Abstract
Magnocellular neuroendocrine cells of the supraoptic nucleus (SON) release the peptides oxytocin (OT) and vasopressin (VP) from their dendrites and terminals. In addition to peptide-containing large dense-core vesicles, axon terminals from these cells contain clear microvesicles that have been shown to contain glutamate. Using multilabelling confocal microscopy, we investigated the presence of vesicular glutamate transporters (VGLUTs) in astrocytes as well as VP and OT neurones of the SON. Simultaneous probing of the SON with antibodies against VGLUT isoforms 1–3, OT, VP and glial fibrillary acidic protein (GFAP) revealed the presence of VGLUT-2 in somata and dendrites of SON neurones. Immunoreactivity (-ir) for VGLUT-3 was also detected in both OT and VP neurones as well as in GFAP-ir astrocytes and other cells of the ventral glial lamina. Colocalisation of VGLUT-2 and VGLUT-3 in individual SON neurones was also examined and VGLUT-ir with both antibodies could be detected in both types of SON neurones. Although VGLUT-1-ir was strong lateral to the SON, only sparse labelling was apparent within the nucleus, and no colocalisation with either SON neurones or astrocytes was observed. The SON or the SON plus its surrounding perinuclear zone was probed using the reverse transcriptase-polymerase chain reaction and the presence of mRNA for all three VGLUT isoforms was detected. These results suggest that similar arrangements of transmitters exist in SON neuronal dendrites and their neurohypophysial terminals and that magnocellular neuroendocrine somata and dendrites may be capable of glutamatergic transmission.
Keywords: VGLUT, hypothalamus, dendritic release, oxytocin, vasopressin, astrocytes, meninges
The supraoptic nucleus (SON) forms a major part of the magnocellular hypothalamic–neurohypophysial neuroendocrine system. Individual SON neurones primarily synthesise either oxytocin (OT) or vasopressin (VP), which are peptide hormones with well-established roles in water balance, parturition and lactation. Stored in dense-core vesicles (DCVs), OT and VP are released from magnocellular neurone terminals in the pituitary neural lobe (NL) and from dendrites in the SON. Depending upon the stimulus, the peptide hormones are released from dendrites, terminals or both (1). Several neurotransmitters influence this release via activation of receptors found both in the SON and NL (2).
In addition to containing DCVs, electron microscopy of the NL has shown that terminals from magnocellular neurones contain clear microvesicles (MVs). These MVs show glutamate immunoreactivity (-ir) and appear clustered for release (3). Additionally, the neural lobe has been found to be immunoreactive for VGLUT-2 (4). The coexistence of glutamate-ir MVs with DCVs in the NL suggests that glutamatergic vesicles might also coexist with the well-documented DCVs in the magnocellular somata and dendrites. Exocytotic release of neuropeptides from SON somata and dendrites, first shown by Pow and Morris (5), accounts for earlier findings describing the presence of OT and VP in the hypothalamus, and their functions in autocrine and retrograde signalling (6). However, anatomical studies addressing the possibility of somatodendritic glutamate release are lacking.
Vesicular glutamate transporters (VGLUTs) account for all of the known glutamatergic transmission in the mammalian brain (7). Currently, three isoforms of VGLUTs are known to exist. These have been found in both neurones and astrocytes, with VGLUT-3 located in serotonergic, cholinergic, dopaminergic and even GABAergic neurones (8, 9). A number of studies (4, 10–12) have demonstrated the presence of VGLUT-2-ir and/or mRNA in the magnocellular neuroendocrine system. This is not surprising because the SON is the destination of myriad glutamate afferents (13). Furthermore, the existence of VGLUTs mRNA has long been known in various types of synaptic terminals and protoplasmic astrocytes (which are especially plentiful in the ventral SON) contain VGLUT-positive MVs (14). Therefore, in the absence of positive magnocellular-neurone and glial identifiers, the precise location of the VGLUT-2-ir and mRNA remains unclear. This is the first study to specifically address the presence of VGLUT-3 immunoreactivity in the magnocellular system and to build upon earlier observations by investigating the presence of different VGLUT isoforms in clearly identified SON neurones and astrocytes.
