Abstract
Anesthesia is an indispensable component of any operative procedure. In this study, we demonstrate that continuous isoflurane anesthesia for 1 h after a lethal dose (20 mg/kg of body weight) of Escherichia coli lipopolysaccharide (LPS) results in a significant increase in survival of C57BL/6J (B6) mice in comparison with survival of nonanesthetized mice. Protection by anesthesia correlates with a delay in plasma LPS circulation, resulting in a delayed inflammatory response, particularly DNA binding activity of NF-κB and serum levels of tumor necrosis factor alpha, interleukin-6 (IL-6), and IL-10. Disparate classes of anesthetic agents produce the same effects on the inflammatory response, which is also independent of the inbred mouse strain used. These results suggest that anesthesia has an important impact on the outcome from endotoxemia. Moreover, the immunomodulatory effects of anesthetics should be considered when interpreting data from experimental animal models.
Anesthesia has become an indispensable component of modern medicine. Despite the now widespread usage of anesthetics, the mechanisms involved in their actions are poorly understood (11, 26). Current concepts suggest that many general anesthetics alter neuronal ion channel activity, specifically synaptic neurotransmitter receptors, such as GABAA, glutamate, and nicotinic acetylcholine receptors (11, 26). In addition to providing analgesia, amnesia, and immobility, anesthetics also appear to play a pivotal role in immune function and inflammation. Recent studies have shown that anesthetic agents modify the neuroendocrine stress response to various operative procedures by decreasing adrenocorticotropic hormone, cortisol, adrenaline, noradrenaline, and growth hormone levels (1, 18). Anesthetics have also been found to maintain the balance between Th1 and Th2 immune responses (27), which is altered in critically ill patients (33, 37). Thus, anesthetics can profoundly influence the hypothalamic-pituitary-adrenal axis, perhaps providing balance between pro- and anti-inflammatory mediators during stress (7, 12).
The alteration in the balance between pro- and anti-inflammatory agents is likely responsible for the development of septic shock. The incidence of sepsis in the United States is approximately 750,000 per year, with mortality of 30 to 50% (29). Sepsis is usually initiated by microbial agents or their products, such as lipopolysaccharide (LPS), an outer membrane component of gram-negative bacteria. LPS induces a rapid release of inflammatory mediators, such as tumor necrosis factor alpha (TNF-α), gamma interferon, interleukin-1β (IL-lβ), and IL-6. The secretion of these proinflammatory mediators is followed by release of counterregulatory cytokines, such as IL-10 and transforming growth factor β (8, 21). In the present study, we show that anesthetics modify the inflammatory response induced by LPS and affect the outcome from endotoxic shock.
MATERIALS AND METHODS
Animal model of anesthesia and endotoxic shock.
Eight-week-old, male B6 mice (20 to 30 g; Jackson Laboratory, Bar Harbor, ME) were housed in a Helicobacter species-free environment with ad libitum access to standard chow and water. The animal housing environment was maintained at a temperature of 22°C and with a 12-h light/dark cycle. Animals were acclimatized to their environment for 5 to 6 days following arrival and were then fasted for 16 h prior to any procedure. All animal experiments were performed in accordance with the Animal Care and Use Committee of the Johns Hopkins University School of Medicine and complied with NIH guidelines for animal experimentation. Mice were anesthetized using 2 to 2.5% vaporized inhaled isoflurane (IsoFlo; Abbott Laboratories, Chicago, IL). LPS from Escherichia coli serotype O26:B6 was obtained by trichloroacetic acid precipitation (Sigma-Aldrich, St. Louis, MO) and given via intraperitoneal (i.p.) injection at the following doses: 20 mg/kg of body weight for mortality studies and 15 mg/kg for all other studies. In one group of mice, anesthesia was immediately induced and then maintained for 1 h with isoflurane inhalation (simultaneous). A second group was anesthetized for 1 h after a 30-min interval following LPS injection (post-LPS injection). The third group received no anesthesia following LPS injection (LPS control). For another experiment, 8-week-old male mice from different strains (A/J, DBA/1J, BALB/CJ, 129S1/SvImJ, and C3H/HeN [20 to 30 g]; Jackson Laboratory, Bar Harbor, ME) all arrived the same week. Mice were randomized into either a simultaneous group or an LPS control group as described above. In a separate experiment, mice were anesthetized for 1 h and then injected with LPS (pretreatment group) or anesthetized for 1 h, allowed to recover for 30 min, and then injected with LPS (pretreatment/recovery group). Simultaneous and LPS control groups identical to those described above were also included in this experiment. For all animals, blood samples were collected via cardiac puncture 1.5 h after LPS (or saline) injection. Plasma was isolated via centrifugation and stored at −80°C. TNF-α, IL-6, and IL-10 plasma levels were determined by an enzyme-linked immunosorbent assay (Biosource, Camarillo, CA). For survival studies, animals were given ad libitum access to standard chow and water postprocedurally.
Other anesthetic agents.
