Abstract
The transcription-coupled repair (TCR) pathway preferentially repairs DNA damage located in the transcribed strand of an active gene. To gain insight into the coupling mechanism between transcription and repair, we have set up an in vitro system in which we isolate an elongating RNA pol IIO, which is stalled in front of a cisplatin adduct. This immobilized RNA pol IIO is used as ‘bait' to sequentially recruit TFIIH, XPA, RPA, XPG and XPF repair factors in an ATP-dependent manner. This RNA pol IIO/repair complex allows the ATP-dependent removal of the lesion only in the presence of CSB, while the latter does not promote dual incision in an XPC-dependent nucleotide excision repair reaction. In parallel to the dual incision, the repair factors also allow the partial release of RNA pol IIO. In this ‘minimal TCR system', the RNA pol IIO can effectively act as a loading point for all the repair factors required to eliminate a transcription-blocking lesion.
Keywords: CSB, NER, RNA pol II, transcription
Introduction
The different mechanisms involved in deciphering the genetic information leading from a gene to a protein are highly regulated and any circumstances that impede them can have severe consequences. Unfortunately, the integrity of cellular DNA is constantly jeopardized by UV radiation, antitumoral drugs (cisplatin) or environmental agents that distort the DNA helix (Hoeijmakers, 2001). Those lesions pose a serious threat to cellular processes such as transcription, as they can obstruct an elongating RNA polymerase II (RNA pol II) and lead to an apoptotic response if left unrepaired (Yamaizumi and Sugano, 1994; Ljungman and Zhang, 1996). Moreover, persistent lesions can also be bound by proteins such as TBP or HMGA/B, which could indirectly lead to an inhibition of transcription and repair (Vichi et al, 1997; Reeves and Adair, 2005). Keeping the gene sequence intact to protect the genetic information then is one of the cell's priorities and is carried out by a transcription-dependent mechanism called transcription-coupled repair (TCR). TCR and global-genome nucleotide excision repair (GG-NER) are subpathways of NER. While GG-NER deals with the removal of lesions present in the overall genome, TCR is specific for removing lesions present on the transcribed strand of active genes (Bohr et al, 1985; Mellon et al, 1987).
At the molecular level, TCR and GG-NER can be distinguished by their damage recognition factor. In GG-NER, the lesion is recognized by the complex XPC–HR23B in an ATP-independent manner, and will further allow for the binding of TFIIH and the subsequent NER factors (Sugasawa et al, 1998; Riedl et al, 2003). In TCR, an elongating RNA pol IIO, likely helped by additional specific factors, is thought to be the switch for engaging the repair mechanism (Christians and Hanawalt, 1992; Sweder and Hanawalt, 1992; Svejstrup, 2003). In Escherichia coli, the stalled RNA polymerase triggers the recruitment of the mutation frequency decline (mfd) protein repair, allowing for the release of RNA polymerase and further recruitment of the repair factors (Selby and Sancar, 1993). In mammals, CSA and CSB (rad28 and rad26 in yeast, respectively) are thought to play this function. Indeed, the trademark characteristic of TCR-deficient Cockayne syndrome (CS) cells is a defect in the mRNA recovery synthesis after UV irradiation (Wade and Chu, 1979; Troelstra et al, 1992; van der Horst et al, 1997). CSB was found to be connected to both the transcription and the repair machineries: (1) CSB, as a member of the SWI/SNF family of proteins, behaves as a chromatin remodeling factor most likely through its DNA-dependent ATPase activity (Citterio et al, 2000); (2) CSB/rad26 stimulates RNA synthesis both in vivo and in vitro (Balajee et al, 1997; Selby and Sancar, 1997b; Lee et al, 2001); (3) CSB interacts with transcriptional complexes containing TFIIH, TFIIE, p53, RNA pol II and I, as well as with repair factors such as XPG, XPA and XAB2 (Iyer et al, 1996; Tantin et al, 1997; Selby and Sancar, 1997b; van Gool et al, 1997; Nakatsu et al, 2000; Bradsher et al, 2002). Therefore, it is not surprising that inherited mutations in proteins specifically involved in the TCR pathway give rise to the rare autosomal recessive disorder CS (Venema et al, 1990; van Hoffen et al, 1993). While the majority of CS cases are caused by mutations in the CSA and CSB genes (Troelstra et al, 1992; Henning et al, 1995), CS phenotypes can also be associated with the xeroderma pigmentosum (XP) arising from mutations in XPB/XPD (TFIIH) and XPG genes. CS patients are characterized by progressive neurodegeneration and developmental defects (Nance and Berry, 1992). They also display sun sensitivity manifested as a severe rash.
We hypothesized that a stalled RNA pol IIO would trigger the TCR reaction. We then set up an in vitro system in which a DNA fragment containing a single site-specific lesion located downstream from a promoter is immobilized on magnetic beads, allowing for both transcription and repair. We showed that an isolated elongating RNA pol IIO stalled at the lesion is able to sequentially recruit the repair factors in the absence of XPC–HR23B factor. Furthermore, we demonstrate that this RNA pol IIO-associated complex initiates and/or mediates an ATP-dependent incision of the damaged DNA in the presence of CSB.
Results
A template for transcription and DNA repair
To investigate the connection between transcription and DNA repair, we have set up an assay in which the DNA template can be used for both reactions. A plasmid, containing a promoter and cisplatin DNA adduct (at position 105 on the transcribed strand), was cut by two restriction enzymes and biotinylated at the extremity, leading to a damage-containing DNA fragment of 709 base pairs (bp), further immobilized on streptavidin magnetic beads (Cax-Pt, Figure 1A).
Figure 1.

A template for both transcription and DNA repair reactions. (A) The transcription/repair template (Cax-Pt) contains a single cisplatin adduct (GTG, Pt) at position +105 nt in the transcribed strand downstream from the adenovirus major late promoter. The TATA box is represented by a triangle and the start site +1 by a bent arrow. The positions of the restriction enzymes sites are indicated. (B) Coomassie staining of highly purified transcription and repair factors. (C) Transcription on Cax (lanes 1–5) and Cax-Pt (lanes 6–10) was performed using either RTS or WCE/XPC as indicated at the top of the panel. Full length (328 nt) or prematurated stopped transcripts (105 nt) were resolved on 8% urea/PAGE. The Addition of α-amanitin is indicated. (D) Dual incision on Cax-Pt was carried out with RIS in either the presence of (lane 1) or the absence of XPC (RISΔXPC; lane 2), WCE/Hela (lane 3) or WCE/XPC (lane 4) supplemented with XPC (lane 5). Dual incision is indicated by the occurrence of a 26–34-nucleotides excision products.
