Abstract
Attenuated molecular clones of simian immunodeficiency virus (SIVmac) are important tools for studying the correlates of protective immunity to lentivirus infection in nonhuman primates. The most highly attenuated SIVmac mutants fail to induce disease but also fail to induce immune responses capable of protecting macaques from challenge with pathogenic virus. We recently described a novel attenuated virus, SIVmac-M4, containing multiple mutations in the transmembrane protein (TM) intracytoplasmic domain. This domain has been implicated in viral assembly, infectivity, and cytopathogenicity. Whereas parental SIVmac239-Nef+ induced persistent viremia and simian AIDS in rhesus macaques, SIVmac-M4 induced transient viremia in juvenile and neonatal macaques, with no disease for at least 1 year postinfection. In this vaccine study, 8 macaques that were infected as juveniles (n = 4) or neonates (n = 4) with SIVmac-M4 were challenged with pathogenic SIVmac251 administered through oral mucosa. At 1 year postchallenge, six of the eight macaques had low to undetectable plasma viremia levels. Assays of cell-mediated immune responses to SIVmac Gag, Pol, Env, and Nef revealed that all animals developed strong CD8+ T-cell responses to Gag after challenge but not before. Unvaccinated control animals challenged with SIVmac251 developed persistent viremia, had significantly weaker SIV-specific T-cell responses, and developed AIDS-related symptoms. These findings demonstrate that SIVmac-M4, which contains a full-length Nef coding region and multiple point mutations in the TM, can provide substantial protection from mucosal challenge with pathogenic SIVmac251.
In the absence of reliable means of inducing protective immunity to human immunodeficiency virus type 1 (HIV-1), there has been substantial interest in using live, attenuated viruses in animal model systems to determine virological correlates of molecular attenuation and immunological correlates of protection (18, 34, 37, 67). Live, attenuated viruses derived from simian immunodeficiency virus (SIV) molecular clones have shown promising levels of protection in macaques (2, 13, 16, 84). However, the ability of an attenuated viral vaccine to induce protective immunity is largely dependent upon the ability of the virus to replicate in the host (17, 35, 45). Enthusiasm for such viruses as potential vaccines has thus been mitigated by the problem of “vaccine perversity”: the possibility that the attenuated virus may replicate and induce AIDS in a portion of recipients. Vaccine perversity is defined in mathematical terms as the fraction of vaccinated individuals who develop disease due to the vaccine virus (9). In studies of SIV, the most highly attenuated viral strains, which replicate poorly in vivo, are unlikely to induce protection while the less-attenuated strains induce solid protection but carry the risk of high perversity (9, 20, 35, 45).
Lentivirus transmembrane proteins (TM) share a conserved structural organization, which includes an N-terminal hydrophobic fusion peptide, an extracellular domain, a hydrophobic membrane anchor domain, and an unusually long C-terminal intracytoplasmic domain (ICD-TM) (33, 59). The ICD-TM region of HIV-1 and SIVmac has been implicated in protein-protein interactions during virion assembly (14, 23), cell surface expression of envelope (8, 40, 65), cell-cell fusion (62, 72, 85), induction of cytopathology (52, 74), and apoptosis (50, 58), perhaps related to binding of calmodulin (50, 73, 76, 77).
Although the precise virologic functions of the ICD-TM remain incompletely understood, several studies have suggested that disruption of this domain leads to an attenuated phenotype (11, 25, 32, 38, 46, 48, 70). In an earlier study, we created a mutant, SIVmac-M4, containing multiple mutation clusters in the ICD-TM. Taken individually, each of these mutation clusters reduced viral replication in vitro, at least in part by reducing envelope stability and/or incorporation into virions (69). We then tested the pathogenic potential of SIVmac-M4 in juvenile and neonatal rhesus macaques. Juvenile macaques intravenously inoculated with SIVmac-M4 developed transient viremia, which dropped below detectable levels by 8 to 12 weeks postinoculation, and the animals remained asymptomatic for 1.5 years (70). Neonatal macaques inoculated with SIVmac-M4 survived the acute phase of infection and maintained a condition of health with no disease signs for over 1 year postinoculation. These results thus demonstrated that the ICD-TM could be a locus for viral attenuation in both juvenile and neonatal rhesus macaques.
Because neonatal and juvenile rhesus macaques survived infection with SIVmac-M4 and remained clinically well, we predicted that these animals would be protected from high viral loads and disease progression upon challenge with pathogenic SIVmac. To test this prediction, eight macaques previously infected with SIVmac-M4 were challenged with two oral doses of pathogenic, uncloned SIVmac251. These animals exhibited a striking reduction in viral load and disease progression compared to naïve control macaques challenged with the same virus. Macaques previously infected with SIVmac-M4 mounted robust SIV-specific CD8+ T-cell responses, which were significantly greater than those observed in unvaccinated control macaques. These findings confirm that the ICD-TM is a locus for SIVmac viral attenuation in rhesus macaques and suggest that strategies to modulate regions other than the nef gene are of interest in the study of virologic and immunologic correlates of protection.
MATERIALS AND METHODS
Construction of SIVmac-M4 and preparation of virus stocks.
Mutant SIVmac-M4 was initially designated SIVmac239-M3 stop and was created by oligonucleotide-directed mutagenesis of SIVmac239-Nef+ (69). A diagram showing the positions of individual mutations was presented in an earlier publication (70). The GenBank accession number for SIVmac239 is M33262 (61). SIVmac-M4 contains point mutations at four sites: a single stop codon at nucleotides (nt) 9056 to 9058 of env (mutation 1); two stop codons at nt 9197 to 9199 and 9203 to 9205 (mutation 2); a single base-pair deletion, nt 9318, creating a (+1) frameshift mutation in the TM reading frame (mutation 3); and three conserved Arg codons changed to Gly codons in the C-terminal amphipathic alpha helix of TM, encompassing nt 9459 to 9470 (mutation 4). Taken individually, mutations 2, 3, and 4 each reduce viral infectivity (69).
Infectious SIVmac-M4 was recovered by transfection of COS-7 cells with a mixture of linearized plasmids encoding the 5′ and 3′ halves of the viral genome, followed by expansion of virus stocks on CEMx174 cells, as previously described (70). The 50% tissue culture infective dose (TCID50) of each virus stock was determined by serial dilution on replicate cultures of CEMx174 cells in a 96-well plate format (36). To perform sequence verification, viral RNA was extracted from all stocks, using the QIAmp viral RNA purification kit (Qiagen, Chatsworth, Calif.), and reverse transcribed, using Superscript II (Gibco-BRL) primed with random hexamers (Pharmacia, Piscataway, N.J.). Amplification products were purified using Qiaquick (Qiagen) and sequenced using sense strand primers SIV-342 (bases 8997 to 9019 [5′-TGCTAGCTAAGTTAAGGCAGGGG-3′]) and SIV-337 (bases 9265 to 9282 [5′-CCAGAGGCTCTCTGCGAC-3′]).
Inoculation of rhesus macaques and collection of samples.
Animals utilized in this study were colony-bred juvenile or newborn rhesus macaques (Macaca mulatta) housed at the California Regional Primate Research Center and determined to be free of simian type D retroviruses, SIV, and simian T-lymphotropic virus. These animals were maintained in accordance with the standards of the Institutional Animal Care and Use Committee. Physical examinations were performed at regular intervals to detect lymphadenopathy, splenomegaly, and opportunistic infections. Clinical criteria for euthanasia consisted of three or more of the following: (i) more than 10% weight loss within 2 weeks or more than 20% in 2 months; (ii) chronic diarrhea unresponsive to treatment; (iii) infections unresponsive to antibiotic treatment; (iv) inability to maintain body heat or fluids without supplementation; and (v) persistent, marked hematological abnormalities (persistent, marked splenomegaly or hepatomegaly).