Mice were injected with LPS intraperitoneally and simultaneously anesthetized for 1.5 h with pentobarbital (70 mg/kg i.p., Nembutal; Abbott Laboratories, Chicago, IL); ketamine (150 mg/kg i.p., Ketaject; Phoenix Pharmaceutical Inc., St. Joseph, MO) plus xylazine (15 mg/kg i.p., Xylaject; Phoenix Pharmaceutical Inc.); medetomidine (1 mg/kg i.p., Domitor; Orion Corp., Finland) plus ketamine (75 mg/kg i.p.); halothane (2% inhaled; Halocarbon Laboratories, River Edge, NJ); or ether (inhaled from gauze-soaked nose cone; J.T. Baker, Phillipsburg, NJ). Blood samples were collected via cardiac puncture immediately following the anesthetic period. Plasma was isolated via centrifugation and stored at −80°C.
Atropine treatment.
B6 mice were injected with atropine (Sigma-Aldrich, St. Louis, MO) prepared in sterile saline. The atropine was injected intraperitoneally at a dose of 1 mg/kg 10 min before the administration of LPS or the simultaneous administration of LPS and anesthesia (for 1 h). Two controls were used: mice injected with saline only (rather than atropine) 10 min prior to LPS, and mice injected with atropine without any further intervention (no LPS). Control animals received a comparable volume of saline.
Electrophoretic mobility shift assay.
Nuclear extracts were isolated using a modification of the protocol described by Dignam et al. (19). Briefly, 100 mg of liver was resuspended in 1 ml of hypotonic buffer (HEPES, 10 mM [pH 7.9]; KCl, 10 mM; EDTA, 0.1 mM; EGTA, 0.1 mM; dithiothreitol, 1 mM; phenylmethylsulfonyl fluoride, 0.5 mM; and 10 μg/ml each of leupeptin, aprotinin, and pepstatin), homogenized, and incubated on ice for 10 min. Samples were centrifuged at 850 × g at 4°C for 10 min, and the pellet was resuspended in 1 ml of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride) and incubated on ice for 10 min. Nonidet P-40 at a final concentration of 0.6% was added, and lysates were vortexed for 1 min. Nuclei were isolated by centrifugation at 2,800 × g for 10 min at 4°C, and the pellet was resuspended in 200 μl of buffer C (HEPES, 20 mM [pH 7.9]; 1 M NaCl; 5% glycerol; 1 mM EDTA; 1 mM EGTA; 1 mM dithiothreitol; 0.5 mM phenylmethylsulfonyl fluoride; and 10 μg/ml each of leupeptin, aprotinin, and pepstatin) and gently agitated on an orbital shaker at 4°C for 30 min. The suspension was centrifuged at 14,000 × g for 10 min at 4°C, and the supernatant was aliquoted and stored at −80°C. An NF-κB DNA binding assay was performed using a double-stranded oligonucleotide containing the consensus binding site for NF-κB (Promega, Madison, WI), and the fragment was end labeled with [γ-32P]ATP (7,000 Ci/mmol; NEN Life Science Products, Boston, MA) by using T4 polynucleotide kinase (Promega, Madison, WI) as described previously (23).
Endotoxin detection.
B6 mice (n = 3) were injected with LPS and simultaneously anesthetized with isoflurane or allowed to remain nonanesthetized (LPS control). Blood samples were obtained via cardiac puncture 0, 5, 10, 20, and 30 min after LPS injection, and samples were stored in sterile, pyrogen-free K+EDTA tubes. Plasma was isolated via centrifugation and stored at −80°C. Endotoxin levels were measured in plasma by use of an automated turbidimetric Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD). Before testing, serum was diluted 1:15 with endotoxin-free water and heated at 70°C for 7 min to remove inhibitors in plasma.
Statistical analysis.
Survival was analyzed via Kaplan-Meier analysis using the log rank test. Cytokine data shown are means ± standard errors of the means (SEM). General differences in cytokine levels among all groups were determined using one-way analysis of variance, with multiple pairwise comparisons by the Student-Newman-Keuls method being used to elucidate specific significances in these parameters between groups. All tests were two-tailed, and differences were considered significant when P was <0.05. Analysis was performed using Microsoft Excel (Microsoft Corporation, Seattle, WA) and SigmaStat (SPSS Incorporated, Chicago, IL) software.
RESULTS
Isoflurane anesthesia increases survival after LPS challenge.