The ability of the Cax-Pt and the undamaged Cax templates to be transcribed was analyzed by using either a reconstituted transcription system (RTS), containing, in addition to RNA pol II, the basal transcription factors TBP, TFIIB, IIE, IIF and TFIIH (Figure 1B, upper panel), or an XPC-deficient cell extract (WCE/XPC). Run-off transcription on Cax-Pt resulted in a 105-nucleotide (nt)-long RNA transcript, suggesting that the cisplatin lesion presents a strong impediment to the progression of RNA pol II (Figure 1C, lanes 5 and 7). On the contrary, transcription on the undamaged Cax template allowed the synthesis of a full 328 nt length of RNA (lanes 1 and 3). The addition of α-amanitin, a specific RNA pol II transcription inhibitor, prevented RNA synthesis (lanes 2, 4, 6 and 8).
We also monitored a dual incision assay on Cax-Pt using either a reconstituted incision system (RIS), containing recombinant XPC–HR23B, TFIIH, XPA, RPA, XPG and XPF/ERCC1 (Figure 1B, lower panel), a HeLa whole-cell extract (WCE/HeLa) or WCE/XPC (Figure 1D). Both the RIS and WCE/HeLa (lanes 1 and 3) were able to release the damaged 26–34 nt dual incision products contrary to WCE/XPC and RIS lacking XPC (RISΔXPC) (lanes 2 and 4). The lack of repair activity of WCE/XPC could be overcome by adding recombinant XPC (lane 5).
Recruitment of NER factors onto the stalled RNA pol II
Having established a system in which both transcription and repair could be carried out on the same substrate, we next wished to isolate a single RNA pol II stalled at the lesion. The Cax-Pt template was first preincubated with RTS for 15 min at 24°C, and then incubated either in the absence of (Figure 2A, lane 1) or in the presence of nucleotide triphosphates for 45 min (lanes 2 and 3). After being washed at different salt concentration, the supernatant was discarded and the remaining proteins bound to the immobilized DNA were submitted to Western blotting. We observed that the 50 mM washed preinitiation complex (PIC-50, lane 1) contained most of the basal transcription factors in addition to the hypophosphorylated RNA pol IIA. After adding the nucleotide triphosphates and further washes at 50 mM KCl, all the transcription factors, in addition to both the RNA pol IIA associated with the transcription initiation complex and the hyperphosphorylated elongating RNA pol IIO (EC-50, lane 2), were present on the immobilized DNA. Following additional washes of EC-50 at 400 mM KCl plus 0.1% sarkosyl, we observed that only RNA pol IIO remained tightly bound to Cax-Pt (EC-400, lane 3), ridding the promoter of the basal transcription factors and RNA pol IIA as well. However, only a small percentage (5–10%) of PIC efficiently promotes transcription; therefore, most of the damaged region of the substrate is not protected by RNA pol IIO (data not shown). To eliminate the unprotected lesion, EC-400 was then incubated with ClaI restriction enzyme, which cuts at position +86 (Figure 1A). When the ClaI-cut EC-400 immobilized DNA was incubated with RIS, no dual incision repair signal could be observed as compared to an uncut Cax-Pt (Figure 2B, lanes 3 and 1, respectively). As a control, untranscribed Cax-Pt cut by ClaI did not exhibit a dual incision signal when incubated with RIS (lane 2). ClaI-cut EC-400 represents therefore a single lesion-stalled RNA pol IIO on the damaged DNA fragment, whose unprotected remaining cisplatin lesions have been removed.
Figure 2.

Recruitment of NER factors onto the stalled RNA pol IIO. (A) Western blots of transcription complexes on immobilized DNA before (PIC, lane 1) and after (EC, lanes 2 and 3) the addition of nucleotide triphosphates. Immobilized protein complexes were washed either at 50 mM KCl (PIC-50 and EC-50) or at 400 mM KCl (EC-400). (B) Dual incision reaction on the immobilized Cax-Pt transcribed or not, and cut by ClaI. (C) Transcription reactions on Cax-Pt (lane 1) were further incubated with TFIIS or CSB (lanes 2 and 3). Arrows indicate the different lengths of RNA transcripts. (D) Immobilized ClaI-cut Cax-Pt (lane 1) as well as ClaI-cut EC-400 complex (lanes 2 and 3) were incubated with XPC. Following a second wash, these templates were tested in a dual incision reaction in which XPC is omitted. Fgt-Pt competitor damaged DNA (lane 2) is added where indicated. Complete NER (lane 4) is used as a control. (E) Immobilized Cax-Pt (lanes 1 and 4) or ClaI-cut EC-400 containing the stalled RNA pol IIO (lanes 2 and 3, 5–8) were pretreated with CIP (as indicated), and further incubated with either WCE/XPC or WCE/XPCΔCSB in the presence of ATP and CSB as indicated. Proteins remaining bound to the immobilized Cax-Pt template were next analyzed by Western blots. (F) Immobilized Cax-Pt (lanes 1) or ClaI-cut EC-400 (lanes 2–6) were treated as indicated at the top of the panel similarly to (E); the RNA pol IIO and CSB remaining on the immobilized Cax-Pt template were next analyzed by Western blots. (G) ClaI-cut Cax-Pt (lane 1) or ClaI-cut EC-400 complex (lane 2) were incubated with RISΔXPC, the dual incision system in which XPC is lacking. Following washes at 50 mM KCl, the remaining bound proteins were analyzed by Western blot.
The dynamics of the stalled RNA pol IIO was studied by incubating the transcription elongation factor TFIIS, which stimulates the nascent RNA cleavage activity intrinsic of RNA pol II (Reines et al, 1993); its addition leads to a 97 nt transcript (Figure 2C, lane 2). Moreover, the addition of CSB helps RNA pol IIO to move forward (Selby and Sancar, 1997a) and to resume transcription from position 97 nt to position 105 nt (lane 3).
We also checked whether XPC was able to target the DNA structure induced by the cisplatin damage when RNA pol IIO was stalled in front. Recombinant XPC was incubated with the ClaI-cut EC-400 complex (see Figure 2A, lane 3), for 30 min at 30°C, and the immobilized template was subsequently rinsed with a buffer containing 50 mM KCl and then further incubated with RISΔXPC. In these conditions, we did not observe the removal of damaged oligonucleotides, suggesting that XPC did not displace RNA pol IIO (Figure 2D, lane 3). Nor did we observe a repair signal when we added a challenge-damaged DNA fragment (Fgt-Pt) to RISΔXPC and the immobilized EC-400 complex (lane 2). However, when a nontranscribed damaged DNA was first incubated with XPC before addition of RISΔXPC, we observed dual incision activity (lane 4); this was not the case when a ClaI-cut DNA fragment was used instead (lane 1). Altogether, our data suggest that XPC neither binds to nor displaces a RNA pol II stalled in front of a DNA damage.