For the initial study of viral attenuation, juvenile rhesus macaques (group A) were inoculated with 103 or 104 TCID50 of cell-free virus by the intravenous route (70). Neonatal rhesus macaques (group B) were inoculated intravenously at 2 days of age with 2 × 103 or 1 × 104 TCID50 of cell-free virus (70). For the vaccine challenge study, all four animals in group A were given a second, booster injection containing 103 TCID50 of SIVmac-M4 at 102 weeks after the initial dose. Two group B animals (Mmu 31342 and Mmu 31346) were given a second intravenous booster injection of SIVmac-M4 (103 TCID50) at 67 weeks. From each juvenile macaque, 8 to 15 ml of blood was collected by venipuncture immediately prior to inoculation and at 1, 2, 4, 8, and 10 weeks postboost.
At 12 weeks following the second injection of SIVmac-M4 (week 114 of the study for group A and week 79 for group B), all animals were challenged with a stock (of previously determined titer) of pathogenic, uncloned SIVmac251 propagated on rhesus macaque peripheral blood mononuclear cells (PBMC) (51, 57). The challenge was administered as two oral doses of 105 TCID50 of SIVmac251, given 4 h apart. This protocol was previously determined to reliably infect rhesus macaques with SIVmac251 (51, 57). Immediately prior to challenge and at 2, 4, 8, 12, 24, 32, and 53 to 56 weeks postchallenge, 8 to 15 ml of blood was collected by venipuncture. Plasma was stored for virus load and antibody determinations. Complete blood count and CD4+/CD8+ T-lymphocyte subset analysis were performed at each time point. Lymph node tissue was obtained by surgical biopsy at 0, 8, 24, and 53 to 56 weeks postchallenge.
Virus load in plasma and mononuclear cells.
Viral RNA in plasma samples was quantified by TaqMan real-time PCR as recently described (44). Results obtained with this method are comparable to those obtained with the branched-chain, or bDNA, assay (44). However, the real-time TaqMan PCR assay can detect as few as 50 copies of SIVmac RNA/ml, while the bDNA method is only sensitive to 1,500 copies of RNA/ml (44). Cell-associated viremia was measured in PBMC and lymph node mononuclear cells (LNMC) by a limiting dilution assay as previously described (48). Briefly, serial 10-fold dilutions of rhesus PBMC or LNMC were cocultivated with SIV-susceptible cell line CEMx174 in replicate wells of a 24-well tissue culture plate. Cultures were maintained for 4 weeks. SIV p27 antigen in culture supernatants was detected using an SIVmac p27gag enzyme-linked immunosorbent assay (ELISA) kit (Coulter Immunology, Hialeah, Fla.). Cell-associated virus load was calculated using the method of Reed and Muench, as described by Marthas et al. (48).
PCR amplification and sequencing of viral DNA.
Genomic DNA was isolated from either 107 PBMC or 107 CEMx174 cells cocultured with PBMC from infected macaques (Qiagen tissue kit). SIVmac TM sequences were amplified in a nested PCR, using first-round primers SIV-340 (nt 8901 to 8920) and SIV-341 (nt 9918 to 9940) and second-round primers SIV-342 (nt 8997 to 9019) and SIV-343 (nt 9816 to 9834), and sequenced on an ABI automated sequence analyzer (Applied Biosystems-Perkin-Elmer, Foster City, Calif.).
Antibody studies.
Antibodies to SIVmac were assessed in longitudinal plasma samples, using an HIV-1/HIV-2 peptide ELISA (Genetic Systems Corporation, Redmond, Wash.). Neutralizing antibodies were assessed using two stocks of SIVmac251: primary SIVmac251 and and T-cell line-adapted (TCLA) SIVmac251. Neutralizing antibodies were measured in CEMx174 cells in the case of TCLA SIVmac251 and in CEMx174-R5 cells in the case of primary SIVmac251 by determination of the reduction in virus-induced cytopathology, as previously described (54). Assay stocks of virus were prepared in H9 cells (TCLA SIVmac251) and human PBMC (primary SIVmac251). Titers were reported as the reciprocal serum dilution at which 50% of cells were protected from virus-induced killing as measured by neutral red uptake.
ELISPOT assay.
Cryopreserved PBMC were tested in duplicate or triplicate using a recombinant vaccinia virus (r-VV)-based enzyme-linked immunospot (ELISPOT) assay (55). Briefly, PBMC (1 × 105 or 2 × 105 per well) were placed in a 96-well sterile culture plate and infected at a multiplicity of infection (MOI) of 2:1 with r-VV expressing SIVmac251 antigens (Gag, vAbT252; Env, vAbT2531; Pol, vAbT258; Nef) or control r-VV (without thymidine kinase) (obtained from Therion Biologicals, Cambridge, Mass.). Phytohemagglutinin was used as a positive control. Following overnight incubation at 37°C, cells were transferred to a 96-well ELISPOT plate (U-Cytech, Utrecht, The Netherlands) previously coated with monoclonal antibody to macaque gamma interferon (IFN-γ). ELISPOTs were incubated for 5 h and then developed as previously described (55). Spot-forming cells (SFC) were quantified using an automated ELISPOT reader (Cell Technology Inc., Bethesda, Md.). Results were calculated as SFC per 106 PBMC after subtracting background SFC for the thymidine kinase-free control vaccinia virus. Based upon background responses to SIV antigens observed in unexposed rhesus macaques, a negative cutoff was established as 50 SFC per 106 PBMC. Statistical comparisons were performed using SigmaStat and SigmaPlot software (SPSS Software, Chicago, Ill.).
RESULTS
Macaques inoculated as juveniles with SIVmac-M4 are substantially protected from pathogenic SIVmac251.
In an earlier report, we demonstrated that SIVmac-M4, containing multiple point mutations in the ICD of the TM subunit of the envelope glycoprotein, was attenuated for replication and pathogenesis in both juvenile and neonatal rhesus macaques (70). In four out of four juvenile macaques, SIVmac-M4 induced transient plasma viremia that declined to undetectable levels by 8 to 12 weeks postinoculation (<1,500 viral RNA copies/ml, as measured by branched DNA assay). Viremia then remained below detectable limits in all four juvenile macaques up to 80 weeks postinoculation (70). More recent analysis of duplicate samples using the more sensitive TaqMan assay confirmed that animals had low viral loads but revealed that one macaque, Mmu 28300, continued to harbor 1 to 200 copies of virus/ml until week 42 (Fig. 1).
FIG. 1.
Viral load in rhesus macaques immunized with SIVmac-M4 and challenged orally with SIVmac251. Animals in group A (left panels) were given booster injections at 102 weeks and challenged at 114 weeks; animals in group B (right panels) were given booster injections at 67 weeks and challenged at 79 weeks. Values on the x axis indicate weeks postimmunization with SIVmac-M4. (A) Plasma viral load as measured by TaqMan PCR. Values on the y axis indicate viral RNA equivalents/ml of plasma. (B) Cell-associated virus in PBMC as measured by coculture with CEMx174 cells. Values on the y axis indicate TCID50 per 106 PBMC. (C) Cell-associated virus in LNMC as measured by coculture with CEMx174 cells. Values on the y axis indicate TCID50 per 106 LNMC.
To determine whether prior exposure to SIVmac-M4 would provide protection from pathogenic challenge, the macaques previously inoculated with attenuated virus were challenged by oral administration of a stock of SIVmac251 of previously determined titer (51, 57). Prior to pathogenic challenge, we attempted to boost immune responses by administering a second intravenous injection of SIVmac-M4. All four macaques in group A were given booster injections at 102 weeks after initial inoculation with 103 TCID50 of SIVmac-M4. Interestingly, none of the macaques showed detectable plasma viremia following this second inoculation with SIVmac-M4 (Fig. 1 and data not shown). At 12 weeks postboost (114 weeks after initial inoculation), all four animals were challenged with two oral doses of 105 TCID50 of SIVmac251 given 4 h apart (51, 57). Viral load in the vaccinated animals was compared with viral load in six unvaccinated controls challenged with the same virus stock (Fig. 1 and 2).
FIG. 2.
Viral load in unimmunized control rhesus macaques challenged orally with SIVmac251. Six unimmunized juvenile rhesus macaques were challenged orally with SIVmac251, as described in the text. Values on the x axis indicate weeks postchallenge. Values on the y axis indicate viral RNA equivalents/ml of plasma, as measured by TaqMan PCR.