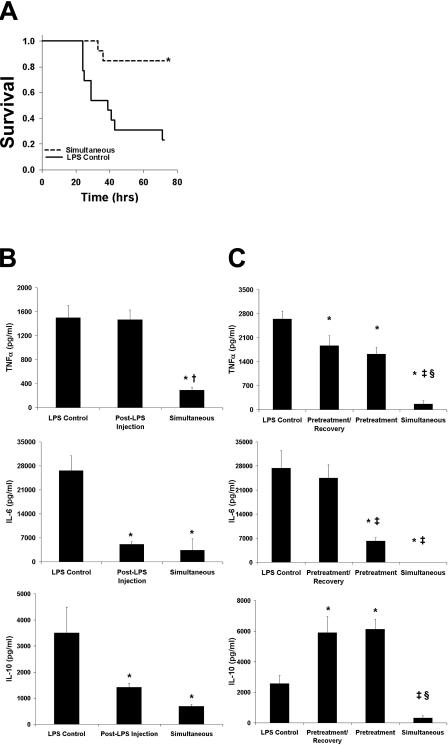
The effects of anesthetics on the outcome of B6 mice after a lethal dose of LPS injection were compared between mice in the absence (control group) or presence of isoflurane for 1 h. Mice that were challenged with LPS and anesthetized developed fewer distress symptoms (e.g., decreased feeding, conjunctivitis, diarrhea, hypoactivity, piloerection, and trembling) than mice that were injected with LPS in the absence of isoflurane anesthesia. An 85% survival rate was observed for mice injected with LPS in the presence of isoflurane, compared to 23% survival in the control group (P < 0.01), within 72 h of the LPS injection (Fig. 1A). Furthermore, mice that survived the LPS challenge in the simultaneous isoflurane group were fully recovered by 48 h, as determined by locomotive activity and feeding, as opposed to 80 h for the nonanesthetized group.
FIG. 1.
Isoflurane ameliorates the response to endotoxic shock. (A) B6 mice (n = 13) were injected with LPS (20 mg/kg) and then (i) were anesthetized for 1 h (simultaneous) or (ii) received no further treatment (LPS control). Mortality was monitored for 72 h following LPS injection. Increased survival was observed among the simultaneous animals (85%) compared to control animals (23%) (P < 0.01). (B) Levels of TNF-α, IL-6, and IL-10 in mice (n = 10) were measured 1.5 h after LPS (15 mg/kg) injection. Lower cytokine levels were observed with the simultaneous group than with the post-LPS injection and control groups (P < 0.05 for all). (C) Mice (n = 8) were anesthetized for 1 h and were injected with LPS (15 mg/kg) immediately (pretreatment group) or after a 30-min recovery (pretreatment/recovery group). Attenuation of cytokine levels was most marked in the simultaneous group. Data are presented as means ± SEM. *, P < 0.05 versus LPS control; †, P < 0.05 versus post-LPS injection; ‡, P < 0.05 versus pretreatment/recovery; §, P < 0.05 versus pretreatment.
Protection from endotoxic shock by isoflurane anesthesia correlates with attenuation of the inflammatory process.
The reduced mortality of anesthetized mice after LPS injection may be due to attenuation of the systemic inflammatory response induced by LPS. To test this idea, mice were injected with LPS and exposed to isoflurane for 1 h immediately (simultaneous group) or 30 min after the LPS injection (post-LPS injection group). These two groups were compared with nonanesthetized mice injected with LPS (control). Plasma samples were collected 1.5 h after LPS injection for each group. A decrease in TNF-α plasma levels was observed for the simultaneous group in comparison with levels for the other two groups (Fig. 1B). IL-6 and IL-10 levels were also reduced in the simultaneous and post-LPS injection groups compared to levels for the control group (Fig. 1B). To further characterize these findings, the effect of isoflurane pretreatment was investigated. Compared to levels in the LPS control group, TNF-α plasma levels 1.5 h after LPS injection were reduced in both the pretreatment/recovery and the pretreatment group. However, TNF-α levels for the two pretreatment groups were still significantly greater than the level for the simultaneous group (Fig. 1C). In contrast, IL-6 plasma levels were reduced only with the pretreatment and simultaneous groups, compared to levels for the control and pretreatment/recovery groups (Fig. 1C). Finally, IL-10 plasma levels in both pretreatment groups were greater than levels in the simultaneous and control groups (Fig. 1C). These data suggest that the suppressive effect of isoflurane on LPS-induced cytokine production increases survival in anesthetized animals. We also evaluated the effect of isoflurane anesthesia duration on the inflammatory response induced by LPS. Mice were injected with LPS and then administered continuous anesthesia for 0, 10, 30, or 60 min. Cytokine plasma levels were measured 1.5 h after injection. Decreasing levels of TNF-α and IL-6 were observed with increasing duration of anesthesia, whereas IL-10 levels decreased only with the 60-min anesthesia group (Fig. 2). Therefore, the timing and duration of isoflurane administration are important in modulating the LPS-induced inflammatory response.
FIG. 2.
Decreasing LPS-induced cytokine production correlates with increasing duration of anesthesia. B6 mice (n = 10) were injected with LPS (15 mg/kg) and anesthetized for 0, 10, 30, or 60 min. Serum levels of TNF-α, IL-6, and IL-10 were measured 1.5 h after LPS injection. Reductions in TNF-α and IL-6 plasma levels were inversely proportional to the anesthetic duration. IL-10 levels were depressed only after 60 min of anesthesia. Data are presented as means ± SEM. *, P < 0.05 versus 0 min; †, P < 0.05 versus 10 min; ‡, P < 0.05 versus 30 min.
Different classes of anesthetics also blunt the inflammatory response induced by LPS.
Our preceding observations prompted us to investigate whether other anesthetics would influence the inflammatory response similarly to isoflurane. Mice were injected with LPS and simultaneously anesthetized for 1.5 h with isoflurane, pentobarbital, or ketamine-xylazine. Plasma samples were collected upon completion of the anesthetic period. Both injectable and volatile anesthetics produced a significant reduction in serum TNF-α, IL-6, and IL-10 compared to levels for the LPS control group (Table 1). Halothane, ether, and ketamine-medetomidine were also found to attenuate cytokine production (data not shown). These results suggest that there is a common pathway of anesthetic attenuation of the inflammatory response.
