Abstract
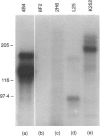
Functional and phenotypic properties of porcine peripheral blood CD4/CD8 double-positive (DP) lymphocytes were examined. In cross-sectional and longitudinal studies involving a total of 103 pigs, this lymphocyte population was found to increase gradually in proportion with age, comprising < 2% of the total peripheral blood lymphocyte pool in 1-week-old swine and reaching 30-55% by 3 years of age. CD4/CD8 DP lymphocytes were able to proliferate in response to stimulation with recall viral antigen. Furthermore, these cells mostly expressed high levels of the surface antigen recognized by monoclonal antibody (mAb) 4B4 (4B4hi), which is specific for the human beta 1 integrin. The CD4+4B4hi lymphocytes from pseudorabies virus-immune swine, proliferated in response to stimulation with the homologous virus, while CD4+4B4lo lymphocytes did not. Stimulation of CD4 single-positive (SP) cells with recall viral antigen, but not with mitogen, resulted in the generation of lymphoblasts which were predominantly of CD4/CD8 DP phenotype, suggesting a role for recall antigen in the generation of this lymphocyte subset. More than half of the CD4+ lymphocytes from palatine tonsils of 6-month-old swine were CD4/CD8 DP, while in the lymph nodes CD4/CD8 DP cells accounted for only one-third or less of CD4+ cells. In contrast, CD4/CD8 DP lymphocytes were absent from the palatine tonsils of 3-day-old swine, which only contained CD4 SP cells. Together, these results indicate that porcine CD4/CD8 DP lymphocytes, exhibit properties of mature antigen-experienced cells, and are inducible by stimulation with recall antigen. These data are consistent with the hypothesis that this population in swine includes memory/effector T cells.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blue M. L., Daley J. F., Levine H., Craig K. A., Schlossman S. F. Biosynthesis and surface expression of T8 by peripheral blood T4+ cells in vitro. J Immunol. 1986 Aug 15;137(4):1202–1207. [PubMed] [Google Scholar]
- Blue M. L., Daley J. F., Levine H., Schlossman S. F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985 Apr;134(4):2281–2286. [PubMed] [Google Scholar]
- Burdick J. F., Beschorner W. E., Smith W. J., McGraw D., Bender W. L., Williams G. M., Solez K. Characteristics of early routine renal allograft biopsies. Transplantation. 1984 Dec;38(6):679–684. doi: 10.1097/00007890-198412000-00026. [DOI] [PubMed] [Google Scholar]
- Clayberger C., Krensky A. M., McIntyre B. W., Koller T. D., Parham P., Brodsky F., Linn D. J., Evans E. L. Identification and characterization of two novel lymphocyte function-associated antigens, L24 and L25. J Immunol. 1987 Mar 1;138(5):1510–1514. [PubMed] [Google Scholar]
- De Maria A., Malnati M., Moretta A., Pende D., Bottino C., Casorati G., Cottafava F., Melioli G., Mingari M. C., Migone N. CD3+4-8-WT31-(T cell receptor gamma+) cells and other unusual phenotypes are frequently detected among spontaneously interleukin 2-responsive T lymphocytes present in the joint fluid in juvenile rheumatoid arthritis. A clonal analysis. Eur J Immunol. 1987 Dec;17(12):1815–1819. doi: 10.1002/eji.1830171221. [DOI] [PubMed] [Google Scholar]
- Devriese L. A., Hommez J., Pot B., Haesebrouck F. Identification and composition of the streptococcal and enterococcal flora of tonsils, intestines and faeces of pigs. J Appl Bacteriol. 1994 Jul;77(1):31–36. doi: 10.1111/j.1365-2672.1994.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Dillender M. J., Lunney J. K. Characteristics of T lymphocyte cell lines established from NIH minipigs challenge inoculated with Trichinella spiralis. Vet Immunol Immunopathol. 1993 Jan;35(3-4):301–319. doi: 10.1016/0165-2427(93)90041-2. [DOI] [PubMed] [Google Scholar]
- Fujihashi K., Yamamoto M., McGhee J. R., Kiyono H. alpha beta T cell receptor-positive intraepithelial lymphocytes with CD4+, CD8- and CD4+, CD8+ phenotypes from orally immunized mice provide Th2-like function for B cell responses. J Immunol. 1993 Dec 15;151(12):6681–6691. [PubMed] [Google Scholar]
- Gianello P. R., Blancho G., Fishbein J. F., Lorf T., Nickeleit V., Vitiello D., Sachs D. H. Mechanism of cyclosporin-induced tolerance to primarily vascularized allografts in miniature swine. Effect of administration of exogenous IL-2. J Immunol. 1994 Nov 15;153(10):4788–4797. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Holers V. M., Ruff T. G., Parks D. L., McDonald J. A., Ballard L. L., Brown E. J. Molecular cloning of a murine fibronectin receptor and its expression during inflammation. Expression of VLA-5 is increased in activated peritoneal macrophages in a manner discordant from major histocompatibility complex class II. J Exp Med. 1989 May 1;169(5):1589–1605. doi: 10.1084/jem.169.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S., Sogn J. A., Kindt T. J. Microdetermination of rabbit immunoglobulin allotypes by ELISA using specific antibodies conjugated with peroxidase or with biotin. J Immunol Methods. 1982;48(3):299–309. doi: 10.1016/0022-1759(82)90331-3. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991 Sep 15;147(6):1746–1751. [PubMed] [Google Scholar]