Earlier ultrastructural analysis of postembedding glutamate immunocytochemistry revealed microvesicles in the NL stain for glutamate (3). Additionally, both magnocellular somata and dendrites are immunoreactive with glutamate antibodies at the light and electron microscopic level (15). However, the amino acid glutamate is present in all cells, functioning in metabolism and incorporated into proteins. Whether or not it is also used as a regulated signalling molecule depends upon whether the cell contains machinery for controlled release of the transmitter. To explore the possibility of glutamate release from neurones and astrocytes of the SON, we studied the presence of the three known VGLUTs and their mRNA, which function in the storage and concentration of glutamate into synaptic vesicles.
Materials and methods
Immunocytochemistry
Adult male rats (aged 50–80 days) were deeply anaesthetised with sodium pentobarbital (100 mg/kg) and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Coronal sections (50–60 µm thick) were cut on a vibratome, treated with 0.3% Triton X-100 in PBS for 30 min and subsequently immunoprocessed (for all antibody identification, see Table 1). Brain sections were incubated with antibodies for either 3 h at room temperature or overnight at 4 °C. All secondary antibodies were made in donkey and were conjugated to AMCA, FITC (Jackson Laboratories, Bar Harbor, ME, USA) or the Alexa Flour® 350, 488, 546, 555, or 647 (Molecular Probes, Eugene, OR, USA). A 5-min histochemical treatment with Hoechst (Sigma, St Louis, MO, USA; 1 : 1000, 1 mg/ml) was also used in several experiments to stain nuclei. Hoechst, which produces a blue fluorescence in response to ultraviolet (UV) excitation, is depicted in grey in the confocal micrographs.
Table 1.
Antibody Dilutions and Sources.
| Antibody | Host | Dilution | Company or PI |
|---|---|---|---|
| Vesicular glutamate transporter type 1 | Rabbit (r) | 1 : 500 | Synaptic Systems |
| Vesicular glutamate transporter type 1 | Rabbit | 1 : 1000 | R.H. Edwards, UCSF |
| Vesicular glutamate transporter type 2 | Rabbit | 1 : 500 | Synaptic Systems |
| Vesicular glutamate transporter type 2 | Guinea Pig (gp) | 1 : 2500 | Chemicon |
| Vesicular glutamate transporter type 3 | Guinea Pig | 1 : 2000 | Chemicon |
| Oxytocin-Neurophysin | Mouse (m) | 1 : 400 | H. Gainer, NIH, PS38 |
| Vasopressin-Neurophysin | Mouse | 1 : 400 | H. Gainer, NIH, PS41 |
| Neurophysin II (marker for VP neurones) | Rabbit | 1 : 500 | Sigma |
| Neurophysin II | Goat (g) | 1 : 20 | Santa Cruz Biotech |
| Glial fibrillary acidic protein (marker for astrocytes) | Mouse | 1 : 500 | Sigma |
| Glial fibrillary acidic protein | Goat | 1 : 20 | Santa Cruz Biotech |
| Neurofilament (marker for neurones) | Mouse | 1 : 100 | Calbiochem |
Confocal microscopy
An upright Leica SP2 UV-equipped laser-scanning confocal microscope (Leica, Heidelberg, Germany) was used in sequential scanning mode with nonoverlapping photomultiplier tube settings for acquiring all images. All data were collected with a pinhole set to 1 airy unit corresponding to Z-optical sections < 300 nm. Z-series were collected with a depth difference of <0.5 µm between successive Z sections.