TABLE 1.
Effects of different anesthetics on plasma cytokine levelsa
| Anesthetic or control | Level (pg/ml) of:
|
||
|---|---|---|---|
| TNF-α | IL-6 | IL-10 | |
| LPS control | 5,249 ± 662 | 27,681 ± 3,403 | 1,166 ± 175 |
| Isoflurane | 1,276 ± 385* | 1,192 ± 662* | 146 ± 37* |
| Pentobarbital | 996 ± 46* | 2,371 ± 1,042* | 451 ± 181* |
| Ketamine-xylazine | 192 ± 66* | 110 ± 110* | 58 ± 43* |
B6 mice (n = 10) were injected with LPS (15 mg/kg) and anesthetized for 90 min. Serum levels of TNF-α, IL-6, and IL-10 were measured 90 min after LPS injection. All anesthetics decreased TNF-α, IL-6, and IL-10 levels. Data are presented as means ± SEM. *, P < 0.05 versus LPS control.
Attenuation of LPS-induced cytokine levels is similar among genetically dissimilar mouse strains.
Different mouse strains have been found to display different sensitivities to anesthetics (42). Moreover, the inflammatory responses induced by LPS also differ among various mouse strains (17). Therefore, we compared the effects of isoflurane anesthesia on cytokine production induced by LPS in B6, A/J, DBA/1J, BALB/CJ, 129S1/SvImJ, and C3H/HeN mice. Among all strains, simultaneous administration of anesthesia and LPS attenuated TNF-α, IL-6, and IL-10 compared to use of LPS alone (Table 2), indicating that the effect of isoflurane is broad with respect to host genetic background.
TABLE 2.
Effect of isoflurane on different inbred mouse strains
| Cytokine | Mouse strain | Cytokine plasma level (pg/ml) for groupa
|
|
|---|---|---|---|
| LPS control | Simultaneous (% of control) | ||
| TNF-α | B6 | 1,503 ± 199 | 291 ± 48* (19.36) |
| A/J | 7,841 ± 1,165 | 216 ± 60* (2.75) | |
| DBA/1J | 2,503 ± 303 | 496 ± 225* (19.82) | |
| BALB/CJ | 5,326 ± 545 | 0 ± 0* (0.00) | |
| 129S1/SvImJ | 918 ± 201 | 151 ± 41* (16.45) | |
| C3H/HeN | 2,857 ± 472 | 478 ± 146* (16.73) | |
| IL-6 | B6 | 26,645 ± 4,303 | 3,389 ± 3,310* (12.72) |
| A/J | 29,510 ± 3,939 | 8 ± 8* (0.03) | |
| DBA/1J | 15,271 ± 1,417 | 161 ± 70* (1.05) | |
| BALB/CJ | 22,991 ± 2,401 | 3,852 ± 699* (16.75) | |
| 129S1/SvImJ | 15,227 ± 1,765 | 64 ± 32* (0.42) | |
| C3H/HeN | 4,078 ± 734 | 1,032 ± 276* (25.31) | |
| IL-10 | B6 | 3,501 ± 991 | 696 ± 66* (19.88) |
| A/J | 2,208 ± 201 | 0 ± 0* (0.00) | |
| DBA/1J | 2,012 ± 200 | 154 ± 35* (7.65) | |
| BALB/CJ | 5,624 ± 393 | 1,817 ± 212* (32.31) | |
| 129S1/SvImJ | 1,417 ± 436 | 257 ± 127* (18.14) | |
| C3H/HeN | 447 ± 61 | 212 ± 48* (47.43) | |
Animals (n = 10) were subjected to simultaneous LPS injection and anesthesia (simultaneous group) or LPS control (no anesthesia). Data are presented as means ± SEM. *, P < 0.05 versus LPS control.
Effect of isoflurane in decreasing LPS-induced cytokine production is not mediated through the cholinergic pathway.
Current hypotheses regarding the molecular mechanism underlying the anesthetic action of isoflurane involve alterations in neuronal synaptic transmission (26), with nicotinic acetylcholine receptors potentially being the most modulating targets (24). Therefore, we tested the hypothesis that inhaled isoflurane anesthesia stimulates the recently described cholinergic anti-inflammatory pathway (nicotinic receptors) (45), thus decreasing cytokine production in LPS-stimulated mice. Compared to saline control 1.5 h after LPS injection, atropine-treated mice displayed reduced plasma TNF-α levels (P < 0.01) and increased IL-10 levels (P < 0.001), with no change in IL-6 levels (Fig. 3). Most notably, isoflurane still significantly attenuated serum production of all three plasma cytokines (to levels comparable to atropine without LPS) in atropine-treated animals (10 min pre-LPS). These observations suggest that the effect of isoflurane on the inflammatory process may be independent of the cholinergic pathway and, thus, is unlikely to be mediated by the vagus nerve or nicotinic receptors.