At 2 weeks postchallenge, peak plasma viremia was significantly lower in group A animals that had received SIVmac-M4 (median, 1.2 × 104 copies/ml) than in unvaccinated controls (median, 6.3 × 107 copies/ml; P = 0.010 [Mann-Whitney rank sum test]) (Fig. 1, left panels, and Fig. 2). Three of four macaques in group A had detectable viremia at 2 weeks postchallenge, but viremia dropped below detectable levels by 4 weeks postchallenge. A fourth macaque, Mmu 28388, had no detectable viremia at any time point postchallenge. The presence of cell-associated virus in PBMC at 2 weeks postchallenge was barely detectable (<5 TCID50/106 PBMC) in three of four animals and was undetectable in Mmu 28388 (Fig. 1B, left panel). Throughout the remainder of the follow-up period, Mmu 28388 had no detectable plasma- or PBMC-associated viremia. Macaques Mmu 28323 and 28543 had plasma viral loads below 3,000 copies/ml and low (<5 TCID50/106 PBMC) to undetectable PBMC-associated virus loads. The fourth animal, Mmu 28300, developed a viral load of 4 × 105 copies/ml by 24 weeks postchallenge (week 138 of the study), with 100 TCID50 of virus per 106 PBMC. To determine the extent of virus replication in lymphoid tissues, lymph node mononuclear cells were assessed for the presence of SIVmac by coculture at 8, 24, and 53 to 54 weeks postchallenge (i.e., weeks 122, 138, and 166 to 167 of the study, respectively) (Fig. 1C, left panel). Mmu 28388 showed no detectable virus in LNMC at any of these time points. Mmu 28323 and 28543 each had a detectable level of ≤25 TCID50/106 LNMC at all time points tested. The fourth animal, Mmu 28300, had a detectable level of 316 TCID50/106 LNMC at 24 weeks, which increased to 4,642 TCID50/106 LNMC at 53 weeks.
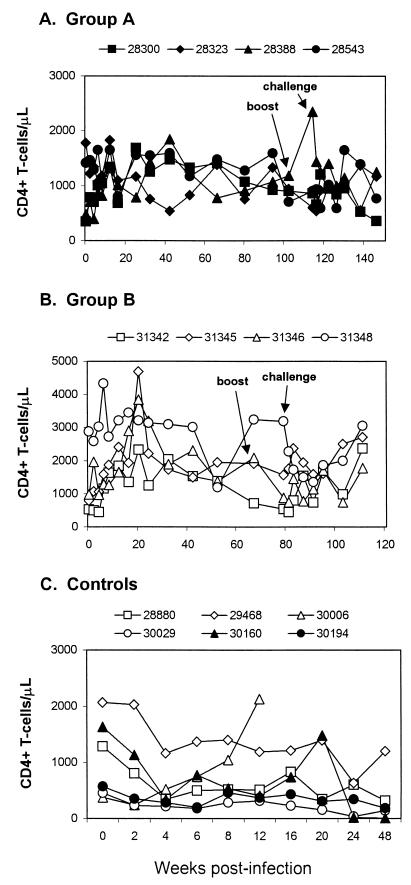
Absolute CD4+ T-cell counts in three of the group A animals generally remained above 900 cells/μl (Fig. 3A). However, Mmu 28300, the animal with the weakest containment of challenge virus, began to exhibit a decline in CD4+ T-cell numbers beginning at 24 weeks postchallenge (week 138 of the study). Taken together, these results indicated that three of four vaccinated juvenile macaques had strong containment of the challenge virus and a fourth had weaker containment but substantial reduction in viral load compared to unvaccinated control macaques.
FIG. 3.
CD4+ T-cell counts in rhesus macaques. Lymphocyte subset analysis was performed by flow cytometry after staining fresh whole blood with monoclonal antibodies recognizing CD4+ T cells (OKT4), CD8+ T cells (Leu2A), CD2+ T cells (Leu5b), and CD19+ B cells (Leu16). The figure shows peripheral blood CD4+ T-cell counts in macaques immunized with SIVmac-M4 as juveniles (A) or as neonates (B) and in unimmunized macaques as controls (C).
Macaques inoculated as neonates with SIVmac-M4 are protected from subsequent challenge with pathogenic SIVmac251.
Earlier studies suggested that infection of neonates with certain highly attenuated SIVmac strains (i.e., SIVmac1A11) could provide protective immunity against challenge with SIVmac251 later in life (57). In our earlier study, four of four neonates that received SIVmac-M4 showed control of viremia and remained healthy for 1 year. These findings were reported previously (70) and contrasted sharply with the persistent viremia and rapid progression to simian AIDS observed in historical control neonates exposed to SIVmac (57, 78-80). We hypothesized that these macaques might have developed immune responses that could provide partial protection from pathogenic challenge as juveniles.
At 67 weeks following the initial inoculation with SIVmac-M4, macaques 31342 and 31346 were administered a booster dose of SIVmac-M4. Neither animal showed detectable viremia following the booster injection. Twelve weeks later, at week 79 (i.e., at 79 weeks of age), these macaques, as well as Mmu 31345 and 31348, were challenged with two oral doses of 105 TCID50 of SIVmac251. At 2 to 4 weeks postchallenge, three of the four animals had low-level but detectable plasma viremia (271 to 1,186 copies/ml) and all four had cell-associated virus by coculture of PBMC (Fig. 1, right panels). Viral loads were significantly lower than those observed in unvaccinated controls (median for group B at 2 weeks, 5.2 × 102; P = 0.010 [Mann-Whitney rank sum test]). At 8 weeks postchallenge, viremia dropped to undetectable levels in plasma and borderline-detectable levels in PBMC in three of the four animals. Of these, one macaque, Mmu 31346, had no detectable virus in plasma, PBMC, or LNMC for the remainder of the study. Mmu 31342 and 31345 had intermittent borderline-detectable levels of SIVmac by coculture of PBMC and LNMC, but both animals were negative for virus coculture at 1 year postchallenge (Fig. 1). The fourth animal, Mmu 31348, showed persistent viremia, in the range of 600 to 5,700 copies/ml, for the remainder of the study. None of the animals in group B displayed an obvious CD4+ T-cell decline during the observation period; CD4+ T-cell counts generally remained above 1,000 cells/μl (Fig. 3B), while those of control animals declined markedly (Fig. 3C).
Taken together, these results demonstrate that three of four animals in group B had very strong containment of SIVmac251 and a fourth had weaker containment of the challenge virus but showed reduction in viral load compared to unvaccinated controls. Interestingly, there was no correlation between induction of acute gastrointestinal symptoms following the initial inoculation with SIVmac-M4 and inability to control the challenge virus. Both Mmu 31342 and 31346 experienced mild to severe gastrointestinal disorders as neonates but survived SIVmac-M4 infection and demonstrated good control of SIVmac251 viremia. There was also no correlation between the magnitude of the initial dose of SIVmac-M4 used to inoculate the neonates and the eventual outcome. Both Mmu 31346, who showed strongest control of SIVmac251, and Mmu 31348, who showed weakest control of the challenge virus, received initial injections of 104 TCID50 of SIVmac-M4. Mmu 31342 and 31345 received 2 × 103 TCID50 of SIVmac-M4. Both of the group B animals that received a booster dose of SIVmac-M4 prior to challenge (Mmu 31342 and 31346) demonstrated very strong containment of challenge virus; however, the sample size was too small to assess a correlation between boosting and outcome in this study.
Unvaccinated control macaques mucosally administered SIVmac251 develop persistent, high-level viremia.
In contrast to the macaques that received SIVmac-M4, unvaccinated control animals challenged with two oral doses of 105 TCID50 of SIVmac251 developed persistent, high-level plasma viremia (Fig. 2). At 2 weeks postchallenge, six control macaques had peak viral loads in the range of 2.1 × 107 to 1.8 × 108 copies/ml. Viral loads remained above 106 copies/ml at 8 weeks postchallenge and above 250,000 at 20 weeks postchallenge. All unvaccinated control macaques showed evidence of SIVmac-related disease at necropsy, including cholecystitis, endocarditis, enterocolitis, lymphadenopathy, Pneumocystis carinii pneumonia, peritonitis, splenomegaly, thymic atrophy, and typhlocolitis.
Genotype of virus isolated from viremic macaques.