Cell cultures
We prepared enriched astrocytic cultures using a modification (16) of the originally described shaking procedure (17). Briefly, visual cortices isolated from 0- to 2-day-old Sprague-Dawley rats were enzymatically treated (papain, 20 IU/ml; 1 h at 36.8 °C). After mechanical dispersion, cells were initially plated into tissue culture flasks (25 cm2) and maintained at 36.8 °C in a humidified 5% CO2 95% air atmosphere in a complete culture medium that consisted of α-MEM (without phenol red; Gibco™, Invitrogen Corp., Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), l-glutamine (2 mM; Invitrogen Corp.), d-glucose (20 mM; Sigma-Aldrich, St Louis, MO, USA), sodium pyruvate (1 mM; Invitrogen Corp.), penicillin (100 IU/ml), streptomycin (100 µg/ml), and sodium bicarbonate (14 mM; Invitrogen Corp.) (pH = 7.4). After 6–24 days in culture, the cells were shaken twice (260 r.p.m. at 36.8 °C), first for 1.5–2 h, and then, after exchange of complete medium, again for 18–20 h. At that time, the remaining attached cells were detached from flasks using a cell scraper and used in preparation of subcellular fractions for Western blotting experiments. The purity (> 99%) of astrocytic culture was confirmed by anti-glial fibrillary acidic protein (GFAP) antibody and indirect immunocytochemistry.
Preparation of subcellular fractions and Western blotting
We obtained non-nuclear membranes and vesicular extracts from purified astrocytes and different brain tissues (occipital lobe, hypothalamus and posterior pituitary gland) as previously described (18). Preparations were subjected to 15% SDS-PAGE, followed by transfer to nitrocellulose membranes that were probed with antibodies against VGLUT-1 (rabbit polyclonal, 1 : 1000; provided by Dr R. H. Edwards, University of California, San Francisco, CA, USA) (19), VGLUT-2 (rabbit polyclonal; 1 : 1000; Synaptic Systems, Goettingen, Germany, cat no. 135102) or VGLUT-3 (guinea pig polyclonal, 1 : 5000; Chemicon International, Temecula, CA, USA, cat No. AB5421). Immunoreactivity of bands was detected using enhanced chemiluminescence (ECL; Amersham Biosciences Corp., Piscataway, NJ, USA). We previously characterised the specificity of these VGLUT antibodies using their respective antigens in adsorption controls (16).
Reverse transcriptase-polymersase chain reaction (RT-PCR)
Five adult (62 day-old) male rats were used to probe both the SON and SON plus perinuclear zone for mRNA corresponding to all three VGLUT isoforms. Total RNA was extracted using TRIzol® Reagent (Invitrogen Corp.) and protocols provided by the manufacturer as previously described (16). Total RNA was used for reverse transcription using Oligo(dT)12−18 and superscriptTM III reverse transcriptase (Invitrogen Corp.). Two pairs of primers (each pair 35 cycles) for each of VGLUTs 1, 2 and 3 (GeneBank accession numbers NM_053859, NM_053427 and NM_153725, respectively) were used to amplify cDNA using PCR Core System I kit (Promega, Madison, WI, USA). For VGLUT-1, in the first round we used, forward 5′-CCG GCA GGA GGA GTT TCG AGG G-3′ and reverse 5′-AGG GAT CAA CAT ATT TAG GGT GGA GGT AGC-3′ primers (478 bp product). Amplified DNA was used as the template for the second round of PCR using nested primers, forward 5′-TAC TGG AGA AGC GGC AGG AAG G-3′ and reverse 5′-CCA GAA AAA GGA GCC ATG TAT GAG G-3′ (311 bp product). For VGLUT-2 we used in the first round, forward 5′-AGC AAG GTT GGC ATG TTG TCT G-3′ and reverse 5′-CGG TCC TTA TAG GAG TAC GCG T-3′ primers (698 bp product), while in the second round the nested primers were forward 5′-TGG TGC AAT GAC GAA GAA CAA G-3′ and reverse 5′-TCC TTT TTC TCC CAG CCG TT-3′ (294 bp product). For VGLUT-3, in the first round the primers were forward 5′-AGG AGT GAA GAA TGC CGT GGG AGA T-3′ and reverse 5′-ACC CTC CAC CAG ACC CTG CAA A-3′ (535 bp product), while the second round nested primers were forward 5′-GAT GGG ACC AAC GAG GAG GGA GAT-3′ and reverse 5′-TGA AAT GAA GCC ACC GGG AAT TTG T-3′ (322 bp product).