FIG. 3.
Mechanism of anesthesia immunomodulation does not involve the cholinergic pathway. B6 mice (n = 7) were injected i.p. with atropine (1 mg/kg) (columns II, III, and IV) or saline (equivalent volume) (column I) 10 min before LPS injection (15 mg/kg). The group depicted by column III received isoflurane anesthesia for 60 min. Cytokine plasma levels were measured 1.5 h after LPS injection. LPS-induced TNF-α plasma levels decreased while IL-10 levels increased after administration of atropine. With chemically vagotomized mice (atropine), anesthesia still decreased TNF-α, IL-6, and IL-10, all to levels similar to that with atropine alone (no LPS). Data are presented as means ± SEM. *, P < 0.05 versus column I; †, P < 0.05 versus column II.
Isoflurane delays the activation of NF-κB, cytokine production, and levels of LPS in circulation.
We also investigated the activation of NF-κB, a central component of the LPS response. B6 mice were injected with LPS alone or with LPS plus simultaneous isoflurane anesthesia for 0, 5, 10, 20, 30, or 60 min. Liver samples were harvested at the end of the anesthetic period, and NF-κB binding to DNA was determined by a gel shift assay. NF-κB normally resides in the cytoplasm bound to the inhibitor IκB complex. After stimulation with LPS, IκB is phosphorylated and degraded, allowing NF-κB to translocate into the nucleus, resulting in the transcription of many inflammatory genes (4, 16). NF-κB DNA binding was retarded in samples from isoflurane-anesthetized mice in comparison with samples from the nonanesthetized group (Fig. 4A). Thus, anesthesia appears to cause a delay in the activation of NF-κB.
FIG. 4.
Anesthetized mice injected with LPS show delayed NF-κB nuclear activity and a shift in cytokine kinetics. B6 mice (n = 3) were injected with LPS alone or with simultaneous anesthetization with isoflurane for 0, 5, 10, 20, 30, or 60 min. Liver samples were harvested at the end of the anesthetic period and nuclei were isolated. (A) NF-κB binding to DNA was monitored by a gel shift assay. A delay in the activation of NF-κB or translocation of NF-κB into the nucleus was observed for mice exposed to isoflurane in comparison with results for nonanesthetized mice. P, probe alone. (B) This delay correlated with a 1- to 2-h shift in cytokine kinetics for anesthetized mice (n = 7) compared to LPS control mice. Data are presented as means ± SEM. *, P < 0.05 versus LPS control.
The peak of plasma TNF-α after LPS injection also shifted to a later time point in anesthetized mice than in nonanesthetized controls (Fig. 4B). IL-6 levels were elevated overall and peaked earlier in the nonanesthetized group than in mice treated with isoflurane (Fig. 4B). Finally, IL-10 levels rose later and peaked higher in the isoflurane-treated group than in the nonanesthetized controls (Fig. 4B). The concentrations of LPS in systemic circulation following its intraperitoneal injection in both anesthetized and nonanesthetized mice were compared. The appearance of LPS in circulation was slower in anesthetized mice than in the control group (Fig. 5), which correlates with the delayed activation of NF-κB and cytokine kinetics.
FIG. 5.
Isoflurane treatment reduces systemic LPS circulation. B6 mice (n = 3) were injected with LPS and simultaneously anesthetized with isoflurane or allowed to remain nonanesthetized (LPS control). Blood samples were obtained via cardiac puncture 0, 5, 10, 20, and 30 min after LPS injection. Endotoxin levels were measured in plasma by use of a turbidimetric Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD). Data are presented as means ± SEM. *, P < 0.05 versus LPS control. EU, endotoxin units.
DISCUSSION
This study provides evidence that anesthetics, specifically isoflurane, dramatically attenuate the inflammatory response induced by LPS. This effect is modulated by altered LPS levels in systemic circulation, which change cytokine kinetics and increase survival. Isoflurane offered the greatest benefit when it was administered simultaneously with LPS injection rather than after the insult, suggesting that the action of this anesthetic occurs at the early stages of the response induced by LPS. These observations are consistent with the fact that the response initiated by gram-negative bacteria is a rapid event in which many inflammatory mediators are released into the circulation within minutes of the initiating event (46). Consequently, isoflurane anesthesia offers a brief therapeutic window during which the development of severe sepsis and septic shock may be avoided. This assumption echoes clinical studies indicating that patients anesthetized within 1 h of injury have better chances for recovery than individuals treated at a later time point (14).
