants (4-methylcylohexanol and 3-octanol) by associating one with an electric shock of different voltage. A preference test for one of the two balanced odors followed in a T-maze choice point. A performance index was calculated by subtracting the number of flies preferring the formerly punished odorant from those preferring the formerly unpunished odorant divided by the total number of flies. This index was multiplied by 100 to generate a half-PI. An average of two half-PIs, measuring avoidance of each shock-associated-odorant, was calculated in generating the final PI. The number of experiments with each condition was six. Tests for shock avoidance similarly followed typical protocols. Shock avoidance used two shock tubes at the T-maze choice point, one of which was used to shock the flies with the same protocol as in the learning paradigm. The number of flies avoiding the shocked tube was determined in a half-experiment. A final PI was calculated pairing avoidance of either one of both shock tubes. The number of experiments was six for each voltage. Tests for olfactory acuity were carried out using the odorants and conditions used for conditioning. Statistics used in this assay were parametric ANOVAs as the data are normally distributed, as is typical of this paradigm.
Acknowledgments
We thank Jan Judy-March for excellent technical assistance. Dr. Jonathan W. King provided insightful comments on an earlier version of this manuscript. This research was supported by a University of Missouri System Research Board Grant and start-up funds from the University of Missouri-Columbia.
Article published online ahead of print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.45506.
References
- Annilo, T., Tammur, J., Hutchinson, A., Rzhetsky, A., Dean, M., and Allikmets, R. 2001. Human and mouse orthologs of a new ATP-binding cassette gene, ABCG4. Cytogenet. Cell Genet. 94: 196-201. [DOI] [PubMed] [Google Scholar]
- Antonov, I., Antonova, I., Kandel, E.R., and Hawkins, R.D. 2003. Activity-dependent presynaptic facilitation and hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron 37: 135-147. [DOI] [PubMed] [Google Scholar]
- Bingham, P.M. and Chapman, C.H. 1986. Evidence that white-blood is a novel type of temperature-sensitive mutation resulting from temperature-dependent effects of a transposon insertion on formation of white transcripts. EMBO J. 5: 3343-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham, P.M. and Judd, B.H. 1981. A copy of copia transposable element is very tightly linked to the w[a] allele at the white locus of D. melanogaster. Cell 25: 705-711. [DOI] [PubMed] [Google Scholar]
- Birchler, J.A. and Hiebert, J.C. 1989. Interaction of the Enhancer of white-apricot with transposable element alleles at the white locus in Drosophila melanogaster. Genetics 122: 129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman, M.E., Menzel, R., Fietz, A., and Schaefer, S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97: 107-119. [PubMed] [Google Scholar]
- Brembs, B. 2000. An analysis of associative learning in Drosophila at the flight simulator. In Department of Genetics and Neurobiology, pp. 40. University of Wuerzburg, Wuerzburg, Germany.
- Campbell, J.L. and Nash, H.A. 2001. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J. Neurobiol. 49: 339-349. [DOI] [PubMed] [Google Scholar]
- Chen, H., Rossier, C., Lalioti, M.D., Lynn, A., Chakravarti, A., Perrin, G., and Antonarakis, S.E. 1996. Cloning of the cDNA for a human homologue of the Drosophila white gene and mapping to chromosome 21q22.3. Am. J. Hum. Genet. 59: 66-75. [PMC free article] [PubMed] [Google Scholar]
- Croop, J.M., Tiller, G.E., Fletcher, J.A., Lux, M.L., Raab, E., Goldenson, D., Son, D., Arciniegas, S., and Wu, R.L. 1997. Isolation and characterization of a mammalian homolog of the Drosophila white gene. Gene 185: 77-85. [DOI] [PubMed] [Google Scholar]
- Dean, M., Rzhetsky, A., and Allikmets, R. 2001. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 11: 1156-1166. [DOI] [PubMed] [Google Scholar]