Results
VGLUT immunocytochemistry positive and negative control experiments
Experiments testing the specificity of the VGLUT antibodies used in this study have been published previously (16). However, further experiments were performed to test their specificity in the tissue under study. Expression of VGLUT-2 in the adult cerebellum has been described (20) as occurring in the large mossy fibre terminals in the granule cell layer and along the thin climbing fibres in the molecular layer. Immunoreactivity for VGLUT-1 occurs in the molecular layer, labelling parallel fibre terminals (Fig. 1a–d). In situ hybridisation and protein expression experiments have shown VGLUT-3 to be present in the hypothalamus and that its protein expression is particularly high in the olfactory tubercles (8, 21). Western blot experiments have bands with molecular weights corresponding to the specific VGLUT being immunoprobed (Fig. 1e). In addition to the nucleus of the lateral olfactory tract (nLOT), strong VGLUT-3-ir can be seen in the SON (Fig. 1f). Note that the punctate labelling in the nLOT is different than the cellular labelling observed in the SON and is seen using the same antibody in the same section.
Fig. 1.
Antibody control experiments. (a–d) The cerebellum shows a stereotypic labelling pattern for vesicular glutamate transporter (VGLUT)-1 and for both VGLUT-2 antibodies. (b,d) Expansions of the areas shown in (a) and (c). (b1) VGLUT-2 labels the granule cell synapses in the granule layer (GL) in addition to the climbing fibres (cf) coursing toward Purkinje cells (pc) in the molecular layer (ML). (d) Demonstration of VGLUT-2 immunoreactivity similar to as seen in (b) using a different VGLUT-2 antibody. (e) Western blots using the same antibodies in addition to the VGLUT-3 antibody used for immunocytochemistry in (f). (f) VGLUT-3 is present in the nucleus of the lateral olfactory tract. Strong immunoreactivity is also seen in the SON. OL, Occipital lobe; H, hypothalamus; AsP, astrocyte culture; PP, pituitary; r, rabbit; m, mouse; guinea pig; gp.
Negative control experiments, performed in the absence of primary antibody exposure, were carried out in parallel with all multilabelling immunocytochemical experiments. Further control experiments, similar to those previously described (16), in which the antibodies were preincubated with their recognised peptide sequences were also performed. Both types of negative control experiments resulted in the absence of stained tissue.
VGLUT-3 in the SON
Cellular labelling for VGLUT-3 appeared throughout the SON (Figs 1f and 2). Experiments using double-labelling for VGLUT-3 and OT or VP resulted in apparent colocalisation of peptide- and VGLUT-3-ir. Dorsal to the SON, scattered cells of the anteriolateral hypothalamic area also displayed VGLUT-3-ir (Fig. 2a). Additionally, lighter VGLUT-3 labelling was apparent in the ventral glial lamina and in cells of the meninges. SON neuronal VGLUT-3-ir was clustered in the perikarya, while labelling was absent in the nuclei of both SON neurones and astrocytes, as determined by Hoechst staining.
Fig. 2.
Vesicular glutamate transporter (VGLUT)-3 expression by neurones, astrocytes, and their underlying meningeal cells. (a) Neurophysin positive cells of the supraoptic nucleus (SON) are immunoreactive for VGLUT-3. Scattered cells of the lateral hypothalamic area also show VGLUT-3-immunoreactivity (ir) (arrows). (b) GFAP-ir of the VGL separates the SON from VGLUT-3 immunoreactive meninges and endothelial cells at the base of the brain (arrows). (c) VGLUT-3 is located in the interior of SON neurones immunoprobed with neurophysin II. Several SON neurones with strong VGLUT-3 labelling also have multiple nucleoli (* and expanded in insets c1 and c2, arrows indicate nucleoli). The scale bar in (c) also applies also to (d). (d) Astrocytes of the VGL express VGLUT-3 (open arrows). (d1–d3) Expanded X–Y, Y–Z and X–Z projections at the crosshair point inside the red square in (d) show that astrocytic VGLUT-3-ir is completely surrounded by GFAP-ir. Z-thickness is 20 µm in (c) and (d). (e–g) Colocalisation of VGLUT-3 (e,g) with oxytocin (OT) (f,g). Open arrows in (e) and (g) identify several oxytocin-positive cells colocalised with VGLUT-3 immunoreactive puncta. The scale bar in (e) also applies to (f) and (g). OC, Optic chiasm; BV, blood vessel.