We next investigated whether the stalled RNA pol IIO could be specifically recognized by NER factors. ClaI-cut EC-400 was then incubated with WCE/XPC in the presence of or in the absence of ATP. Most of the DNA repair factors including XPG, TFIIH, XPA, XPF/ERCC1 and RPA were recruited onto RNA pol IIO in the presence of ATP (Figure 2E, compare lanes 1 and 2). Interestingly, when ATP was omitted from the incubation, we observed a decrease in the recruitment of XPG, TFIIH, XPA and XPF/ERCC1 (lane 3), while RNA pol IIO was still bound to the template (Figure 2F, lanes 2 and 3). We also tested the influence of CSB in recruiting NER factors on RNA pol IIO. CSB has been suggested to be part of a complex containing RNA pol II, TFIIH and XPG (Iyer et al, 1996; Bradsher et al, 2002), and to allow for TFIIH recruitment onto the RNA pol II (Tantin et al, 1998). However, adding recombinant CSB to WCE/XPCΔCSB (WCE/XPC immunodepleted for CSB; Figure 2F, lane 5 and 6), as well as ATP on ClaI-cut EC-400, did not modify the recruitment of the NER factors (Figure 2E, lanes 7 and 8). In parallel, the amount of RNA pol IIO on the immobilized DNA remained constant (Figure 2F, lanes 5 and 6).
To verify that the recruitment was dependent on the presence of the RNA pol IIO on the DNA, we treated RNA pol IIO with a phosphatase as dephosphorylation of a stalled RNA pol IIO CTD destabilizes the RNA pol II from the template when incubated next with a cellular extract (Tremeau-Bravard et al, 2004) (Figure 2F, compare lane 4 with lanes 2, 5 and 6). In these circumstances, we observed a drop in the presence of NER factors (Figure 2E, lanes 5 and 6) and CSB (Figure 2F, lane 4) associated with the loss of RNA pol IIO.
Similarly, we showed that when the incubation was carried out in the presence of the five recombinant repair proteins and ATP, an increased recruitment of each protein onto RNA pol IIO could be detected compared to the nontranscribed template (Figure 2G). This further suggests that their recruitment does not necessarily require intermediate proteins.
Altogether, our results show the ability of the lesion-stalled RNA pol IIO to specifically recruit DNA repair factors whose recruitment is stimulated by ATP. Furthermore, while CSB appears to be part of the complex, it does not influence the recruitment of the NER factors.
CSB together with the stalled RNA pol II promote dual incision
Having shown that RNA pol IIO can recruit repair factors, we next wondered whether this complex could mediate the removal of the lesion. Knowing that the lengths of the repair patches were similar for TC-NER and for GG-NER (Bowman et al, 1997), we used our in vitro DNA repair system to check for the release of a damaged single-strand fragment (Aboussekhra et al, 1995). When EC-400 was first incubated with RISΔXPC, no incision signal was detected. However, when increasing amounts of CSB were also added (Figure 3A), we detected a low but significant increased amount of released damaged oligonucleotides (lanes 3–5) characteristic of a repair signal. Nevertheless, to check whether such a dual incision pattern would have been nonspecifically generated by XPG and XPF, we incubated EC-400 and CSB with varying combinations of four of the five NER factors. The omission of TFIIH, XPA, RPA, XPG, or XPF completely abolished the repair reaction, suggesting that the removal of the damaged oligonucleotide is highly specific and not promoted by unspecific endonuclease cuts (Figure 3B, lanes 3–7).
Figure 3.

RNA pol IIO/CSB-mediated incision. (A) EC-400 was incubated with RISΔXPC and increasing amounts of CSB, and subjected to a dual incision assay. Quantification was made as described in Materials and methods. A graphic depicts the relative intensity of each signal. (B) Dual incision assays were performed on EC-400, which was incubated with different combinations of NER factors and CSB as indicated. (C) EC-400 transcription complex (lanes 1–12) and Cax-Pt (lanes 13–15) pretreated or not with CIP (lanes 5 and 6) were incubated with RISΔXPC (lanes 2–15) and with either wild-type CSB (lanes 3–6, 9 and 15), mutated CSBΔ440, CSBΔ378, CSBR670W (lanes 10–12) or XPC (lane 13), and subjected to a 3′-incision primer extension assay. The position and scans of sensitive bands relative to cisplatin lesion are denoted by asterisks and indicated at the right of the gel. (D) Dual incisions were performed on the untranscribed Cax-Pt with RISΔXPC in the presence (lanes 1, 3–11) or the absence of XPC (lanes 2, 12 and 13), with either a limiting amount of TFIIH (lanes 3–5) or XPG (lanes 9–11). CSB was added in the reaction where indicated. The relative amounts of TFIIH and XPG are indicated by thick boxes (saturating amounts) and thin boxes (limiting amounts).
The RNA pol IIO-mediated dual incision is rather weak compared to an XPC-mediated dual incision and might reflect either the low efficiency of the reaction (and the absence of putative additional stimulatory repair factors) and/or a different incision pattern, which cannot be fully detected by our repair system due to the design of our probe. Therefore, instead of using a probe, the DNA fragments generated from the 3′-incision were amplified by primer extension using a radiolabeled oligonucleotide annealed 100 bp upstream from the site of the lesion. Increasing amounts of CSB together with the recruited factors on RNA pol IIO stimulated the 3′-incision activity (Figure 3C, compare lanes 3 and 4 to lane 2). We observed a particular increase in the intensity of the hypersensitive sites at positions +9, +12, +14, +16, +17 and +20 (Figure 3C, lanes 4 and 9), also depicted in the histogram in which the intensity of the bands were quantified and normalized to the background bands located at positions +8 and +24 found in every samples. Dephosphorylation of RNA pol IIO, which leads to its partial destabilization, resulted in a decreased recruitment of the NER factors (Figure 2E) and in a decreased of the 3′-incision pattern (Figure 3C, lanes 5 and 6). The 3′-incision positions mediated on the one hand by RNA pol IIO and CSB and on the other hand by XPC are rather similar (lanes 13 and 9). However, we reproducibly observed in the RNA pol IIO-mediated repair a more sensitive site at position C+11 and less-sensitive sites at positions T+17 and A+20 compared to GG-NER (Figure 3C, right scans). Furthermore, to evaluate the specificity of the CSB-dependent incision reaction, we tested several mutated CSB (provided by A Lehmann): (CSBΔ440, whose 440–446 glycine residues were deleted; CSBΔ378, which lacks seven glutamine residues (from position 378 to position 384); and CSBR670W, found within a CS patient, in which the arginine located in the conserved motif III of the ATPase domain was changed into a tryptophane). We observed a slight decrease incision activity with CSBΔ440, and an inhibition with CSBΔ378 and CSBR670W (Figure 3C, lanes 10–12).
To further investigate the specificity of CSB for the RNA pol IIO-mediated incision, we tested the influence of CSB on an XPC-mediated repair reaction, and more specifically its effect on TFIIH and XPG as CSB interacts with these two factors (Iyer et al, 1996) (Figure 3D). Cax-Pt and increasing amounts of CSB were then incubated with RIS (lanes 6–8), RISΔXPC (lanes 12 and 13) or RIS containing limited amounts of either TFIIH (lanes 3–5) or XPG (lanes 9–11). CSB did not affect the efficiency of the repair reaction by stimulating TFIIH or XPG, nor did it stimulate the overall rate of the reaction. Moreover, when CSB alone was incubated with RISΔXPC (lanes 12 and 13), we did not observe any repair signal, suggesting that CSB did not promote the dual incision and that the CSB fraction was not contaminated by XPC.