- de Belle, J.S. and Heisenberg, M. 1994. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263: 692-695. [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh, S.D., Badner, J.A., Goldin, L.R., Berrettini, W.H., Sanders, A.R., Rollins, D.Y., Turner, G., Moses, T., Haerian, H., Muniec, D., et al. 1996. Affected-sib-pair analyses reveal support of prior evidence for a susceptibility locus for bipolar disorder, on 21q. Am. J. Hum. Genet. 58: 1279-1285. [PMC free article] [PubMed] [Google Scholar]
- Detera-Wadleigh, S.D., Badner, J.A., Yoshikawa, T., Sanders, A.R., Goldin, L.R., Turner, G., Rollins, D.Y., Moses, T., Guroff, J.J., Kazuba, D., et al. 1997. Initial genome scan of the NIMH genetics initiative bipolar pedigrees: Chromosomes 4, 7, 9, 18, 19, 20, and 21q. Am. J. Med. Genet. 74: 254-262. [DOI] [PubMed] [Google Scholar]
- Dill, M., Wolf, R., and Heisenberg, M. 1995. Behavioral analysis of Drosophila landmark learning in the flight simulator. Learn. Mem. 2: 152-160. [DOI] [PubMed] [Google Scholar]
- Dragoi, V. and Staddon, J.E. 1999. The dynamics of operant conditioning. Psychol. Rev. 106: 20-61. [DOI] [PubMed] [Google Scholar]
- Dreesen, T.D., Johnson, D.H., and Henikoff, S. 1988. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol. Cell. Biol. 8: 5206-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dura, J.M., Preat, T., and Tully, T. 1993. Identification of linotte, a new gene affecting learning and memory in Drosophila melanogaster. J. Neurogenet. 9: 1-14. [DOI] [PubMed] [Google Scholar]
- Ephrussi, B. and Herold, J.L. 1945. Studies of eye pigments of Drosophila. II. Effect of temperature on the red and brown pigments in the mutant blood (wbl). Genetics 30: 62-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, A., Liu, L., Xia, S.-z., Feng, C.-h., Wolf, R., and Heisenberg, M. 1996. Conditioned visual flight orientation in Drosophila: Dependence on age, practice, and diet. Learn. Mem. 3: 49-59. [DOI] [PubMed] [Google Scholar]
- Hammer, M. 1993. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366: 59-63. [DOI] [PubMed] [Google Scholar]
- Hammer, M. and Menzel, R. 1998. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5: 146-156. [PMC free article] [PubMed] [Google Scholar]
- Han, P.L., Levin, L.R., Reed, R.R., and Davis, R.L. 1992. Preferential expression of the Drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron 9: 619-627. [DOI] [PubMed] [Google Scholar]
- Hazelrigg, T., Levis, R., and Rubin, G.M. 1984. Transformation of white locus DNA in Drosophila: Dosage compensation, zeste interaction, and position effects. Cell 36: 469-481. [DOI] [PubMed] [Google Scholar]
- Heimbeck, G., Bugnon, V., Gendre, N., Keller, A., and Stocker, R.F. 2001. A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. 98: 15336-15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg, M. and Wolf, R. 1984. Vision in Drosophila: Genetics of microbehavior. Springer-Verlag, Berlin.
- Herrnstein, R.J. 1997. The matching law: Papers in psychology and economics. Harvard University Press, Cambridge, MA.
- Hing, A.L. and Carlson, J.R. 1996. Male-male courtship behavior induced by ectopic expression of the Drosophila white gene: Role of sensory function and age. J. Neurobiol. 30: 454-464. [DOI] [PubMed] [Google Scholar]
- Hollerman, J.R. and Schultz, W. 1998. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat. Neurosci. 1: 304-309. [DOI] [PubMed] [Google Scholar]
- Ikemoto, S. and Panksepp, J. 1996. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav. Neurosci. 110: 331-345. [DOI] [PubMed] [Google Scholar]
- Isabel, G., Pascual, A., and Preat, T. 2004. Exclusive consolidated memory phases in Drosophila. Science 304: 1024-1027. [DOI] [PubMed] [Google Scholar]
- Kandel, E.R. 2001. The molecular biology of memory storage: A dialogue between genes and synapses. Science 294: 1030-1038. [DOI] [PubMed] [Google Scholar]
- Killeen, P.R. 1994. Mathematical principles of reinforcement. Behav. Brain Sci. 17: 105-172. [Google Scholar]
- Lechner, H.A. and Byrne, J.H. 1998. New perspectives on classical conditioning: A synthesis of Hebbian and non-Hebbian mechanisms. Neuron 20: 355-358. [DOI] [PubMed] [Google Scholar]
- Levin, L.R., Han, P.L., Hwang, P.M., Feinstein, P.G., Davis, R.L., and Reed, R.R. 1992. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell 68: 479-489. [DOI] [PubMed] [Google Scholar]