To determine if the VGLUT-3-ir was present intracellularly, Z-series through SON sections immunoprobed for neurophysin II, GFAP, OT and VGLUT-3 were taken. Extranuclear puncta representing VGLUT-3 are surrounded by both the VP-specific peptide neurophysin II- (Fig. 2c) and GFAP-ir (Fig. 2d). Shown in all three dimensions in Fig. 2(d, insets 1–3) VGLUT-3-ir is localised within the boundaries of GFAP-ir, suggesting SON astrocytes express this transporter. OT-positive cells were also seen to contain VGLUT-3-ir (Fig. 2e–g). Interestingly, many of the magnocellular neurones that are most immunoreactive for VGLUT-3 displayed 2 or 3 nucleoli and regions of ribosome synthesis (Fig. 2c, insets).
The VGLUT-3-ir did not appear to extend into the dendrites or along axons. Rather, it was limited to the perikarya. VGLUT-3-ir was also seen in astrocytic somata but not processes. Strong labelling was also apparent in the endothelial and meningeal cells at the base of the brain. There, the labelling appears more distributed and not necessarily restricted to perinuclear areas.
VGLUT-2 in the SON
Two polyclonal antibodies to VGLUT-2 were used to localise the protein. Punctate labelling by both guinea pig and rabbit antibodies can be seen throughout the SON and was also particularly strong dorsal to the nucleus. Multilabelling experiments, including probing for the VP cell marker neurophysin II, revealed colocalisation of VGLUT-2-ir with many of the neurophysin II-positive cell processes (Figs 3 and 4). Examples of neurophysin II-positive dendrites and somata containing intracellular VGLUT-2-ir are shown in Fig. 3(a–e). Because these cells are contacted by many glutamatergic terminals, it was necessary to perform Z-series acquisitions to be sure the VGLUT immunoreactivity was occurring within the boundaries of specific magnocellular neurones as judged by their peptide immunoreactivity. Additionally, strong punctate labelling, appearing just outside much of the neurophysin II-ir, morphologically reveals putative glutamatergic synapses onto these cells (Fig. 3a,c,e).
Fig. 3.
Vasopressinergic magnocellular neurone expression of vesicular glutamate transporter (VGLUT)-2-ir. Upper-left inset: area of supraoptic nucleus expanded in the figure. Further expansions of outlined areas (a–e) are shown in panels on right. The X–Z and Y–Z projections of neurophysin II-immunoreactivity (ir) completely surrounding that for VGLUT-2 can be seen at the points of the crosshairs (Z-thickness is 12 µm). Expansions of internally localised VGLUT-2-ir found at one such point can be seen on the right (circled in a–c). Putative afferent synapses represented by large VGLUT-2 puncta are adjacent to the peptide-labelled cells (arrows in a,c,e). OC, Optic chiasm.
Fig. 4.
(a–c) Colocalisation of vesicular glutamate transporter (VGLUT)-2- and peptide hormone-immunoreactivity can be seen with a different VGLUT-2 antibody (arrows). Colocalisation occurs in somata and dendrites of magnocellular neurones. OT, Oxytocin; NP-II, neurophysin II.
Similar multilabelling experiments were performed to investigate VGLUT-2 and OT colocalisation. OT-positive cells also appeared to receive glutamatergic input from VGLUT-2 immunoreactive terminals (Fig. 5d, arrows). Intracellular VGLUT-2-ir was apparent in several OT-positive dendrites (Fig. 5). Unlike VGLUT-3-ir, colocalisation of VGLUT-2 with GFAP within the VGL was not evident. Furthermore, meningeal and endothelial cells did not show detectable levels of VGLUT-2-ir with either VGLUT-2 antibody.
Fig. 5.