Altogether, these results strongly suggest that the RNA pol IIO-mediated incision reaction is CSB and NER factor specific, as well as XPC-independent.
The coming of the TCR components
We further investigated whether the recruitment of each of the repair proteins was interconnected. The EC-400 complex (Figure 4B, upper panel) was incubated with different combinations of the five NER factors (in which the omitted factor is referred to by Δ as indicated at the top of each lane), and the remaining RNA pol IIO-associated proteins were next detected by Western blot. Incubation of TFIIH, XPA, RPA, XPG and XPF resulted in their recruitment on the RNA pol IIO (Figure 4B, lane 1; see also Figure 2). We next found that TFIIH, revealed by the p62 subunit (Figure 4B, panel 2), could still be detected in the absence of RPA (lane 4), XPG (lane 5), and XPF (lane 6). In the latter case, we observed a slight decrease of TFIIH. Similarly, XPA (panel 3) could also be detected in ΔRPA (lane 4), ΔXPG (lane 5) and ΔXPF (lane 6). Interestingly, we observed an interdependence between TFIIH and XPA in which, in the absence of one, the other is less present on the damaged complex. We found that if TFIIH was almost completely absent in ΔXPA (lane 2), there was a significant drop of XPA recruitment in ΔTFIIH (lane 2). Since the omission of RPA, XPG or XPF did not prevent them from binding to the RNA pol IIO (panels 2 and 3), we conclude that TFIIH and XPA might be recruited first onto the RNA pol IIO. The level of RPA (panel 4) remained the same in the absence of ΔTFIIH (lane 2), ΔXPA (lane 3) or ΔXPG (lane 5), and only decreased in ΔXPF (lane 6), suggesting that the RPA is able to load independently from the rest of the NER factors onto the complex. As previously observed in GG-NER (Riedl et al, 2003), XPG (panel 5) is no longer recruited onto the RNA pol IIO in ΔTFIIH (lane 2) or ΔXPA (lane 3), and strongly decreases in ΔRPA (lane 4). However, XPG is still present when XPF is omitted (lane 6), suggesting that XPG is recruited late on the RNA pol IIO and likely independently of, or before, XPF. Regarding the recruitment of XPF (panel 6), its presence was strongly diminished when either TFIIH or XPA was omitted (lanes 2 and 3, respectively). Neither the omission of RPA nor the absence of XPG affected the presence of XPF onto the RNA pol IIO (lane 4). Therefore, XPF might be recruited late onto the RNA pol IIO and positioned in relation with XPG.
Figure 4.
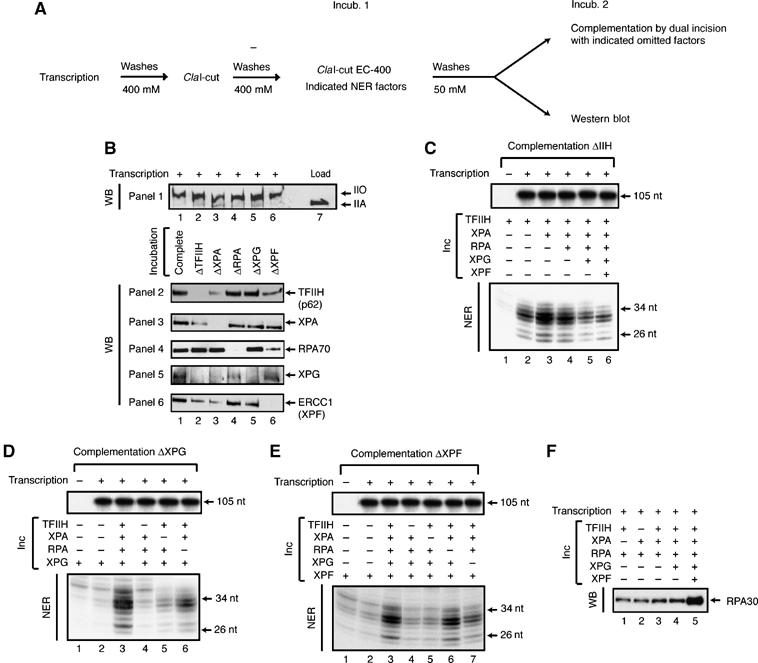
Sequential assembly of NER factors onto RNA pol IIO. (A) Scheme depicting the reaction. Cax-Pt was transcribed and then cut by ClaI to form the ClaI-cut EC-400, which was further incubated with different combinations of four repair factors (the omitted factor is referred to by Δ in each lane), or with different combinations of indicated NER factors. After soft washes (50 mM KCl), the recruited proteins were analyzed by Western blots (B) and (F) or by a functional dual incision complementation assay (see text for details). (C–E) Either RNA pol IIO (B) or RNA synthesis levels (C), (D) and (E) are used as a loading control.
The presence of an NER factor onto the RNA pol IIO following the first incubation can be further investigated for its requirement in the dual incision reaction in which the factor of interest is omitted (Riedl et al, 2003). In our experimental conditions, RNA synthesis, and consequently the amount of the elongating RNA pol IIO, was similar in every sample (Figure 4C–E). Similar to what we observed by Western blot, omission of either TFIIH or XPA in the first incubation strongly prevented the recruitment of XPG and XPF (Figure 4D and E, lanes 4 and 5, respectively), underlying the requirement of TFIIH and XPA for the further recruitment of NER factors on RNA pol IIO. Furthermore, we also observed a slight decrease of XPF when XPG was omitted (Figure 4E, lane 7). The recruitment of XPF slightly modified the concentration of TFIIH on the damaged DNA (Figure 4C) as it was observed in GG-NER, where the release of TFIIH from the damage DNA is concomitant with the arrival of XPF (Riedl et al, 2003). We also noticed that the presence of XPF did enhance the recruitment of RPA (Figure 4F, lane 5), a factor that would be later involved in DNA resynthesis.
NER factor-mediated partial release of RNA pol II
Next, questions arose regarding what becomes of RNA pol IIO during the reaction. EC-400 was incubated with the NER factors for 30 min at 30°C. The supernatant was then separated from the beads and analyzed by Western blot for the presence of RNA pol IIO. We also checked for the presence of remaining RNA pol IIO on the immobilized DNA. However, because of a very low percentage of RNA pol IIO released from the immobilized DNA (5–10%), the remaining RNA pol IIO on the immobilized DNA was in saturating amounts. The addition of each NER factor separately did not significantly promote the release of RNA pol IIO, even in the presence of ATP (Figure 5A, compare lane 1 to lanes 2–6). On the contrary, the sequential addition and recruitment of NER factors led to an increased release of RNA pol IIO from the template (Figure 5A, lanes 7–10). A maximum was obtained when all the NER factors were incubated together (lane 10). We also noticed that when incubated either alone or together with the NER factors, CSB does not help release RNA pol IIO from the DNA (Selby and Sancar, 1997b) (Figure 5B). Furthermore, while RNA pol IIO is ‘destabilized' by the five NER factors in the absence of ATP, its removal from the template is stimulated by the addition of increasing amounts of ATP (Figure 5C, lanes 3–6). Both TFIIH and CSB exhibit a DNA-dependent ATPase activity; however, because CSB is not implicated in the release of RNA pol IIO, TFIIH is the most likely to use ATP to unwind the DNA. This hypothesis was confirmed by incubating RNA pol IIO with the repair factors either in the absence of TFIIH or in the presence of TFIIH/XPB-f99s, whose mutation is detrimental for TFIIH DNA-opening activity (Coin et al, 1999). In both cases the RNA pol IIO release is much weaker than in the presence of wild-type TFIIH (Figure 5D and E), likely due to the absence of the ‘TFIIH helicase functioning'.