The multiple detrimental effects of severe sepsis are attributed to an overwhelming inflammatory response. We found that administration of anesthetics simultaneously with LPS injection delayed the peak of TNF-α from 1.5 to 2 h (Fig. 4B) without affecting the peak level. Similarly, the time of maximum IL-6 expression changed from 2 to 4 h in the control group versus the anesthetized group. This delay in cytokine profile correlated with delayed NF-κB DNA binding activity. We also found a decrease in cytokine production proportional to an increase in the duration of anesthesia. The anti-inflammatory cytokine IL-10 displayed higher levels in anesthetized mice than in nonanesthetized mice. Prior studies have shown that IL-10 decreases TNF-α levels and increases survival in the cecal ligation and puncture and LPS models of sepsis (31, 38). It is possible that elevation of IL-10 may be at least in part responsible for the decrease in mortality in mice treated with isoflurane. Our findings regarding changes in cytokine kinetics after LPS injection are consistent with prior studies using the anesthetic ketamine, which decreased systolic arterial pressure, acid-base changes, and cytokine production when administered immediately before LPS injection (43). Isoflurane pretreatment has also been shown to prevent endothelial and vascular smooth muscle cell injury in vitro (15) and in vivo (36). Ketamine-xylazine has been shown to attenuate gastrointestinal inducible nitric oxide synthase (iNOS) expression (28) and activity in activated alveolar macrophages exposed to LPS (32). Furthermore, volatile anesthetics have been implicated in the reduction of mRNA and protein levels of iNOS and NOS activity after LPS or gamma interferon stimulation (47). The anesthetic protection that we observed could be due to attenuation of iNOS expression, thereby preventing the cardiovascular collapse during sepsis partially caused by upregulation of iNOS and cytokine release (20). Another potential mechanism by which anesthetics may exert their protection against endotoxic shock is by blocking major signal pathways for cytokine production. We found that isoflurane delayed the activation of NF-κB, a central component of the LPS response (4), which is consistent with previous reports (40).
LPS enters circulation slowly in anesthetized mice in comparison with nonanesthetized animals. This delayed appearance of LPS in circulation may be explained by alterations of hemodynamics. Indeed, prior studies have shown that anesthetized mice have reduced heart rate, mean arterial pressure, and plasma volume compared to conscious mice (5). A slower delivery of LPS to target tissues in the anesthetized mice may allow cells to clear the endotoxin more efficiently, avoiding secondary toxic effects. We speculate that anesthesia protects against endotoxic shock through a mechanism similar to ischemia preconditioning. In fact, several reports have indicated that anesthetics offer cardioprotection (44) and neuroprotection (25, 48) to subsequent ischemic events through a concept known as “anesthesia preconditioning” (15). The mechanism of this protective role of anesthetics has been attributed to adenosine A1 receptor activation and the opening of mitochondrial ATP-regulated potassium channels (39, 44). These channels may have an antiapoptotic effect by inhibiting cytochrome c release and preventing the loss of mitochondrial membrane potential (2). Other studies suggest that volatile anesthetics prime mitochondrial KATP channels through protein kinase C-coupled signaling pathways (49) or through the release of reactive oxygen species (3, 35, 41). The concept of anesthesia preconditioning has been extended to endothelial and vascular smooth muscle cells (10, 15, 36). Furthermore, anesthetics have been shown to prevent lymphocyte and neuronal apoptosis and increase survival during ischemia/reperfusion and under septic conditions (13, 22, 30).
The observed effect of anesthesia on the LPS response could also be related to a direct effect on the nervous system. A correlation between the inflammatory response and the nervous system was recently reported and coined the “cholinergic anti-inflammatory pathway” (45). Stimulation of the peripheral vagus nerve releases acetylcholine, which has been shown to block TNF-α release in LPS-stimulated animals (9). In contrast, others have shown that subdiaphragmatic vagotomy blocks LPS toxicity (34). Chemical vagotomy using atropine, a muscarine receptor antagonist, has been shown to attenuate the decrease of TNF-α production in LPS-challenged animals (6). One hypothesis is that isoflurane stimulates the release of acetylcholine, resulting in protection from endotoxic shock; thus, the protective effect of isoflurane should be blocked by administration of atropine. We found that the decrease in cytokine production after isoflurane anesthesia was not modified by administration of atropine. This observation suggests that the cholinergic anti-inflammatory pathway is not responsible for the effect of isoflurane on the inflammatory process.
Overall, our observations suggest that general anesthesia may be of vital importance in the treatment of critically ill patients. Finally, because anesthetics are an intrinsic component of any experimental animal model of injury or inflammation, special consideration should be made for the use of anesthetics in the design and interpretation of results from such experiments.
Acknowledgments
This study was supported by R01-GM062899-02, National Institutes of Health, Bethesda, Maryland.
We thank Rebecca Torres for her editorial assistance.