- Loo, S.K. and Bitterman, M.E. 1992. Learning in honeybees (Apis mellifera) as a function of sucrose concentration. J. Comp. Psychol. 106: 29-36. [DOI] [PubMed] [Google Scholar]
- Malleret, G., Haditsch, U., Genoux, D., Jones, M.W., Bliss, T.V., Vanhoose, A.M., Weitlauf, C., Kandel, E.R., Winder, D.G., and Mansuy, I.M. 2001. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell 104: 675-686. [DOI] [PubMed] [Google Scholar]
- Martin, K.C., Casadio, A., Zhu, H., Yaping, E., Rose, J.C., Chen, M., Bailey, C.H., and Kandel, E.R. 1997. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: A function for local protein synthesis in memory storage. Cell 91: 927-938. [DOI] [PubMed] [Google Scholar]
- Menzel, R. and Erber, J. 1972. The influence of the quantity of reward on the learning performance in honeybees. Behaviour 41: 27-42. [Google Scholar]
- Menzel, R., Heyne, A., Kinzel, C., Gerber, B., and Fiala, A. 1999. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav. Neurosci. 113: 744-754. [PubMed] [Google Scholar]
- Montague, P.R., Hyman, S.E., and Cohen, J.D. 2004. Computational roles for dopamine in behavioural control. Nature 431: 760-767. [DOI] [PubMed] [Google Scholar]
- Nakamura, M., Ueno, S., Sano, A., and Tanabe, H. 1999. Polymorphisms of the human homologue of the Drosophila white gene are associated with mood and panic disorders. Mol. Psychiatry 4: 155-162. [DOI] [PubMed] [Google Scholar]
- Nestler, E.J., Barrot, M., DiLeone, R.J., Eisch, A.J., Gold, S.J., and Monteggia, L.M. 2002. Neurobiology of depression. Neuron 34: 13-25. [DOI] [PubMed] [Google Scholar]
- Nilsson, E.E., Asztalos, Z., Lukacsovich, T., Awano, W., Usui-aoki, K., and Yamamoto, D. 2000. Fruitless is in the regulatory pathway by which ectopic mini-white and transformer induce bisexual courtship in Drosophila. J. Neurogenet. 13: 213-232. [DOI] [PubMed] [Google Scholar]
- O'Hare, K., Alley, M.R., Cullingford, T.E., Driver, A., and Sanderson, M.J. 1991. DNA sequence of the Doc retroposon in the white-one mutant of Drosophila melanogaster and of secondary insertions in the phenotypically altered derivatives white-honey and white-eosin. Mol. Gen. Genet. 225: 17-24. [DOI] [PubMed] [Google Scholar]
- Oldfield, S., Lowry, C., Ruddick, J., and Lightman, S. 2002. ABCG4: A novel human white family ABC-transporter expressed in the brain and eye. Biochim. Biophys. Acta 1591: 175-179. [DOI] [PubMed] [Google Scholar]
- Pirotta, V., Hadfield, C., and Pretorius, G.H.J. 1983. Microdissection and cloning of the white locus and the 3B1-3C2 region of the Drosophila X chromosome. EMBO J. 2: 927-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz, G. 2002. Characterization of memories and ignorant (S6KII) mutants in operant conditioning in the heat-box. In Department of Genetics and Neurobiology, pp. 115. Julius Maximillians Universitaet Wuerzburg, Wuerzburg, Germany.
- Putz, G. and Heisenberg, M. 2002. Memories in Drosophila heat-box learning. Learn. Mem. 9: 349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary, S., Denizot, F., Luciani, M., Mattei, M., and Chimini, G. 1996. Molecular cloning of a mammalian ABC transporter homologous to Drosophila white gene. Mamm. Genome 7: 673-676. [DOI] [PubMed] [Google Scholar]
- Sayeed, O. and Benzer, S. 1996. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl. Acad. Sci. 93: 6079-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz, H., Ramond, J., Singh, C.M., and Heberlein, U. 2000. Functional ethanol tolerance in Drosophila. Neuron 28: 261-271. [DOI] [PubMed] [Google Scholar]
- Schultz, W. 2004. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr. Opin. Neurobiol. 14: 139-147. [DOI] [PubMed] [Google Scholar]
- Schwaerzel, M., Monastirioti, M., Scholz, H., Friggi-Grelin, F., Birman, S., and Heisenberg, M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23: 10495-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, B.F. 1950. Are theories of learning necessary? Psychol. Rev. 57: 193-216. [DOI] [PubMed] [Google Scholar]
- Smith, R.F. and Konopka, R.J. 1981. Circadian clock phenotypes of chromosome aberrations with a breakpoint at the per locus. Mol. Gen. Genet. 183: 243-251. [DOI] [PubMed] [Google Scholar]