Figure 5.

NER factors-mediated release of RNA pol IIO. (A–E) EC-400 were incubated for 30 min at 30°C, either alone or together with NER factors and CSB as indicated at the top of each panel. The removal of RNA pol IIO from the DNA template in the supernatant was further analyzed by Western blots. (B) EC-400 was incubated with indicated factors in the absence or the presence of increasing amounts of ATP. IIH/XPB-f99s was also used (E). Quantification was made as described in Materials and methods. A graphic at the bottom of each panel depicts the relative intensity of each signal.
Discussion
Depending on the type of DNA damage, the elongating RNA pol IIO can stall and therefore face a situation in which it might trigger factors to allow for either the bypass or the removal of the DNA-blocking lesion. To understand the TCR pathway in which DNA repair is coupled to transcription, we have set up an in vitro system: first, a 3′-end immobilized DNA is used as a template by RNA pol II and its highly purified transcription factors; second, to further repair the damaged DNA, the stalled RNA pol IIO recruits the NER factors upon encountering a cisplatin lesion.
RNA pol IIO, the target
TCR requires five of the seven factors already involved in global genome nucleotide excision repair (GG-NER), explaining at least partially some similarities in the sequential recruitment of the NER factors between both mechanisms. In TCR, the stalled RNA pol II supplant for XPC in its role as a DNA damage recognition factor in addition to providing an open DNA structure, which can be targeted by structure-specific factors. In the absence of ATP, RNA pol IIO efficiently allows for the arrival of TFIIH, XPA and RPA proteins. Moreover, the addition of ATP not only increases their proper recruitment, but also attracts and/or stabilizes the two other factors, XPG and XPF/ERCC1. It is possible that ATP is used by both the XPB and the XPD helicases of TFIIH to unwind the DNA, thus offering and/or improving an accurate DNA/protein structure for the forthcoming factors. Similar to what was observed in GG-NER, TFIIH and XPA are likely the first factors recruited in TCR, since the omission of either of them affects the binding of XPG and XPF to RNA pol IIO, and vice versa. Furthermore, it seems that TFIIH and XPA may be recruited together and stabilize each other onto the RNA pol IIO. The omission of RPA, XPG or XPF does not affect TFIIH or XPA recruitment. Surprisingly, the level of RPA remained constant. The recruitment of RPA, therefore, is more likely due to the single-strand DNA region created by the elongating RNA pol IIO, rather than the presence of the first NER factors. While this would make RPA one of the first proteins to be present around RNA pol IIO, we do not exclude the role of XPA in further stabilizing and possibly positioning RPA, a situation that was previously described in GG-NER (Asahina et al, 1994; Wakasugi and Sancar, 1999; Missura et al, 2001; Reardon and Sancar, 2002). As observed in GG-NER, XPG and XPF join the complex formed around RNA pol IIO late. The recruitment of XPG might first be mediated through its interaction with TFIIH, and then further stabilized by RPA, as they physically interact with each other (de Laat et al, 1998b). We also observed that the recruitment of XPF is directed not only by the presence of the ssDNA/dsDNA junction on the 5′ side within the transcription bubble already formed by the stalled RNA pol IIO (de Laat et al, 1998a; Tapias et al, 2004), but also upon XPG binding, underlining the connection between both endonucleases.
Next, concerns might arise regarding the specificity of the recruitment of NER factors. However, we have shown that RNA pol IIO recruits the NER factors in an XPC-independent manner, as RNA pol IIO cannot be substituted by XPC. In addition, as dephosphorylation of RNA pol IIO partially destabilizes it from the template (Tremeau-Bravard et al, 2004), it led to a decrease in the recruitment of the NER factors. We also noticed the additional role of ATP in increasing such a recruitment by probably inducing the unwinding of the DNA template via TFIIH rather than modifying the phosphorylation state of RNA pol IIO, which remains stable.
RNA pol IIO/CSB-mediated incision activity
The RNA pol IIO/NER factor complex was not able to initiate the removal of the oligonucleotide-containing lesion, except in the presence of the TCR-specific factor CSB. To that matter, we observed a specific recruitment of CSB onto RNA pol IIO, which is not surprising since CSB-containing RNA pol II complexes have been previously described in vivo as well as in vitro (Tantin et al, 1997; Bradsher et al, 2002; van den Boom et al, 2004). We detected a weak but significant signal of dual incision in the presence of CSB. We have also shown that RNA pol IIO dephosphorylation resulted in a loss of the 3′-incision signal, strongly suggesting that the reaction relied on RNA pol IIO. These reactions occur in a CSB-dependent manner, since the absence of or the use of mutated CSB does not allow dual incision. However, the role (if any) of ATP hydrolysis generated by CSB and its putative helicase activity could be to rearrange the interface between the DNA and the NER factors.
We are aware of the low yield (around 1–2%) of our in vitro ‘TCR reaction', keeping in mind that only 5–10% of DNA template is transcribed in vitro. This results in a very low percentage of NER factors/CSB using RNA pol IIO as a repair-initiating factor. We do not exclude that some additional factors are missing, such as CSA, XAB2 and MMS19 (Lauder et al, 1996; Lombaerts et al, 1997; Nakatsu et al, 2000; Groisman et al, 2003). Nevertheless, at first glance, the CSB-dependent RNA pol IIO-mediated incision pattern looks rather similar to a XPC-mediated incision pattern, as repair patches from both GG-NER and TCR have been shown to be similar in vivo (Bowman et al, 1997). The discrepancies in the incision sites could reflect a different bending of the DNA by the RNA pol IIO compared to XPC, or might be associated with a different positioning of the repair factors in the two systems. Interestingly, while our system includes all the putative known repair proteins involved in TCR, a recent study has also shown an incision activity around the RNA pol IIO mediated by XPG and TFIIH (Sarker et al, 2005).