- Lunney J. K., Pescovitz M. D. Phenotypic and functional characterization of pig lymphocyte populations. Vet Immunol Immunopathol. 1987 Dec;17(1-4):135–144. doi: 10.1016/0165-2427(87)90134-6. [DOI] [PubMed] [Google Scholar]
- Lunney J. K., Walker K., Goldman T., Aasted B., Bianchi A., Binns R., Licence S., Bischof R., Brandon M., Blecha F. Overview of the First International Workshop to Define Swine Leukocyte Cluster of Differentiation (CD) Antigens. Vet Immunol Immunopathol. 1994 Oct;43(1-3):193–206. doi: 10.1016/0165-2427(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K., Horovitz D. A., Fletcher F. A., Watson J. D. Analysis of the intra-epithelial lymphocyte compartment in SCID mice that received co-isogenic CD4+ T cells. Evidence that mature post-thymic CD4+ T cells can be induced to express CD8 alpha in vivo. J Immunol. 1995 Mar 15;154(6):2678–2686. [PubMed] [Google Scholar]
- Mosley R. L., Styre D., Klein J. R. CD4+CD8+ murine intestinal intraepithelial lymphocytes. Int Immunol. 1990;2(4):361–365. doi: 10.1093/intimm/2.4.361. [DOI] [PubMed] [Google Scholar]
- Ortolani C., Forti E., Radin E., Cibin R., Cossarizza A. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem Biophys Res Commun. 1993 Mar 15;191(2):601–609. doi: 10.1006/bbrc.1993.1260. [DOI] [PubMed] [Google Scholar]
- Patel S. S., Wacholtz M. C., Duby A. D., Thiele D. L., Lipsky P. E. Analysis of the functional capabilities of CD3+CD4-CD8- and CD3+CD4+CD8+ human T cell clones. J Immunol. 1989 Aug 15;143(4):1108–1117. [PubMed] [Google Scholar]
- Pescovitz M. D., Hsu S. M., Katz S. I., Lunney J. K., Shimada S., Sachs D. H. Characterization of a porcine CD1-specific mAb that distinguishes CD4/CD8 double-positive thymic from peripheral T lymphocytes. Tissue Antigens. 1990 Apr;35(4):151–156. doi: 10.1111/j.1399-0039.1990.tb01772.x. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985 Jan;134(1):37–44. [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984 Jul;133(1):368–375. [PubMed] [Google Scholar]
- Pescovitz M. D., Sakopoulos A. G., Gaddy J. A., Husmann R. J., Zuckermann F. A. Porcine peripheral blood CD4+/CD8+ dual expressing T-cells. Vet Immunol Immunopathol. 1994 Oct;43(1-3):53–62. doi: 10.1016/0165-2427(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Quiding M., Granström G., Nordström I., Ferrua B., Holmgren J., Czerkinsky C. High frequency of spontaneous interferon-gamma-producing cells in human tonsils: role of local accessory cells and soluble factors. Clin Exp Immunol. 1993 Jan;91(1):157–163. doi: 10.1111/j.1365-2249.1993.tb03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmüller A., Bryant J. Characteristics of porcine T lymphocytes and T-cell lines. Vet Immunol Immunopathol. 1994 Oct;43(1-3):45–52. doi: 10.1016/0165-2427(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Hirt W., Reddehase M. J. Phenotypic discrimination between thymic and extrathymic CD4-CD8- and CD4+CD8+ porcine T lymphocytes. Eur J Immunol. 1989 Nov;19(11):2011–2016. doi: 10.1002/eji.1830191107. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Jonjic S., Bühring H. J., Reddehase M. J., Koszinowski U. H. Monoclonal antibodies reactive with swine lymphocytes. II. Detection of an antigen on resting T cells down-regulated after activation. J Immunol. 1987 Mar 15;138(6):1852–1857. [PubMed] [Google Scholar]
- Saalmüller A., Reddehase M. J., Bühring H. J., Jonjić S., Koszinowski U. H. Simultaneous expression of CD4 and CD8 antigens by a substantial proportion of resting porcine T lymphocytes. Eur J Immunol. 1987 Sep;17(9):1297–1301. doi: 10.1002/eji.1830170912. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Weiland F., Reddehase M. J. Resting porcine T lymphocytes expressing class II major histocompatibility antigen. Immunobiology. 1991 Sep;183(1-2):102–114. doi: 10.1016/S0171-2985(11)80190-7. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Leight G., Cone J., Schwarz S., Stuart L., Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976 Dec;22(6):559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Secrist H., Egan M., Peters M. G. Tissue-specific regulation of IL-4 mRNA expression in human tonsil. J Immunol. 1994 Feb 1;152(3):1120–1126. [PubMed] [Google Scholar]
- Takimoto H., Nakamura T., Takeuchi M., Sumi Y., Tanaka T., Nomoto K., Yoshikai Y. Age-associated increase in number of CD4+CD8+ intestinal intraepithelial lymphocytes in rats. Eur J Immunol. 1992 Jan;22(1):159–164. doi: 10.1002/eji.1830220124. [DOI] [PubMed] [Google Scholar]
- Wood R. L., Pospischil A., Rose R. Distribution of persistent Salmonella typhimurium infection in internal organs of swine. Am J Vet Res. 1989 Jul;50(7):1015–1021. [PubMed] [Google Scholar]
- Zuckermann F. A., Schabacker D., Binns R. M. Biochemical analysis of molecules reactive with monoclonal antibodies specific for porcine CD45. Vet Immunol Immunopathol. 1994 Oct;43(1-3):307–313. doi: 10.1016/0165-2427(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Zuckermann F. A., Zsak L., Mettenleiter T. C., Ben-Porat T. Pseudorabies virus glycoprotein gIII is a major target antigen for murine and swine virus-specific cytotoxic T lymphocytes. J Virol. 1990 Feb;64(2):802–812. doi: 10.1128/jvi.64.2.802-812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]