- Smyth, C., Kalsi, G., Curtis, D., Brynjolfsson, J., O'Neill, J., Rifkin, L., Moloney, E., Murphy, P., Petursson, H., and Gurling, H. 1997. Two-locus admixture linkage analysis of bipolar and unipolar affective disorder supports the presence of susceptibility loci on chromosomes 11p15 and 21q22. Genomics 39: 271-278. [DOI] [PubMed] [Google Scholar]
- Straub, R.E., Lehner, T., Luo, Y., Loth, J.E., Shao, W., Sharpe, L., Alexander, J.R., Das, K., Simon, R., Fieve, R.R., et al. 1994. A possible vulnerability locus for bipolar affective disorder on chromosome 21q22.3. Nat. Genet. 8: 291-296. [DOI] [PubMed] [Google Scholar]
- Strauss, R. and Heisenberg, M. 1993. A higher control center of locomotor behavior in the Drosophila brain. J. Neurosci. 13: 1852-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto, H., Heisenberg, M., and Gerber, B. 2004. Experimental psychology: Event timing turns punishment to reward. Nature 430: 983. [DOI] [PubMed] [Google Scholar]
- Tearle, R.G., Belote, J.M., McKeown, M., Baker, B.S., and Howells, A.J. 1989. Cloning and characterization of the scarlet gene of Drosophila melanogaster. Genetics 122: 595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully, T. and Quinn, W.G. 1985. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. [A] 157: 263-277. [DOI] [PubMed] [Google Scholar]
- Waelti, P., Dickinson, A., and Schultz, W. 2001. Dopamine responses comply with basic assumptions of formal learning theory. Nature 412: 43-48. [DOI] [PubMed] [Google Scholar]
- Wang, H., Pineda, V.V., Chan, G.C., Wong, S.T., Muglia, L.J., and Storm, D.R. 2003. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. J. Neurosci. 23: 9710-9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, F., Qiu, C.S., Kim, S.J., Muglia, L., Maas, J.W., Pineda, V.V., Xu, H.M., Chen, Z.F., Storm, D.R., Muglia, L.J., et al. 2002. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 36: 713-726. [DOI] [PubMed] [Google Scholar]
- Wolf, R. and Heisenberg, M. 1986. Visual orientation in motion-blind flies in an operant behavior. Nature 323: 154-156. [Google Scholar]
- ____. 1991. Basic organization of operant behavior as revealed in Drosophila flight orientation. J. Comp. Physiol. [A] 169: 699-705. [DOI] [PubMed] [Google Scholar]
- Wolf, R., Voss, A., Hein, S., and Heisenberg, M. 1992. Can a fly ride a bicycle? Phil. Trans. R. Soc. Lond. B 337: 261-269. [Google Scholar]
- Wu, Z.L., Thomas, S.A., Villacres, E.C., Xia, Z., Simmons, M.L., Chavkin, C., Palmiter, R.D., and Storm, D.R. 1995. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc. Natl. Acad. Sci. 92: 220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wustmann, G. and Heisenberg, M. 1997. Behavioral manipulation of retrieval in a spatial memory task for Drosophila melanogaster. Learn. Mem. 4: 328-336. [DOI] [PubMed] [Google Scholar]
- Wustmann, G., Rein, K., Wolf, R., and Heisenberg, M. 1996. A new paradigm for operant conditioning of Drosophila melanogaster. J. Comp. Physiol. [A] 179: 429-436. [DOI] [PubMed] [Google Scholar]
- Zachar, Z. and Bingham, P.M. 1982. Regulation of white locus expression: The structure of mutant alleles at the white locus of Drosophila melanogaster. Cell 30: 529-541. [DOI] [PubMed] [Google Scholar]
- Zars, T. 2001. Two thermosensors in Drosophila have different behavioral functions. J. Comp. Physiol. [A] 187: 235-242. [DOI] [PubMed] [Google Scholar]
- Zars, T., Fischer, M., Schulz, R., and Heisenberg, M. 2000a. Localization of a short-term memory in Drosophila. Science 288: 672-675. [DOI] [PubMed] [Google Scholar]
- Zars, T., Wolf, R., Davis, R., and Heisenberg, M. 2000b. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: In search of the engram. Learn. Mem. 7: 18-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S.D. and Odenwald, W.F. 1995. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc. Natl. Acad. Sci. 92: 5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Gainetdinov, R.R., Beaulieu, J.M., Sotnikova, T.D., Burch, L.H., Williams, R.B., Schwartz, D.A., Krishnan, K.R., and Caron, M.G. 2005. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 45: 11-16. [DOI] [PubMed] [Google Scholar]