Past and future of the RNA pol IIO/CSB couple
How and when CSB joins the RNA pol II, knowing its ability to bind to either the initiating or the elongating RNA pol II. At this stage of our study, we have not yet determined whether CSB travels along with RNA pol II or transiently binds to both forms of RNA pol II when needed. However, only a small percentage of CSB-containing complexes were found to be immobilized in a transcription-dependent fashion (van den Boom et al, 2004), arguing in favor of a mechanism in which CSB monitors progression of RNA pol II by regularly probing its elongation. Therefore, when RNA pol IIO encounters DNA damage, CSB becomes more tightly recruited (van den Boom et al, 2004) to participate in remodeling the DNA/RNA pol IIO interface (Troelstra et al, 1992; Citterio et al, 2000). Depending on the type of damage, this would result in either a bypass (our unpublished results and Lee et al, 2002) and/or a stabilization of RNA pol IIO to allow for the recruitment of the repair factors. Such reorganization of the DNA repair machinery might then play a central role in the release of the stalled RNA pol IIO, as it occurs concomitantly with the arrival of XPF, the last factor to join the complex. This is associated with an increase recruitment of RPA, which is probably needed to stabilize the open DNA structure. CSB, which does not participate in the release of RNA pol IIO, must therefore be recruited before the formation of the entire complex: CSB interacts with TFIIH, XPA and XPG, and could be recruited before the arrival of XPF.
In addition, while we cannot exclude that the release was a consequence of the steric hindrance from all the NER factors, part of it was driven by an ATP-dependent reaction. TFIIH is likely to be partially responsible for this phenomenon, since the release of RNA pol IIO is less efficient in either the absence of TFIIH or in the presence of a mutated TFIIH complex. Whether the release of RNA pol IIO is associated with its recycling by a FCP1 phosphatase or with its degradation directed by CSA upon ubiquitylation (Groisman et al, 2003) has to be further investigated.
Materials and methods
Cell culture and extract preparation
Lymphoblastoid XP-C cells (GM2246D) are cultured in suspension with Dulbecco's modified Eagle medium high glucose with 2 mM L-glutamine supplemented with 10% fetal bovine serum. The whole cell extract is prepared as described previously (Sugasawa et al, 2001).
Damaged DNA template
The covalently closed circular DNA-Pt containing a single 1,3-intrastrand d(GpTpG) cisplatin-DNA crosslink is prepared as described previously (Shivji et al, 1995), based on the 105.TS plasmid (Frit et al, 2002). The immobilized damaged DNA template is generated by digesting the DNA-Pt plasmid with FokI and AseI, resulting in a 722-bp fragment containing the AdML promoter, with the cisplatin adduct located at position 105 bp from the start site (Cax-Pt). The damaged strand is biotinylated at the FokI site by Klenow fill-in reaction with Bio-dUTP (Roche) and purified after separation on agarose gel with a QIAEX gel extraction kit (Quiagen). Undamaged DNA is prepared following the same procedure. For each sample, 50 ng of purified biotinylated Cax-Pt is then bound to 10 mg magnetic streptavidin beads (Dynabeads M-280 Streptavidin, Dynal) and equilibrated with a dual incision assay buffer prior to use.
Immunoblotting
Proteins were separated on 6 or 10% SDS–PAGE gels and transferred to nitrocellulose membranes. The following mouse monoclonal (Mab) and rabbit polyclonal (Pab) antibodies are used as primary antibodies: p62: Mab 3C9; XPA: Mab 1E11 raised against peptide aa 242–261; RPA 70/32: Pab N2.2 (Henricksen et al, 94); RPA32 Mab 1 E9 XPG: Mab 1B5 raised against peptide aa 1167–1186; XPF: Mab Ab-5 (Neomarkers); RNA pol II: Mab 7C2 raised against the Ctd domain and recognizing both forms of RNA pol II and CSB: Pab H300 (Santa Cruz Biotechnology).
In vitro transcription assay
Runoff transcription assays are performed in transcription buffer 50 (20 mM Tris–HCl, pH 7.8, 10% glycerol, 0.1 mM EDTA, 0.5 mM DTT, 50 mM KCl), as described previously (Gerard et al, 1991), but on immobilized damaged Cax-Pt.
Dual incision assay
Reconstituted dual incision reactions (RIS, 25 μl) are carried out in a buffer containing 20 mM HEPES–KOH, pH 7.6, 20 mM Tris–HCl, pH 7.6, 50 mM KCl, 2.5 mM MgCl2, 0.5 mM DTT, 0.5 EDTA mM, 10% glycerol, 0.02% NP40 and 2 mM ATP. NER factors are purified as described (Araujo et al, 2000) and used in saturating amounts: XPC–HR23B (10 ng), TFIIH (50 ng) (Gerard et al, 1991), XPA (30 ng), RPA (200 ng), XPG (50 ng) and XPF-ERCC1 (10 ng) per reaction. The NER factors are incubated with 50 ng of either free or immobilized Cax-Pt, for 90 min at 30°C. Reactions are stopped by boiling for 5 min after the addition of 9 ng of a 32-nt-long oligonucleotide complementary to the excised DNA fragment with a 5′-extension of four extra G residues (Shivji et al, 1995). After annealing the oligonucleotide to the excised DNA fragment, excision products are radiolabeled by extension with 0.15 U of sequenase version 2.0 polymerase (USB) and 2 μCi of (α-32P)dCTP (3000 Ci/mmol). Labeled excision products are separated on a denaturing 14% polyacrylamide gel and visualized by autoradiography.
Dual incision reactions with HeLa WCE and XPC-WCE (30 μg) (Frit et al, 2002) are performed in the presence of 15 μM wortmannin, 60 μM aphidicolin and 2% DMSO, which are preincubated all together for 10 min at 30°C before DNA substrate addition. DNA substrate is then added and the reactions are incubated at 30°C for 40 min.
Quantification was made after scanning the autoradiographies of three independent experiments using the Genetool software (Syngene). Background bands were used to normalize the values obtained for each experiments.
Protein binding studies on immobilized DNA
Reaction mixtures of 50 ng each of previously transcribed (when indicated) immobilized Cax-Pt are washed twice in transcription buffer 400 (20 mM Tris–HCl, pH 7.8, 10% glycerol, 0.1 mM EDTA, 0.5 mM DTT, 400 mM KCl), supplemented with 0.1% sarkosyl, and twice in transcription buffer 50. Cax-Pt was then cut by ClaI (New England Biolabs) in a reaction volume of 40 ml.
The transcribed and ClaI-cut immobilized Cax-Pt is washed again in transcription buffer 400 and 50, before incubation with either purified NER factors or XPC-WCE under dual incision assay condition for 30 min at 30°C. Washed beads in transcription buffer 50 are further analyzed for bound proteins either functionally or by Western blot. Functional protein-binding studies are carried out as described previously (Riedl et al, 2003) by omission of the factor interest in the subsequent dual incision reaction, with the equivalent of one dual incision reaction volume. Western blot analyses were conducted by re-suspending the washed beads in 25 μl of protein loading buffer 1 × and loading them on SDS–PAGE protein gel. The amount of proteins (equivalent to five dual incision reactions) is loaded for each condition. Dephosphorylation of the RNA pol IIO is carried out after the cut by ClaI, by incubating 1 U of calf intestinal phosphatase (CIP) at 37°C for 1 h. CIP is washed off the DNA and the immobilized Cax-Pt is further treated as described above.