REFERENCES
- 1.Adams, H. A., C. S. Schmitz, and B. Baltes-Gotz. 1994. Endocrine stress reaction, hemodynamics and recovery in total intravenous and inhalation anesthesia. Propofol versus isoflurane. Anaesthesist 43:730-737. (In German.) [DOI] [PubMed] [Google Scholar]
- 2.Akao, M., A. Ohler, B. O'Rourke, and E. Marban. 2001. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ. Res. 88:1267-1275. [DOI] [PubMed] [Google Scholar]
- 3.An, J., A. Stadnicka, W. M. Kwok, and Z. J. Bosnjak. 2004. Contribution of reactive oxygen species to isoflurane-induced sensitization of cardiac sarcolemmal adenosine triphosphate-sensitive potassium channel to pinacidil. Anesthesiology 100:575-580. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle, P. A., and D. Baltimore. 1996. NF-kappa B: ten years after. Cell 87:13-20. [DOI] [PubMed] [Google Scholar]
- 5.Barbee, R. W., B. D. Perry, R. N. Re, and J. P. Murgo. 1992. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am. J. Physiol. 263:R728-R733. [DOI] [PubMed] [Google Scholar]
- 6.Bernik, T. R., S. G. Friedman, M. Ochani, R. DiRaimo, L. Ulloa, H. Yang, S. Sudan, C. J. Czura, S. M. Ivanova, and K. J. Tracey. 2002. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besedovsky, H., A. del Rey, E. Sorkin, and C. A. Dinarello. 1986. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233:652-654. [DOI] [PubMed] [Google Scholar]
- 8.Beutler, B., and G. E. Grau. 1993. Tumor necrosis factor in the pathogenesis of infectious diseases. Crit. Care Med. 21:S423-S435. [PubMed] [Google Scholar]
- 9.Borovikova, L. V., S. Ivanova, M. Zhang, H. Yang, G. I. Botchkina, L. R. Watkins, H. Wang, N. Abumrad, J. W. Eaton, and K. J. Tracey. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458-462. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard, J. F., and D. Lamontagne. 1996. Mechanisms of protection afforded by preconditioning to endothelial function against ischemic injury. Am. J. Physiol. 271:H1801-H1806. [DOI] [PubMed] [Google Scholar]
- 11.Campagna, J. A., K. W. Miller, and S. A. Forman. 2003. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 348:2110-2124. [DOI] [PubMed] [Google Scholar]
- 12.Chrousos, G. P. 1995. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 332:1351-1362. [DOI] [PubMed] [Google Scholar]
- 13.Chung, C. S., Y. X. Xu, W. Wang, I. H. Chaudry, and A. Ayala. 1998. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis? Arch. Surg. 133:1213-1220. [DOI] [PubMed] [Google Scholar]
- 14.Collins, R., R. Peto, C. Baigent, and P. Sleight. 1997. Aspirin, heparin, and fibrinolytic therapy in suspected acute myocardial infarction. N. Engl. J. Med. 336:847-860. [DOI] [PubMed] [Google Scholar]
- 15.de Klaver, M. J., M. G. Buckingham, and G. F. Rich. 2003. Isoflurane pretreatment has immediate and delayed protective effects against cytokine-induced injury in endothelial and vascular smooth muscle cells. Anesthesiology 99:896-903. [DOI] [PubMed] [Google Scholar]
- 16.Delhalle, S., R. Blasius, M. Dicato, and M. Diederich. 2004. A beginner's guide to NF-κB signaling pathways. Ann. N. Y. Acad. Sci. 1030:1-13. [DOI] [PubMed] [Google Scholar]
- 17.De Maio, A., M. L. Mooney, L. E. Matesic, C. N. Paidas, and R. H. Reeves. 1998. Genetic component in the inflammatory response induced by bacterial lipopolysaccharide. Shock 10:319-323. [DOI] [PubMed] [Google Scholar]
- 18.Demirbilek, S., S. Ganidagli, N. Aksoy, C. Becerik, and Z. Baysal. 2004. The effects of remifentanil and alfentanil-based total intravenous anesthesia (TIVA) on the endocrine response to abdominal hysterectomy. J. Clin. Anesth. 16:358-363. [DOI] [PubMed] [Google Scholar]
- 19.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimmeler, S., and A. M. Zeiher. 1997. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide 1:275-281. [DOI] [PubMed] [Google Scholar]
- 21.Dinarello, C. A. 1997. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112:321S-329S. [DOI] [PubMed] [Google Scholar]
- 22.Engelhard, K., C. Werner, E. Eberspacher, M. Pape, M. Blobner, P. Hutzler, and E. Kochs. 2004. Sevoflurane and propofol influence the expression of apoptosis-regulating proteins after cerebral ischaemia and reperfusion in rats. Eur. J. Anaesthesiol. 21:530-537. [DOI] [PubMed] [Google Scholar]
- 23.Ferlito, M., O. G. Romanenko, S. Ashton, F. Squadrito, P. V. Halushka, and J. A. Cook. 2001. Effect of cross-tolerance between endotoxin and TNF-α or IL-1β on cellular signaling and mediator production. J. Leukoc. Biol. 70:821-829. [PubMed] [Google Scholar]
- 24.Flood, P., J. M. Sonner, D. Gong, and K. M. Coates. 2002. Isoflurane hyperalgesia is modulated by nicotinic inhibition. Anesthesiology 97:192-198. [DOI] [PubMed] [Google Scholar]
- 25.Franks, N. P., and E. Honore. 2004. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol. Sci. 25:601-608. [DOI] [PubMed] [Google Scholar]