At 24 weeks postchallenge (week 138 of the study), four animals, including three from group A and one from group B, had detectable plasma viremia. In addition, one animal, Mmu 31345, lacked plasma- or PBMC-associated virus but showed detectable virus in LNMC coculture. Two animals, Mmu 28388 and 31346, lacked detectable virus in plasma, PBMC, or LNMC. To establish whether the viremia observed in these animals was due to SIVmac-M4 or to the challenge virus, SIVmac251, genomic DNA was isolated from PBMC or LNMC coculture material at 24 weeks postchallenge. SIVmac TM sequences were amplified in a nested PCR, using first- and second-round primers designed to amplify the region of SIVmac-M4 containing point mutations (i.e., first-round amplification, nt 8901 to 9940; second-round amplification, nt 8997 to 9834). PCR amplification, followed by sequence analysis of viral DNA, revealed that Mmu 28300, 28323, 28543, and 31348 were infected with SIVmac251 (Table 1). However, analysis of genomic DNA from Mmu 31345 revealed that LNMC from this macaque contained only the attenuated mutant, SIVmac-M4, and not the challenge virus. Thus, despite an absence of detectable plasma viremia, this animal continued to harbor SIVmac-M4 viral sequences in lymphoid tissues.
TABLE 1.
Virus persistence in vaccinated macaques
| Macaque | Age status when inoculated with SIVmac-M4 | Virus persistence at 24 wk postchallengea |
|---|---|---|
| Group A | ||
| Mmu 28300 | Juvenile | PBMC positive (SIVmac251) |
| Mmu 28323 | Juvenile | PBMC positive (SIVmac251) |
| Mmu 28388 | Juvenile | Plasma, PBMC, LNMC negative |
| Mmu 28543 | Juvenile | PBMC positive (SIVmac251) |
| Group B | ||
| Mmu 31342 | Neonate | Plasma, PBMC, LNMC negative |
| Mmu 31345 | Neonate | LNMC positive (SIVmac-M4) |
| Mmu 31346 | Neonate | Plasma, PBMC, LNMC negative |
| Mmu 31348 | Neonate | PBMC positive (SIVmac251) |
As determined by PCR and sequence analysis of ICD-TM (see text).
Neutralizing antibody production.
Antibody responses to SIVmac were assessed in plasma samples from all eight vaccinees and six unvaccinated controls, using a standard ELISA. SIVmac-specific antibodies were detected in all eight macaques in groups A and B after SIVmac-M4 infection (70) and persisted following challenge with SIVmac251. Antibody titers following challenge with SIVmac251 were similar in vaccinated and unvaccinated macaques (Fig. 4).
FIG. 4.
Plasma antibody titers to SIVmac. Antibodies to SIV were assessed in longitudinal samples from experimentally inoculated macaques, using an HIV-1/HIV-2 peptide ELISA (Genetic Systems Corporation). (A) Antibody titers for macaques vaccinated with SIVmac-M4 as juveniles and challenged with SIVmac251. (B) Antibody titers for macaques vaccinated with SIVmac-M4 as neonates and challenged as juveniles with SIVmac251. (C) Antibody titers for unvaccinated macaques as controls challenged with SIVmac251. Values on the x axis indicate weeks postinfection.
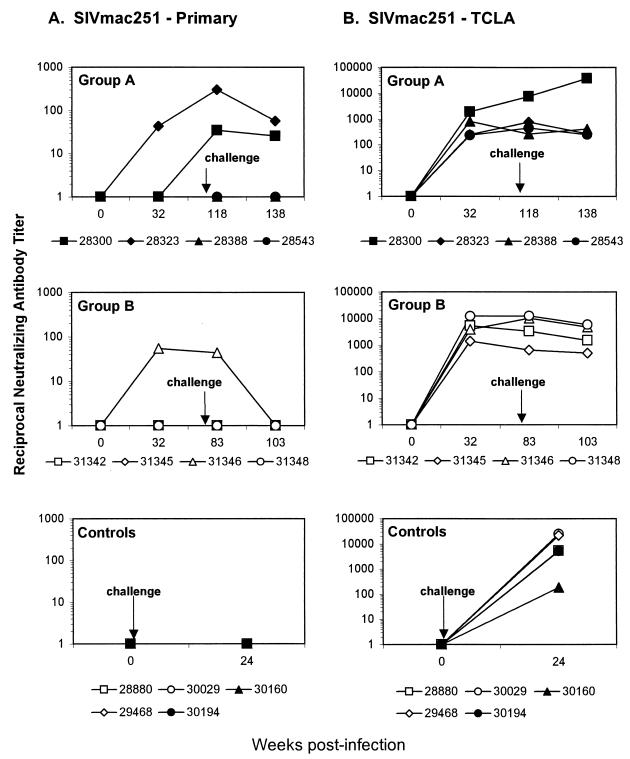
Neutralizing antibody production was assessed using two stocks of SIVmac251: a TCLA stock, which is highly sensitive to neutralization, and a primary stock of the same virus, which is extremely difficult to neutralize. As shown in Fig. 5, antibodies to the latter stock of virus were detected in only three of the vaccinated animals (Mmu 31346, 28300, and 28323) and in none of the controls. Antibodies to the TCLA strain of SIVmac251 were detected in plasma from all animals. Among vaccinated macaques, antibody titers to the TCLA strain were generally higher in group B animals than in group A animals and were highest in the two animals demonstrating the weakest control of viremia (i.e., Mmu 28300 and 31348).
FIG. 5.
Neutralizing antibody titers. Neutralizing antibodies capable of reducing the infectivity of primary SIVmac251 and TCLA SIVmac251 were assessed in macaque plasma. Titers are reported as the reciprocal plasma dilution at which 50% of cells were protected from virus-induced killing. (A) Neutralization titers to primary SIVmac251. (B) Neutralization titers to TCLA SIVmac251. Results are shown for animals in group A (top), group B (center), and the control group (bottom).
SIV-specific CD8+ T-cell responses prior to pathogenic challenge.
To assess T-cell responses to SIV antigens, we utilized an ELISPOT assay measuring IFN-γ production by SIV-specific T cells. Although this assay utilizes whole PBMC containing both CD4+ and CD8+ T cells, depletion experiments using magnetic beads have demonstrated that 80 to 90% of IFN-γ producing responder cells are CD8+ T cells when antigen is supplied via r-VV (43). In this assay, peripheral blood monocytes are the principal antigen-presenting cell population and most antigen presentation occurs via the major histocompatibility complex (MHC) class I restricted pathway when cells are infected with r-VV (43).
During the first 80 weeks after exposure to attenuated SIVmac-M4, three of four juveniles in group A had detectable T-cell responses to SIV Gag (Fig. 6, left panels) ranging from 70 to 205 SFC/106 PBMC. One animal, Mmu 28323, had no detectable responses. Mmu 28388 had a detectable Gag-specific response at 12 weeks postinoculation only (205 SFC/106 PBMC). In Mmu 28300 and 28543, Gag responses were more sustained over time but remained at or below 250 SFC/106 PBMC (78 to 140 and 70 to 203 SFC/106 PBMC, respectively). All four animals in group A received a second intravenous dose of SIVmac-M4 at 102 weeks. This booster injection induced increased CD8+ T-cell responses in the absence of detectable viremia. All four animals, including Mmu 28323, developed enhanced responses to SIVmac Gag at between 102 and 114 weeks. Two animals, Mmu 28300 and 28543, developed responses to SIVmac Nef (103 and 60 SFC/106 PBMC, respectively). Mmu 28300 also developed a response to SIVmac Env (68 and 185 SFC/106 PBMC at 106 and 114 weeks, respectively).
FIG. 6.
SIV-specific IFN-γ production by CD8+ T cells from rhesus macaques immunized as juveniles with SIVmac-M4. PBMC from group A macaques were tested in an ELISPOT assay for IFN-γ production in response to stimulation with SIVmac antigens Gag, Pol, Nef, and Env. Results are reported as IFN-γ SFC per 106 PBMC. Left panels: immune responses after initial infection with SIVmac-M4. Right panels: immune responses after challenge with SIVmac251. Values on the x axis indicate weeks postvaccination.
