Abstract
1. Voltage clamp experiments were carried out on squid giant axons internally perfused with 300 mM-NaF + sucrose. K-free artificial sea-water, -0·3 to 3·5° C, was used externally.
2. Membrane currents were corrected for capacitative and leakage components, and the resulting Na current was converted to Na conductance, gNa. An attempt was made to fit changes in gNa according to the Hodgkin—Huxley model, namely [Formula: see text].
According to the model ḡNa is a constant, m∞ and h∞ are steady-state values which depend only on voltage, τm-1 and τh-1 are rate constants which also are functions only of voltage.
3. Stepwise depolarizations from the holding potential (-67 to -83 mV) to a potential which varied from -10 to +63 mV resulted in an exponential decline of h from its initial level to a final, non-zero level. If the test depolarization was preceded by a positive prepulse (duration, 19-105 msec; voltage, -6 to 94 mV) the rate constant for h, τh-1, was increased roughly threefold with practically no change in the final level.
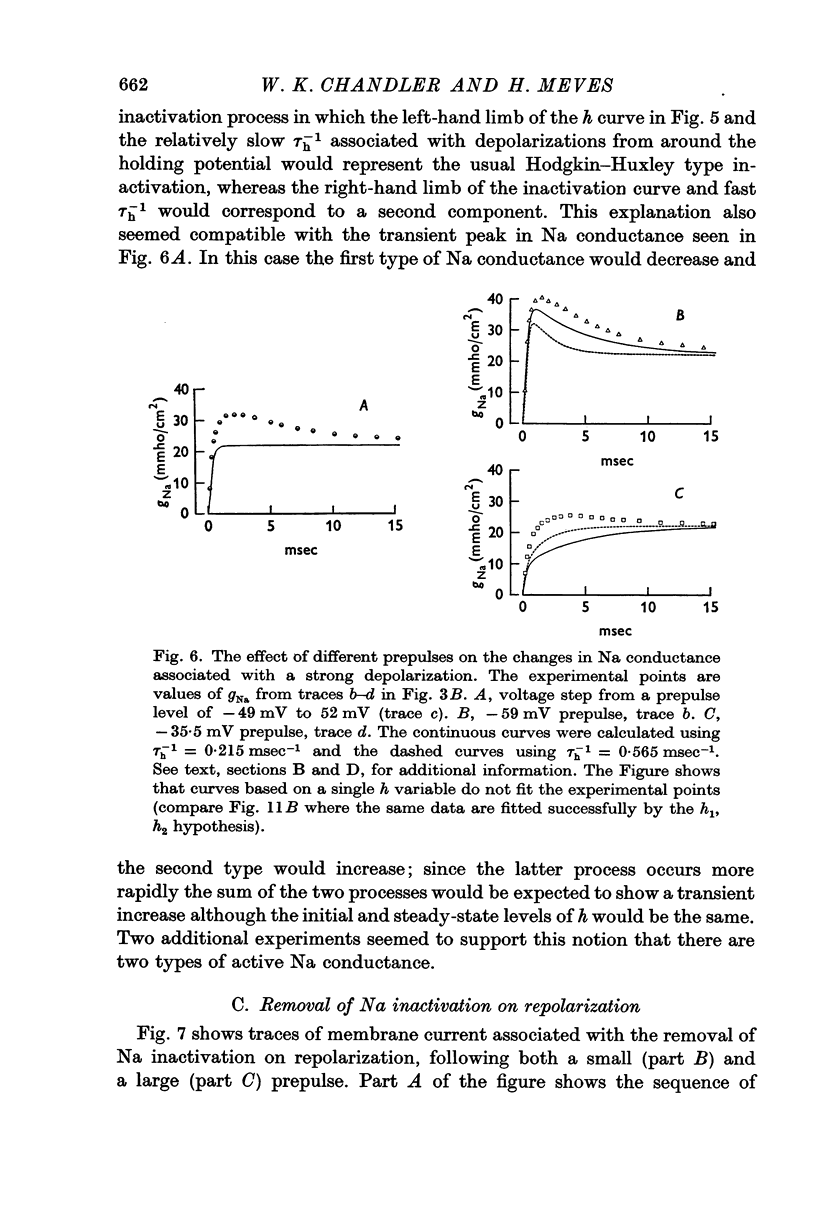
4. The steady-state level of h was studied by using prepulses of varying amplitude followed by a test depolarization. In one such experiment a value of 0·34 was obtained for a 105 msec prepulse to -49 mV. The same value for the steady level of h was obtained from analysing a record taken at +52 mV. If the potential was switched from -49 to +52 mV there was a transient increase in gNa although h∞ had the same value at these two potentials.
5. Recovery from depolarization was studied by repolarizing the fibre for varying lengths of time, then applying a test depolarization. If the first depolarization was strongly positive (for example, 70 mV), so that the steady level of h was large (0·39), the currents associated with the test pulse could not be fitted on the basis of an exponential increase in h during the recovery period. Rather, the results suggested that on repolarization h rapidly decreased initially, then slowly increased.
6. These results can be explained by assuming that h is given by the sum of two components, h1 and h2. Changes are represented kinetically by h1 ⇌ x ⇌ h2, where x signifies the inactive state. The distribution is shifted to the left at negative potentials and to the right for positive ones. The resulting Na conductance is comprised of two types: the first type, ḡNam3h1, is similar to the Hodgkin—Huxley system and underlines the usual transient increase in gNa associated with depolarization; the second type, ḡNam3h2, is maintained with depolarization and gives rise to a steady level of gNa.
Full text
PDF
























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Sodium and potassium currents in squid axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):623–652. doi: 10.1113/jphysiol.1970.sp009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. INACTIVATION OF THE SODIUM-CARRYING MECHANISM IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:445–451. doi: 10.1113/jphysiol.1963.sp007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]