- 26.Franks, N. P., and W. R. Lieb. 1994. Molecular and cellular mechanisms of general anaesthesia. Nature 367:607-614. [DOI] [PubMed] [Google Scholar]
- 27.Galley, H. F., M. A. DiMatteo, and N. R. Webster. 2000. Immunomodulation by anaesthetic, sedative and analgesic agents: does it matter? Intensive Care Med. 26:267-274. [DOI] [PubMed] [Google Scholar]
- 28.Helmer, K. S., Y. Cui, A. Dewan, and D. W. Mercer. 2003. Ketamine/xylazine attenuates LPS-induced iNOS expression in various rat tissues. J. Surg. Res. 112:70-78. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss, R. S., and I. E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138-150. [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss, R. S., K. W. Tinsley, P. E. Swanson, K. C. Chang, J. P. Cobb, T. G. Buchman, S. J. Korsmeyer, and I. E. Karl. 1999. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl. Acad. Sci. USA 96:14541-14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard, M., T. Muchamuel, S. Andrade, and S. Menon. 1993. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 177:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, C. Y., T. C. Chou, C. S. Wong, S. T. Ho, C. C. Wu, M. H. Yen, and Y. A. Ding. 1997. Ketamine inhibits nitric oxide synthase in lipopolysaccharide-treated rat alveolar macrophages. Can. J. Anesth. 44:989-995. [DOI] [PubMed] [Google Scholar]
- 33.Mack, V. E., M. D. McCarter, H. A. Naama, S. E. Calvano, and J. M. Daly. 1996. Dominance of T-helper 2-type cytokines after severe injury. Arch. Surg. 131:1303-1309. [DOI] [PubMed] [Google Scholar]
- 34.Maier, S. F., L. E. Goehler, M. Fleshner, and L. R. Watkins. 1998. The role of the vagus nerve in cytokine-to-brain communication. Ann. N. Y. Acad. Sci. 840:289-300. [DOI] [PubMed] [Google Scholar]
- 35.Mullenheim, J., D. Ebel, J. Frassdorf, B. Preckel, V. Thamer, and W. Schlack. 2002. Isoflurane preconditions myocardium against infarction via release of free radicals. Anesthesiology 96:934-940. [DOI] [PubMed] [Google Scholar]
- 36.Plachinta, R. V., J. K. Hayes, L. A. Cerilli, and G. F. Rich. 2003. Isoflurane pretreatment inhibits lipopolysaccharide-induced inflammation in rats. Anesthesiology 98:89-95. [DOI] [PubMed] [Google Scholar]
- 37.Powrie, F., and R. L. Coffman. 1993. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol. Today 14:270-274. [DOI] [PubMed] [Google Scholar]
- 38.Rongione, A. J., A. M. Kusske, S. W. Ashley, H. A. Reber, and D. W. McFadden. 1997. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J. Surg. Res. 70:107-112. [DOI] [PubMed] [Google Scholar]
- 39.Roscoe, A. K., J. D. Christensen, and C. Lynch III. 2000. Isoflurane, but not halothane, induces protection of human myocardium via adenosine A1 receptors and adenosine triphosphate-sensitive potassium channels. Anesthesiology 92:1692-1701. [DOI] [PubMed] [Google Scholar]
- 40.Sun, J., X. D. Wang, H. Liu, and J. G. Xu. 2004. Ketamine suppresses endotoxin-induced NF-κB activation and cytokines production in the intestine. Acta Anaesthesiol. Scand. 48:317-321. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, K., D. Weihrauch, F. Kehl, L. M. Ludwig, J. F. LaDisa, Jr., J. R. Kersten, P. S. Pagel, and D. C. Warltier. 2002. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology 97:1485-1490. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka, T., K. Ogli, H. Komatsu, J. Nogaya, and S. Yokono. 1993. Strain-differences of sensitivity to volatile anesthetics and their genetic character in mice. J. Anesth. 7:75-81. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi, T., K. Shibata, and K. Yamamoto. 2001. Ketamine inhibits endotoxin-induced shock in rats. Anesthesiology 95:928-932. [DOI] [PubMed] [Google Scholar]
- 44.Tonkovic-Capin, M., G. J. Gross, Z. J. Bosnjak, J. S. Tweddell, C. M. Fitzpatrick, and J. E. Baker. 2002. Delayed cardioprotection by isoflurane: role of KATP channels. Am. J. Physiol. Heart Circ. Physiol. 283:H61-H68. [DOI] [PubMed] [Google Scholar]
- 45.Tracey, K. J. 2002. The inflammatory reflex. Nature 420:853-859. [DOI] [PubMed] [Google Scholar]
- 46.Tracey, K. J., and A. Cerami. 1993. Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell Biol. 9:317-343. [DOI] [PubMed] [Google Scholar]
- 47.Tschaikowsky, K., J. Ritter, K. Schroppel, and M. Kuhn. 2000. Volatile anesthetics differentially affect immunostimulated expression of inducible nitric oxide synthase: role of intracellular calcium. Anesthesiology 92:1093-1102. [DOI] [PubMed] [Google Scholar]
- 48.Xiong, L., Y. Zheng, M. Wu, L. Hou, Z. Zhu, X. Zhang, and Z. Lu. 2003. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth. Analg. 96:233-237. [DOI] [PubMed] [Google Scholar]
- 49.Zaugg, M., E. Lucchinetti, D. R. Spahn, T. Pasch, and M. C. Schaub. 2002. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial KATP channels via multiple signaling pathways. Anesthesiology 97:4-14. [DOI] [PubMed] [Google Scholar]