During the first 52 weeks after exposure to SIVmac-M4, three of four group B neonatal macaques lacked detectable CD8+ T-cell responses to SIV antigens (Fig. 7, left panels). Mmu 31348 had measurable Gag-specific responses beginning at 20 weeks postinoculation. The magnitude of this response continued to increase until 67 weeks postinoculation (maximum, 805 SFC/106 PBMC) and increased again following pathogenic challenge at 79 weeks.
FIG. 7.
SIV-specific IFN-γ production by CD8+ T cells from rhesus macaques immunized as neonates with SIVmac-M4. PBMC from group B macaques were tested in an ELISPOT assay for IFN-γ production in response to stimulation with SIVmac antigens Gag, Pol, Nef, and Env. Results are reported as IFN-γ SFC per 106 PBMC. Left panels: immune responses after initial infection with SIVmac-M4. Right panels: immune responses after challenge with SIVmac251. Values on the x axis indicate weeks postvaccination.
SIV Gag-specific T-cell responses predominate after challenge.
CD8+ T-cell responses to Gag, and in some cases to Nef and/or Env, were detectable on the day of challenge in all animals that received SIVmac-M4 (Fig. 6 and 7, left panels). After challenge with SIVmac251, responses in most macaques increased in both magnitude and breadth (Fig. 6 and 7, right panels). However, Gag-specific responses predominated in six of eight vaccinated animals. Two animals from group A (Mmu 28323 and 28388) and one from group B (Mmu 31348) responded only to Gag. Two animals, Mmu 28543 and 31342, had strong Nef responses, and one animal, Mmu 31346, had a strong Env response. Molecular HLA typing of all eight macaques revealed that only one, Mmu 31348, showed positive results for the Mamu-A∗01 allele. Accordingly, PBMC from this macaque were tested for CD8+ T-cell responses to a peptide corresponding to the immunodominant Mamu-A∗01 p11C(C-M) epitope (39). Prior to challenge, a weak response to this peptide was detected; however, after challenge, the p11C-specific response increased, apparently accounting for the majority of the postchallenge Gag-specific response. Interestingly, the three group B animals that did not mount strong prechallenge T-cell responses had low-to-undetectable viremia following challenge, whereas Mmu 31348, which had a strong Gag-specific response before challenge, had persistent SIVmac251 viremia postchallenge.
SIV-specific T-cell responses in unvaccinated control macaques.
The six unvaccinated control animals fell into three groups according to the magnitude of CD8+ T-cell responses after challenge (Fig. 8). Mmu 28880 had high T-cell responses beginning early postinoculation, with peak Gag responses greater than 400 SFC per 106 PBMC. Macaques Mmu 30029, 30160, and 29468 had relatively weak responses (generally below 250 SFC per 106 PBMC), which peaked early after infection and quickly waned. Finally, Mmu 30194 and 30006 lacked significant responses. Mmu 30006 was euthanized at 11 weeks postinoculation with inanition and dehydration and on necropsy was found to have lymphoid depletion, thymic atrophy, enterocolitis, and probable Pneumocystis carinii. Taken as a group, unvaccinated control macaques had significantly lower SIV-specific CD8+ T-cell responses after challenge than either the group A macaques (P = 0.015, Student's t test) or the group B macaques (P = 0.004, Student's t test) (Fig. 9). When responses to all antigens (i.e., Gag, Pol, Nef, and Env) were combined, the average postchallenge ELISPOT responses among macaques in group A ranged from 285 to 728 SFC/106 PBMC. Average postchallenge responses among macaques in group B ranged from 356 to 535 SFC/106 PBMC. With one exception (Mmu 28880), average combined responses in unvaccinated control macaques were much lower, ranging from 35 (not significant) to 300 SFC/106 PBMC.
FIG. 8.
SIV-specific IFN-γ production by CD8+ T cells from unimmunized rhesus macaques as controls challenged with SIVmac251. As described in the legends to Fig. 6 and 7, PBMC from six unimmunized macaques as controls were tested in an ELISPOT assay for IFN-γ production in response to stimulation with SIVmac antigens. Results are reported as IFN-γ SFC per 106 PBMC. Values on the x axis indicate weeks postchallenge.
FIG. 9.
Comparison of SIV-specific CD8+ T-cell responses in vaccinated and unvaccinated macaques. Results of ELISPOT assays are summarized in box plots. For all box plots, the boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median value, and the boundary of the box farthest from zero indicates the 75th percentile. Lines above and below the box indicate the 90th and 10th percentiles. P values represent results of Student's t tests, as described in the text.
DISCUSSION
SIVmac-M4 induces substantial protection from SIVmac251 challenge.
This report demonstrates that prior exposure to live, attenuated SIVmac-M4, which contains mutations in the ICD of Env-TM, reduced viral load and delayed disease progression in macaques challenged mucosally with pathogenic SIVmac251. We did not observe a strict correlation between strong CD8+ T-cell responses and suppression of viremia, as has been suggested by some studies of individuals infected with HIV (56) (Fig. 6 and 7). However, macaques that received live, attenuated SIVmac-M4 had stronger, broader (i.e., responding to more SIVmac antigens), and more sustained CD8+ T-cell responses to SIVmac antigens postchallenge than unvaccinated control animals (Fig. 8 and 9). Interestingly, although most other attenuated viruses tested to date contain lesions in the nef gene, SIVmac-M4 encodes an intact nef reading frame. Two out of four macaques in group A and three of four macaques in group B had detectable Nef-specific T-cell responses at several time points, and Nef responses dominated in one animal, Mmu 31342.
Kinetics of induction of SIV-specific CD8+ T-cell responses in juvenile macaques.
Use of the vaccinia virus-based ELISPOT assay allowed us to perform a detailed assessment of the kinetics of induction of CD8+ T-cell responses in macaques following infection with a live, attenuated virus. Most prior studies have assessed responses to a limited number of antigens, using bulk 51Cr release assay or limiting dilution analysis, which are technically cumbersome and require lengthy (10 to 14 days) restimulation of effector cells. Recent studies have demonstrated that limiting dilution analysis may underestimate the frequency of antigen-specific T cells by 1 to 2 orders of magnitude (21, 22, 41, 43). The ELISPOT assay is particularly well suited to the study of SIVmac infection, as it allows sensitive quantification of cytokine release in response to peptides or viral proteins irrespective of MHC restriction.
Our results demonstrated that all vaccinated macaques developed strong SIV-specific T-cell responses following pathogenic challenge. Prior to challenge, SIV-specific T-cell responses were generally weak but Gag-specific T cells were detected in three of four juveniles at maximal frequencies of 140 to 205 IFN-γ producing cells per million PBMC (Fig. 6). These results are similar to SIV-specific precursor frequencies previously reported for macaques infected with SIVmacΔNef (35). Administering a second intravenous booster injection of SIVmac-M4 at 102 weeks appeared to enhance the magnitude of T-cell responses and increase the breadth of viral antigens recognized.
Development of SIV-specific CD8+ T-cell responses in neonatal macaques.
Simian AIDS in macaque neonates is generally more severe than in juveniles or adults; similarly, HIV-1-infected children show more severe disease than adults. Furthermore, a proportion of macaque neonates infected with most attenuated SIV strains also progress to simian AIDS (4, 5, 49, 66). However, neonates infected with the very highly attenuated SIVmac1A11 do not develop disease (49). In one study, neonates infected with SIVmac1A11 were protected from oral challenge with pathogenic SIVmac251 more than 1 year after inoculation with attenuated virus (57). This observation suggests that (i) very highly attenuated strains of SIVmac are not inevitably pathogenic in neonates and that (ii) the immune system of very young rhesus macaques can generate protective immune responses. Cellular immune responses in neonatal macaques have not been thoroughly characterized. However, several reports suggest that some human infants infected with HIV-1, in some cases as young as 2 to 6 months of age, are capable of mounting HIV-1-specific cytotoxic T lymphocyte (CTL) responses (10, 47, 60, 63).