Release of RNA pol II from immobilized DNA
The immobilized Cax-Pt is first transcribed (where indicated) and cut by ClaI as described above, and then incubated with NER factors as described in the dual incision assay. The reactions were then incubated at 30°C for 30 min. The supernatant is then collected, supplemented with 5 μl of 6 × protein loading dye and further analyzed on a 6% SDS–PAGE protein gel. Gels are scanned with the Genetool software (Syngene) and the amount of RNA pol IIO released in supernatant was calculated after subtraction of the negative background and correlated to the total amount of RNA pol IIO.
RNA pol II-mediated incision assay
Reaction mixtures of 50 ng each of previously transcribed (when indicated) immobilized DNA are washed twice in transcription buffer 400 and twice in transcription buffer 50. NER factors and others (see figure legends) are incubated as described in the dual incision assay at 30°C for 90 min. Reactions are then stopped by adding 100 μl of phenol and boiled for 5 min. In all, 100 μl of chloroform and 200 μl of stop solution (1% SDS, 300 mM sodium acetate and 25 μg/μl glycogen) are added next and DNA fragments are further ethanol precipitated. The pellet is subjected to 30 cycles (95°C 30 s, 55°C 30 s, 72°C 1 min) of primer extension using a 22-nt-long end-radiolabeled primer, which binds to the transcribed strand 105 nt downstream from the lesion. After phenol–chloroform extraction, ethanol precipitation and wash, the samples are loaded onto a 6% sequencing gel. The gel is dried and autoradiographed. Gels are scanned with the Genetool software (Syngene) and experiments were quantified following the same protocol described in dual incision assay.
Acknowledgments
We thank D Mallery, A Lehman, JH Hoeijmakers and R Wood for providing some of the recombinant proteins. We thank V Mocquet, N Charlet, F Coin and S Feuerhahn for fruitful discussions. We are grateful to I Kolb-Cheynel for the production of recombinant baculoviruses and C Braun and A Larnicol for their high technical expertise. This study was supported by funds from La Ligue contre le Cancer (equipe labelisée, Contract No. EL2004), the Ministere de l'Education National et de la Recherche for ACI grants (BCMS No. 03 2 535). JPh L is supported by grants from the Association pour la Recherche contre le Cancer (ARC) and the Fondation pour La Recherche Medicale (FRM).
References
- Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hubscher U, Egly JM, Wood RD (1995) Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell 80: 859–868 [DOI] [PubMed] [Google Scholar]
- Araujo SJ, Tirode F, Coin F, Pospiech H, Syvaoja JE, Stucki M, Hubscher U, Egly JM, Wood RD (2000) Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev 14: 349–359 [PMC free article] [PubMed] [Google Scholar]
- Asahina H, Kuraoka I, Shirakawa M, Morita EH, Miura N, Miyamoto I, Ohtsuka E, Okada Y, Tanaka K (1994) The XPA protein is a zinc metalloprotein with an ability to recognize various kinds of DNA damage. Mutat Res 315: 229–237 [DOI] [PubMed] [Google Scholar]
- Balajee AS, May A, Dianov GL, Friedberg EC, Bohr VA (1997) Reduced RNA polymerase II transcription in intact and permeabilized Cockayne syndrome group B cells. Proc Natl Acad Sci USA 94: 4306–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr VA, Smith CA, Okumoto DS, Hanawalt PC (1985) DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell 40: 359–369 [DOI] [PubMed] [Google Scholar]
- Bowman KK, Smith CA, Hanawalt PC (1997) Excision-repair patch lengths are similar for transcription-coupled repair and global genome repair in UV-irradiated human cells. Mutat Res 385: 95–105 [DOI] [PubMed] [Google Scholar]
- Bradsher J, Auriol J, Proietti de Santis L, Iben S, Vonesch JL, Grummt I, Egly JM (2002) CSB is a component of RNA pol I transcription. Mol Cell 10: 819–829 [DOI] [PubMed] [Google Scholar]
- Christians FC, Hanawalt PC (1992) Inhibition of transcription and strand-specific DNA repair by alpha-amanitin in Chinese hamster ovary cells. Mutat Res 274: 93–101 [DOI] [PubMed] [Google Scholar]
- Citterio E, Van Den Boom V, Schnitzler G, Kanaar R, Bonte E, Kingston RE, Hoeijmakers JH, Vermeulen W (2000) ATP-dependent chromatin remodeling by the Cockayne syndrome B DNA repair-transcription-coupling factor. Mol Cell Biol 20: 7643–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coin F, Bergmann E, Tremeau-Bravard A, Egly JM (1999) Mutations in XPB and XPD helicases found in xeroderma pigmentosum patients impair the transcription function of TFIIH. EMBO J 18: 1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat WL, Appeldoorn E, Jaspers NG, Hoeijmakers JH (1998a) DNA structural elements required for ERCC1-XPF endonuclease activity. J Biol Chem 273: 7835–7842 [DOI] [PubMed] [Google Scholar]
- de Laat WL, Appeldoorn E, Sugasawa K, Weterings E, Jaspers NG, Hoeijmakers JH (1998b) DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev 12: 2598–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frit P, Kwon K, Coin F, Auriol J, Dubaele S, Salles B, Egly JM (2002) Transcriptional activators stimulate DNA repair. Mol Cell 10: 1391–1401 [DOI] [PubMed] [Google Scholar]
- Gerard M, Fischer L, Moncollin V, Chipoulet JM, Chambon P, Egly JM (1991) Purification and interaction properties of the human RNA polymerase B(II) general transcription factor BTF2. J Biol Chem 266: 20940–20945 [PubMed] [Google Scholar]
- Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, Kisselev AF, Tanaka K, Nakatani Y (2003) The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 113: 357–367 [DOI] [PubMed] [Google Scholar]
- Henning KA, Li L, Iyer N, McDaniel LD, Reagan MS, Legerski R, Schultz RA, Stefanini M, Lehmann AR, Mayne LV, Friedberg EC (1995) The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell 82: 555–564 [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374 [DOI] [PubMed] [Google Scholar]
- Iyer N, Reagan MS, Wu KJ, Canagarajah B, Friedberg EC (1996) Interactions involving the human RNA polymerase II transcription/nucleotide excision repair complex TFIIH, the nucleotide excision repair protein XPG, and Cockayne syndrome group B (CSB) protein. Biochemistry 35: 2157–2167 [DOI] [PubMed] [Google Scholar]
- Lauder S, Bankmann M, Guzder SN, Sung P, Prakash L, Prakash S (1996) Dual requirement for the yeast MMS19 gene in DNA repair and RNA polymerase II transcription. Mol Cell Biol 16: 6783–6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Yu SL, Prakash L, Prakash S (2001) Requirement for yeast RAD26, a homolog of the human CSB gene, in elongation by RNA polymerase II. Mol Cell Biol 21: 8651–8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Yu SL, Prakash L, Prakash S (2002) Yeast RAD26, a homolog of the human CSB gene, functions independently of nucleotide excision repair and base excision repair in promoting transcription through damaged bases. Mol Cell Biol 22: 4383–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman M, Zhang F (1996) Blockage of RNA polymerase as a possible trigger for u.v. light-induced apoptosis. Oncogene 13: 823–831 [PubMed] [Google Scholar]