In our study, the vaccinia virus ELISPOT assay did not detect SIV-specific CD8+ T-cell responses in three of four neonatal macaques during the first year following exposure to SIVmac-M4. However, one (Mmu 31348) of four neonates mounted a strong Gag-specific CD8+ T-cell response beginning as early as 20 weeks postinfection (Fig. 7). By week 79, all four neonates had detectable responses. It is intriguing that none of the three neonates who lacked SIV-specific CD8+ T-cell responses after initial infection succumbed to SIVmac-M4. This observation suggests that other immune responses, perhaps including β-chemokine production and/or innate immune responses, may be critical components of the neonatal immune response (28, 30, 82).
What immune responses correlate with protection?
The correlates of protective immunity induced by live, attenuated retroviruses remain a subject of debate. Some studies have suggested a role for neutralizing antibodies in protection from pathogenic challenge (12, 84), but this has not been confirmed by more recent analyses (42). In the present study, all macaques vaccinated with SIVmac-M4 produced SIV-specific antibodies that persisted throughout the study. However, only three macaques had antibodies capable of neutralizing the infectivity of primary SIVmac251. This observation suggests that factors other than neutralizing antibodies were responsible for the reduced viral load of vaccinees.
Gauduin et al. demonstrated an inverse correlation between viral attenuation and SIV p55-specific CD4+ Th1-type responses (27, 28). Similarly, Lohman et al. showed that the magnitude and breadth of SIV-specific CTL responses were inversely related to the degree of attenuation of the immunizing virus (45). Other studies showed strong SIV-specific CTL responses in macaques that resisted pathogenic challenge (15, 26, 35). However, a direct correlation between CTL responses and protection of vaccinated animals from pathogenic challenge has not been demonstrated. In addition to MHC-restricted cytotoxicity, soluble factors, including MIP-1α, MIP-1β, and RANTES as well as undefined suppressor factors secreted by CD8+ T cells, have been implicated in control of SIV replication (1, 28, 29). PBMC from macaques infected with live, attenuated SIVmac clones produced 8- to 10-fold more of these chemokines than uninfected controls.
In this study, macaques that received live, attenuated SIVmac-M4 had stronger, broader, and more sustained CD8+ T-cell responses to SIVmac antigens postchallenge than unvaccinated control animals. However, outliers were present in each group of infected macaques. In the vaccinated groups, Mmu 31348 and 28300 showed poor containment of viral replication despite strong CD8+ T-cell responses. Interestingly, these two macaques also had the highest titers of antibodies capable of neutralizing the TCLA strain of SIVmac251. In these animals, immune responses appeared to be “antigen-driven”: that is, increasing as a function of increased antigenic load. In addition, one control animal, Mmu 28880, mounted robust CD8+ T-cell responses postchallenge but was unable to contain viral replication. Genetic factors affecting susceptibility to SIVmac infection (such as coreceptor polymorphisms), or regulating immune responses (such as MHC class I or class II haplotypes), may partially explain these differences.
Several recent studies have emphasized the importance of mucosal immune responses in protection of macaques from SIVmac challenge at mucosal surfaces (7, 24, 31, 83). Furthermore, the frequency of SIV-specific CD8+ T cells in cervicovaginal and intestinal mucosae of acutely and chronically infected macaques has been shown to exceed that in peripheral blood (75, 81). Accordingly, another possibility is that the macaques that demonstrated strong containment of SIVmac251 replication had particularly strong, broad SIV-specific cellular and/or antibody responses in mucosal tissues. Further studies will be required to assess these responses in macaques vaccinated with SIVmac-M4.
Live, attenuated viruses and protective immunity.
Virologic and immunologic factors may contribute to viral attenuation and protection from challenge. Several authors have demonstrated an inverse relationship between the degree of virologic attenuation and induction of protective immune responses to SIV (19, 20, 35, 45). Desrosiers and colleagues proposed a hierarchy for some of the best-characterized live, attenuated SIVmac239 deletion mutants, based on in vivo replication kinetics, induction of immune responses, and protection from pathogenic challenge (20). The proposed hierarchy was as follows: SIVmac239 ΔVpr (least attenuated) > ΔVpx > ΔVprΔVpx = ΔNef > Δ3 > Δ3x ≥ Δ4 > ΔVif > Δ5 (most attenuated). As noted previously (70), direct comparison of these studies with our own results is problematic, due to the differences in methodologies used for evaluating viral titers, viral load measurements, and CTL responses and to the different challenge stocks and routes of administration. Nevertheless, some consistencies are apparent: following initial intravenous infection with SIVmac-M4, viral loads in juvenile macaques were comparable to those observed in macaques infected with SIVmacΔ3 (70). Protection induced by SIVmac-M4 against oral challenge with SIVmac251 appeared to be intermediate between that induced by SIVmacΔ3 and that induced by SIVmacΔ3x against vaginal challenge with SIVmac251 (35).
It should be emphasized that, although vaccination with SIVmac-M4 resulted in decreased viral load and induction of strong SIV-specific immune responses postchallenge, five of eight animals showed evidence of persistent infection. In four of those animals, DNA sequence analysis revealed infection with SIVmac251. Thus, in these animals, disease progression may have been delayed but not prevented. In one macaque, Mmu 31345, SIVmac-M4 sequences were detected in a lymph node. In light of previous studies showing eventual pathogenicity or reversion of live, attenuated viruses, this macaque might also be predicted to progress to simian AIDS (5, 68).
Because of safety considerations, the development of live, attenuated lentivirus vaccines for human use is unlikely (4, 9, 53). Progress is being made in development of DNA vaccines and alternative vector systems for delivery of HIV-1 antigens (3, 6, 24, 64, 71). Nevertheless, the study of live, attenuated viruses may provide important insights into immune correlates of protection from mucosal challenge with pathogenic SIV or simian-human immunodeficiency viruses. Although CD8+ T-cell responses appear to be an important component of this protection, additional studies will be required to determine the extent to which other elements of the adaptive and/or innate immune responses also contribute to protective immunity.
Acknowledgments
This research was supported by National Institutes of Health (NIH) grant RO1-AI39415 to M.B.G., American Foundation for AIDS Research (AmFAR) grant 02781-28-RGV to B.L.S., the base grant to CNRS UPR 0415 (P.S.), and the base grant to the California Regional Primate Research Center, RR-00169.
We thank the veterinary and colony services staffs of the California Regional Primate Research Center for expert assistance with the animals. We acknowledge C. J. Weber, E. M. Keddie, V. M. Doucette (University of California, Davis), and B. Boson (Institut Cochin, Paris, France) for technical support during the initial phases of this project. We thank Christian Leutenegger and Thomas North (University of California, Davis) for assistance with TaqMan PCR assays for viral RNA and Joann Yee (University of California, Davis) for performing antibody ELISAs.
REFERENCES
- 1.Ahmed, R. K., C. Nilsson, Y. Wang, T. Lehner, G. Biberfeld, and R. Thorstensson. 1999. Beta-chemokine production in macaques vaccinated with live attenuated virus correlates with protection against simian immunodeficiency virus (SIVsm) challenge. J. Gen. Virol. 80:1569-1574. [DOI] [PubMed] [Google Scholar]
- 2.Almond, N., K. Kent, M. Cranage, E. Rud, B. Clarke, and E. J. Stott. 1995. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet 345:1342-1344. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., and N. L. Letvin. 2000. DNA vaccination for HIV-1 and SIV. Intervirology 43:282-287. [DOI] [PubMed] [Google Scholar]
- 7.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 8.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blower, S. M., K. Koelle, D. E. Kirschner, and J. Mills. 2001. Live attenuated HIV vaccines: predicting the tradeoff between efficacy and safety. Proc. Natl. Acad. Sci. USA 98:3618-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buseyne, F., M. Burgard, J. P. Teglas, E. Bui, C. Rouzioux, M. J. Mayaux, S. Blanche, and Y. Riviere. 1998. Early HIV-specific cytotoxic T lymphocytes and disease progression in children born to HIV-infected mothers. AIDS Res. Hum. Retrovir. 14:1435-1444. [DOI] [PubMed] [Google Scholar]
- 11.Chakrabarti, L., V. Baptiste, E. Khatissian, M. C. Cumont, A. M. Aubertin, L. Montagnier, and B. Hurtrel. 1995. Limited viral spread and rapid immune response in lymph nodes of macaques inoculated with attenuated simian immunodeficiency virus. Virology 213:535-548. [DOI] [PubMed] [Google Scholar]
- 12.Cole, K. S., J. L. Rowles, B. A. Jagerski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor, R., D. Montefiori, J. Binley, J. Moore, S. Bonhoeffer, A. Gettie, E. Fenamore, K. Sheridan, D. Ho, P. Dailey, and P. Marx. 1998. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol. 72:7501-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosson, P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783-5788. [PMC free article] [PubMed] [Google Scholar]
- 15.Cranage, M. P., A. M. Whatmore, S. A. Sharpe, N. Cook, N. Polyanskaya, S. Leech, J. D. Smith, E. W. Rud, M. J. Dennis, and G. A. Hall. 1997. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology 229:143-154. [DOI] [PubMed] [Google Scholar]
- 16.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 17.Denesvre, C., R. Le Grand, F. Boissin-Cans, L. Chakrabarti, B. Hurtrel, B. Vaslin, D. Dormont, and P. Sonigo. 1995. Highly attenuated SIVmac142 is immunogenic but does not protect against SIVmac251 challenge. AIDS Res. Hum. Retrovir. 11:1397-1406. [DOI] [PubMed] [Google Scholar]
- 18.Desrosiers, R. 1998. Prospects for live attenuated HIV. Nat. Med. 4:982. [DOI] [PubMed] [Google Scholar]
- 19.Desrosiers, R. C. 1992. HIV with multiple gene deletions as a live attenuated vaccine for AIDS. AIDS Res. Hum. Retrovir. 8:411-421. [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. Howe, L. O. Arthur, and R. P. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty, P. C. 1998. The numbers game for virus-specific CD8+ T cells. Science 280:227. [DOI] [PubMed] [Google Scholar]
- 22.Doherty, P. C. 1998. The new numerology of immunity mediated by virus-specific CD8+ T cells. Curr. Opin. Microbiol. 1:419-422. [DOI] [PubMed] [Google Scholar]
- 23.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller, D. H., P. A. Rajakumar, L. A. Wilson, A. M. Trichel, J. T. Fuller, T. Shipley, M. S. Wu, K. Weis, C. R. Rinaldo, J. R. Haynes, and M. Murphey-Corb. 2002. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J. Virol. 76:3309-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fultz, P. N., P. J. Vance, M. J. Endres, B. Tao, J. D. Dvorin, I. C. Davis, J. D. Lifson, D. C. Montefiori, M. Marsh, M. H. Malim, and J. A. Hoxie. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 75:278-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallimore, A., M. Cranage, N. Cook, N. Almond, J. Bootman, E. Rud, P. Silvera, M. Dennis, T. Corcoran, J. Stott, et al. 1995. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat. Med. 1:1167-1173. [DOI] [PubMed] [Google Scholar]
- 27.Gauduin, M. C., R. L. Glickman, S. Ahmad, T. Yilma, and R. P. Johnson. 1999. Characterization of SIV-specific CD4+ T-helper proliferative responses in macaques immunized with live-attenuated SIV. J. Med. Primatol. 28:233-241. [DOI] [PubMed] [Google Scholar]
- 28.Gauduin, M. C., R. L. Glickman, S. Ahmad, T. Yilma, and R. P. Johnson. 1999. Immunization with live attenuated simian immunodeficiency virus induces strong type 1 T helper responses and beta-chemokine production. Proc. Natl. Acad. Sci. USA 96:14031-14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauduin, M.-C., R. L. Glickman, R. Means, and R. P. Johnson. 1998. Inhibition of simian immunodeficiency virus (SIV) replication by CD8+ T lymphocytes from macaques immunized with live attenuated SIV. J. Virol. 72:6315-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hariharan, D., W. Ho, J. Cutilli, D. E. Campbell, and S. D. Douglas. 2000. C-C chemokine profile of cord blood mononuclear cells: selective defect in RANTES production. Blood 95:715-718. [PubMed] [Google Scholar]
- 31.Hel, Z., J. Nacsa, B. Kelsall, W.-P. Tsai, N. Letvin, R. W. Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 75:11483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch, V. M., P. Edmondson, M. Murphey-Corb, B. Arbeille, P. R. Johnson, and J. I. Mullins. 1989. SIV adaptation to human cells. Nature 341:573-574. [DOI] [PubMed] [Google Scholar]
- 33.Hunter, E. 1994. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin. Virol. 5:71-83. [Google Scholar]
- 34.Johnson, R. P. 1999. Live attenuated AIDS vaccines: hazards and hopes. Nat. Med. 5:154-155. [DOI] [PubMed] [Google Scholar]
- 35.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson, V., and R. Byington. 1990. Quantitative assays for virus infectivity, p. 71-76. In A. Aldovini and B. Walker (ed.), Techniques in HIV research. Stockton Press, New York, N.Y.
- 37.Kestler, H. W., and K. T. Jeang. 1995. Attenuated retrovirus vaccines and AIDS. Science 270:1220-1222. [PubMed] [Google Scholar]
- 38.Kodama, T., D. P. Wooley, Y. M. Naidu, H. W. Kestler III, M. D. Daniel, Y. Li, and R. C. Desrosiers. 1989. Significance of premature stop codons in env of simian immunodeficiency virus. J. Virol. 63:4709-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaBranche, C. C., M. M. Sauter, B. S. Haggarty, P. J. Vance, J. Romano, T. K. Hart, P. J. Bugelski, M. Marsh, and J. A. Hoxie. 1995. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J. Virol. 69:5217-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langlois, A. J., R. C. Desrosiers, M. G. Lewis, V. N. KewalRamani, D. R. Littman, J. Y. Zhou, K. Manson, M. S. Wyand, D. P. Bolognesi, and D. C. Montefiori. 1998. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J. Virol. 72:6950-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson, M., X. Jin, B. Ramratnam, G. S. Ogg, J. Engelmayer, M. A. Demoitie, A. J. McMichael, W. I. Cox, R. M. Steinman, D. Nixon, and N. Bhardwaj. 1999. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS 13:767-777. [DOI] [PubMed] [Google Scholar]
- 44.Leutenegger, C. M., J. Higgins, T. B. Matthews, A. F. Tarantal, P. A. Luciw, N. C. Pedersen, and T. W. North. 2001. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res. Hum. Retrovir. 17:243-251. [DOI] [PubMed] [Google Scholar]
- 45.Lohman, B. L., M. B. McChesney, C. J. Miller, E. McGowan, S. M. Joye, K. K. Van Rompay, E. Reay, L. Antipa, N. C. Pedersen, and M. L. Marthas. 1994. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J. Virol. 68:7021-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luciw, P. A., K. E. Shaw, B. L. Shacklett, and M. L. Marthas. 1998. Importance of the intracytoplasmic domain of the simian immunodeficiency virus (SIV) envelope glycoprotein for pathogenesis. Virology 252:9-16. [DOI] [PubMed] [Google Scholar]
- 47.Luzuriaga, K., D. Holmes, A. Hereema, J. Wong, D. L. Panicali, and J. L. Sullivan. 1995. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J. Immunol. 154:433-443. [PubMed] [Google Scholar]