- Lombaerts M, Tijsterman M, Verhage RA, Brouwer J (1997) Saccharomyces cerevisiae mms19 mutants are deficient in transcription-coupled and global nucleotide excision repair. Nucleic Acids Res 25: 3974–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I, Spivak G, Hanawalt PC (1987) Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell 51: 241–249 [DOI] [PubMed] [Google Scholar]
- Missura M, Buterin T, Hindges R, Hubscher U, Kasparkova J, Brabec V, Naegeli H (2001) Double-check probing of DNA bending and unwinding by XPA-RPA: an architectural function in DNA repair. EMBO J 20: 3554–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu Y, Asahina H, Citterio E, Rademakers S, Vermeulen W, Kamiuchi S, Yeo JP, Khaw MC, Saijo M, Kodo N, Matsuda T, Hoeijmakers JH, Tanaka K (2000) XAB2, a novel tetratricopeptide repeat protein involved in transcription-coupled DNA repair and transcription. J Biol Chem 275: 34931–34937 [DOI] [PubMed] [Google Scholar]
- Nance MA, Berry SA (1992) Cockayne syndrome: review of 140 cases. Am J Med Genet 42: 68–84 [DOI] [PubMed] [Google Scholar]
- Reardon JT, Sancar A (2002) Molecular anatomy of the human excision nuclease assembled at sites of DNA damage. Mol Cell Biol 22: 5938–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R, Adair JE (2005) Role of high mobility group (HMG) chromatin proteins in DNA repair. DNA Repair (Amst) 4: 926–938 [DOI] [PubMed] [Google Scholar]
- Reines D, Ghanouni P, Gu W, Mote J Jr, Powell W (1993) Transcription elongation by RNA polymerase II: mechanism of SII activation. Cell Mol Biol Res 39: 331–338 [PubMed] [Google Scholar]
- Riedl T, Hanaoka F, Egly JM (2003) The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J 22: 5293–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker AH, Tsutakawa SE, Kostek S, Ng C, Shin DS, Peris M, Campeau E, Tainer JA, Nogales E, Cooper PK (2005) Recognition of RNA Polymerase II and Transcription Bubbles by XPG, CSB, and TFIIH: Insights for Transcription-Coupled Repair and Cockayne Syndrome. Mol Cell 20: 187–198 [DOI] [PubMed] [Google Scholar]
- Selby CP, Sancar A (1993) Molecular mechanism of transcription-repair coupling. Science 260: 53–58 [DOI] [PubMed] [Google Scholar]
- Selby CP, Sancar A (1997a) Cockayne syndrome group B protein enhances elongation by RNA polymerase II. Proc Natl Acad Sci USA 94: 11205–11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby CP, Sancar A (1997b) Human transcription-repair coupling factor CSB/ERCC6 is a DNA-stimulated ATPase but is not a helicase and does not disrupt the ternary transcription complex of stalled RNA polymerase II. J Biol Chem 272: 1885–1890 [DOI] [PubMed] [Google Scholar]
- Shivji MK, Podust VN, Hubscher U, Wood RD (1995) Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry 34: 5011–5017 [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, Hanaoka F, Bootsma D, Hoeijmakers JH (1998) Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell 2: 223–232 [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Okamoto T, Shimizu Y, Masutani C, Iwai S, Hanaoka F (2001) A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev 15: 507–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup JQ (2003) Rescue of arrested RNA polymerase II complexes. J Cell Sci 116: 447–451 [DOI] [PubMed] [Google Scholar]
- Sweder KS, Hanawalt PC (1992) Preferential repair of cyclobutane pyrimidine dimers in the transcribed strand of a gene in yeast chromosomes and plasmids is dependent on transcription. Proc Natl Acad Sci USA 89: 10696–10700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantin D (1998) RNA polymerase II elongation complexes containing the Cockayne syndrome group B protein interact with a molecular complex containing the transcription factor IIH components xeroderma pigmentosum B and p62. J Biol Chem 273: 27794–27799 [DOI] [PubMed] [Google Scholar]
- Tantin D, Kansal A, Carey M (1997) Recruitment of the putative transcription-repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol Cell Biol 17: 6803–6814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias A, Auriol J, Forget D, Enzlin JH, Scharer OD, Coin F, Coulombe B, Egly JM (2004) Ordered conformational changes in damaged DNA induced by nucleotide excision repair factors. J Biol Chem 279: 19074–19083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau-Bravard A, Riedl T, Egly JM, Dahmus ME (2004) Fate of RNA polymerase II stalled at a cisplatin lesion. J Biol Chem 279: 7751–7759 [DOI] [PubMed] [Google Scholar]
- Troelstra C, van Gool A, de Wit J, Vermeulen W, Bootsma D, Hoeijmakers JH (1992) ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell 71: 939–953 [DOI] [PubMed] [Google Scholar]
- van den Boom V, Citterio E, Hoogstraten D, Zotter A, Egly JM, van Cappellen WA, Hoeijmakers JH, Houtsmuller AB, Vermeulen W (2004) DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J Cell Biol 166: 27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst GT, van Steeg H, Berg RJ, van Gool AJ, de Wit J, Weeda G, Morreau H, Beems RB, van Kreijl CF, de Gruijl FR, Bootsma D, Hoeijmakers JH (1997) Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition. Cell 89: 425–435 [DOI] [PubMed] [Google Scholar]
- van Gool AJ, Citterio E, Rademakers S, van Os R, Vermeulen W, Constantinou A, Egly JM, Bootsma D, Hoeijmakers JH (1997) The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. EMBO J 16: 5955–5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoffen A, Natarajan AT, Mayne LV, van Zeeland AA, Mullenders LH, Venema J (1993) Deficient repair of the transcribed strand of active genes in Cockayne's syndrome cells. Nucleic Acids Res 21: 5890–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J, van Hoffen A, Natarajan AT, van Zeeland AA, Mullenders LH (1990) The residual repair capacity of xeroderma pigmentosum complementation group C fibroblasts is highly specific for transcriptionally active DNA. Nucleic Acids Res 18: 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichi P, Coin F, Renaud JP, Vermeulen W, Hoeijmakers JH, Moras D, Egly JM (1997) Cisplatin- and UV-damaged DNA lure the basal transcription factor TFIID/TBP. EMBO J 16: 7444–7456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade MH, Chu EH (1979) Effects of DNA damaging agents on cultured fibroblasts derived from patients with Cockayne syndrome. Mutat Res 59: 49–60 [DOI] [PubMed] [Google Scholar]
- Wakasugi M, Sancar A (1999) Order of assembly of human DNA repair excision nuclease. J Biol Chem 274: 18759–18768 [DOI] [PubMed] [Google Scholar]
- Yamaizumi M, Sugano T (1994) U.v.-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene 9: 2775–2784 [PubMed] [Google Scholar]