- 48.Marthas, M.-L., R. A. Ramos, B. L. Lohman, K. K. A. Van Rompay, R. E. Unger, C. J. Miller, B. Banapour, N. C. Pedersen, and P. A. Luciw. 1993. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J. Virol. 67:6047-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marthas, M. L., K. K. van Rompay, M. Otsyula, C. J. Miller, D. R. Canfield, N. C. Pedersen, and M. B. McChesney. 1995. Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques. J. Virol. 69:4198-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Micoli, K. J., G. Pan, Y. Wu, J. P. Williams, W. J. Cook, and J. M. McDonald. 2000. Requirement of calmodulin binding by HIV-1 gp160 for enhanced FAS-mediated apoptosis. J. Biol. Chem. 275:1233-1240. [DOI] [PubMed] [Google Scholar]
- 51.Miller, C. J. 1994. Mucosal transmission of simian immunodeficiency virus. Curr. Top. Microbiol. Immunol. 188:107-122. [DOI] [PubMed] [Google Scholar]
- 52.Miller, M. A., M. W. Cloyd, J. Liebmann, C. R. Rinaldo, Jr., K. R. Islam, S. Z. Wang, T. A. Mietzner, and R. C. Montelaro. 1993. Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology 196:89-100. [DOI] [PubMed] [Google Scholar]
- 53.Mills, J., R. Desrosiers, E. Rud, and N. Almond. 2000. Live attenuated HIV vaccines: a proposal for further research and development. AIDS Res. Hum. Retrovir. 16:1453-1461. [DOI] [PubMed] [Google Scholar]
- 54.Montefiori, D. C., T. W. Baba, A. Li, M. Bilska, and R. M. Ruprecht. 1996. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239δ3 in adult and infant rhesus monkeys. J. Immunol. 157:5528-5535. [PubMed] [Google Scholar]
- 55.Moretto, W. J., L. A. Drohan, and D. F. Nixon. 2001. Rapid quantification of SIV-specific CD8 T cell responses with recombinant vaccinia virus ELISPOT or cytokine flow cytometry. AIDS 14:2625-2627. [DOI] [PubMed] [Google Scholar]
- 56.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 57.Otsyula, M. G., C. J. Miller, A. F. Tarantal, M. L. Marthas, T. P. Greene, J. R. Collins, K. K. van Rompay, and M. B. McChesney. 1996. Fetal or neonatal infection with attenuated simian immunodeficiency virus results in protective immunity against oral challenge with pathogenic SIVmac251. Virology 222:275-278. [DOI] [PubMed] [Google Scholar]
- 58.Pan, Z., W. Radding, T. Zhou, E. Hunter, J. Mountz, and J. M. McDonald. 1996. Role of calmodulin in HIV-potentiated Fas-mediated apoptosis. Am. J. Pathol. 149:903-910. [PMC free article] [PubMed] [Google Scholar]
- 59.Pancino, G., H. Ellerbrok, M. Sitbon, and P. Sonigo. 1994. Conserved framework of envelope glycoproteins among lentiviruses. Curr. Top. Microbiol. Immunol. 188:77-105. [DOI] [PubMed] [Google Scholar]
- 60.Pikora, C. A., J. L. Sullivan, D. Panicali, and K. Luzuriaga. 1997. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J. Exp. Med. 185:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 62.Ritter, G. D., Jr., M. J. Mulligan, S. L. Lydy, and R. W. Compans. 1993. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology 197:255-264. [DOI] [PubMed] [Google Scholar]
- 63.Riviere, Y., and F. Buseyne. 1998. Cytotoxic T lymphocytes generation capacity in early life with particular reference to HIV. Vaccine 16:1420-1422. [DOI] [PubMed] [Google Scholar]
- 64.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 65.Rowell, J. F., P. E. Stanhope, and R. F. Siliciano. 1995. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J. Immunol. 155:473-488. [PubMed] [Google Scholar]
- 66.Ruprecht, R. M., T. W. Baba, V. Liska, R. Bronson, D. Penninck, and M. F. Greene. 1996. “Attenuated” simian immunodeficiency virus in macaque neonates. AIDS Res. Hum. Retrovir. 12:459-460. [DOI] [PubMed] [Google Scholar]
- 67.Ruprecht, R. M., T. W. Baba, R. Rasmussen, Y. Hu, and P. L. Sharma. 1996. Murine and simian retrovirus models: the threshold hypothesis. AIDS 10:S33-S40. [DOI] [PubMed] [Google Scholar]
- 68.Sawai, E. T., M. S. Hamza, M. Ye, K. E. S. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live, attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 74:854-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shacklett, B. L., C. Denesvre, B. Boson, and P. Sonigo. 1998. Features of the SIVmac transmembrane glycoprotein cytoplasmic domain that are important for Env functions. AIDS Res. Hum. Retrovir. 14:373-383. [DOI] [PubMed] [Google Scholar]
- 70.Shacklett, B. L., C. J. Weber, K. E. S. Shaw, E. M. Keddie, M. B. Gardner, P. Sonigo, and P. A. Luciw. 2000. The intracytoplasmic domain of the Env transmembrane protein is a locus for attenuation of simian immunodeficiency virus SIVmac in rhesus macaques. J. Virol. 74:5836-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 72.Spies, C. P., and R. W. Compans. 1994. Effects of cytoplasmic domain length on cell surface expression and syncytium-forming capacity of the simian immunodeficiency virus envelope glycoprotein. Virology 203:8-19. [DOI] [PubMed] [Google Scholar]
- 73.Srinivas, S. K., R. V. Srinivas, G. M. Anantharamaiah, R. W. Compans, and J. P. Segrest. 1993. Cytosolic domain of the human immunodeficiency virus envelope glycoproteins binds to calmodulin and inhibits calmodulin-regulated proteins. J. Biol. Chem. 268:22895-22899. [PubMed] [Google Scholar]
- 74.Srinivas, S. K., R. V. Srinivas, G. M. Anantharamaiah, J. P. Segrest, and R. W. Compans. 1992. Membrane interactions of synthetic peptides corresponding to amphipathic helical segments of the human immunodeficiency virus type-1 envelope glycoprotein. J. Biol. Chem. 267:7121-7127. [PubMed] [Google Scholar]
- 75.Stevceva, L., B. Kelsall, J. Nacsa, M. Moniuszko, Z. Hel, E. Tryniszewska, and G. Franchini. 2002. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J. Virol. 76:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tencza, S. B., T. A. Mietzner, and R. C. Montelaro. 1997. Calmodulin-binding function of LLP segments from the HIV type 1 transmembrane protein is conserved among natural sequence variants. AIDS Res. Hum. Retrovir. 13:263-269. [DOI] [PubMed] [Google Scholar]
- 77.Tencza, S. B., M. A. Miller, K. Islam, T. A. Mietzner, and R. C. Montelaro. 1995. Effect of amino acid substitutions on calmodulin binding and cytolytic properties of the LLP-1 peptide segment of human immunodeficiency virus type 1 transmembrane protein. J. Virol. 69:5199-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Rompay, K. K., C. J. Berardi, S. Dillard-Telm, R. P. Tarara, D. R. Canfield, C. R. Valverde, D. C. Montefiori, K. S. Cole, R. C. Montelaro, C. J. Miller, and M. L. Marthas. 1998. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J. Infect. Dis. 177:1247-1259. [DOI] [PubMed] [Google Scholar]
- 79.Van Rompay, K. K. A., M. B. McChesney, N. L. Aguirre, K. A. Schmidt, N. Bischofberger, and M. L. Marthas. 2001. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J. Infect. Dis. 184:429-438. [DOI] [PubMed] [Google Scholar]
- 80.Van Rompay, K. K. A., M. D. Miller, M. L. Marthas, N. A. Margot, P. J. Dailey, D. R. Canfield, R. P. Tarara, J. M. Cherrington, N. L. Aguirre, N. Bischofberger, and N. C. Pedersen. 2000. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J. Virol. 74:1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veazey, R. S., M.-C. Gauduin, K. G. Mansfield, I. C. Tham, J. D. Altman, J. D. Lifson, A. A. Lackner, and R. P. Johnson. 2001. Emergence and kinetics of simian immunodeficiency virus-specific CD8+ T cells in the intestines of macaques during primary infection. J. Virol. 75:10515-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wasik, T. J., J. Bratosiewicz, A. Wierzbicki, V. E. Whiteman, R. R. Rutstein, S. E. Starr, S. D. Douglas, D. Kaufman, A. V. Sison, M. Polansky, H. W. Lischner, and D. Kozbor. 1999. Protective role of beta-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J. Immunol. 162:4355-4364. [PubMed] [Google Scholar]
- 83.Wilson, L. A., M. Murphey-Corb, L. N. Martin, R. M. Harrison, M. S. Ratterree, and R. P. Bohm. 2000. Identification of SIV env-specific CTL in the jejunal mucosa in vaginally exposed, seronegative rhesus macaques (Macaca mulatta). J. Med. Primatol. 29:173-181. [DOI] [PubMed] [Google Scholar]
- 84.Wyand, M., K. Manson, M. Garcia, D. Montefiori, and R. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of SIV. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases env incorporation into particles and fusogenicity and infectivity. J. Virol. 67:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]