Oxytocin (OT)-immunoreactive dendrites show strong labelling for vesicular glutamate transporter (VGLUT)-2. Upper-left inset: area of supraoptic nucleus expanded in the figure. Projections in the X–Y, X–Z, and Y–Z planes from the points of the crosshairs show OT-ir completely surrounding that for VGLUT-2 (Z-thickness is 12 = m). Expansions of internally localised VGLUT-2 at one such point can be seen below (circled in a–c). VGLUT-2 labelling was not as apparent in OT-positive somata. Large puncta (arrows in d) indicate putative afferent glutamatergic synapses onto an OT-immunoreactive process. OC, Optic chiasm.
OT and VP neurones of the SON can express VGLUT-2 or VGLUT-3. However, the question remained as to whether or not an individual magnocellular neurone could express both VGLUTs. Multilabelling for the two peptide hormones along with the two VGLUTs revealed VGLUT-2- and VGLUT-3-ir in both types of magnocellular neurones (Fig. 6). Although strong VGLUT-3-ir appeared in virtually all labelled neurones (Figs 2, 6 and 7), this was not the case for VGLUT-2. Some magnocellular neurones were clearly more immunoreactive than others for VGLUT-2 (Fig. 6d, inset), suggesting that VGLUT expression may be a regulated phenomenon.
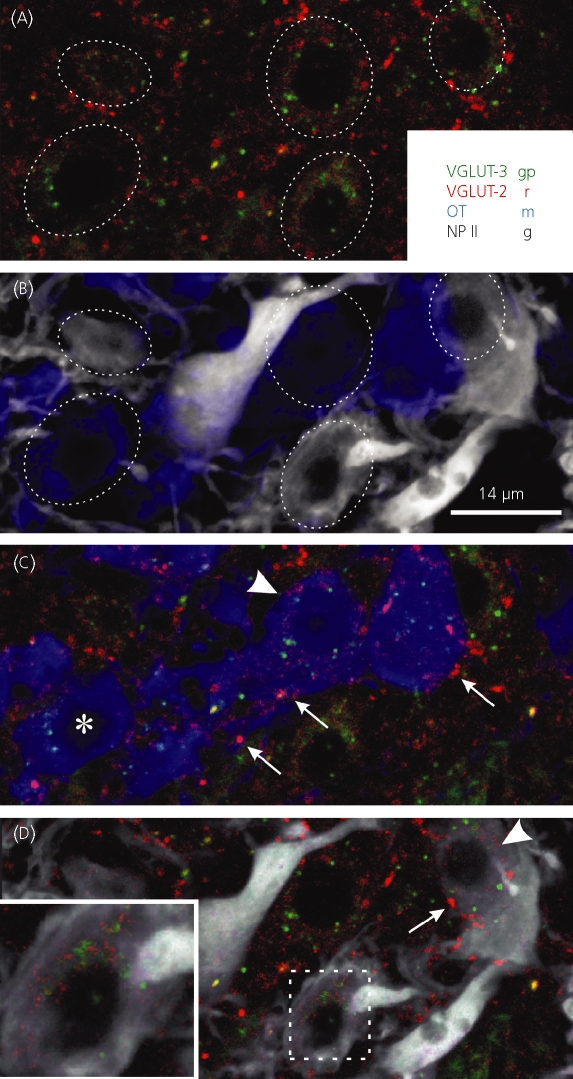
Fig. 6.
Colocalisation of vesicular glutamate transporter (VGLUT)-2 and 3 in magnocellular supraoptic nucleus (SON) neurones. (a) VGLUT-2- and 3-immunoreactivity appears in the perikarya of SON cells. Dashed lines in A and B approximate cell boundaries. (b) Oxytocin (OT)- and neurophysin II (NP-II)-ir reveals a heterogeneous population of peptidergic neurones in this section. (c) An OT-positive cell is positive for both VGLUT-2 and -3 (arrowhead). In the same field, a VGLUT-3-positive, although only faintly VGLUT-2-positive cell is indicated (asterisk). A few large puncta representing the clustering of numerous VGLUT-2 positive vesicles forming a putative glutamatergic synapses are indicated (arrows in c and d). (d) Neurophysin-II-positive cells were immunoreactive to both VGLUTs (arrowhead and inset).
VGLUT-1 in the SON
Just lateral to the SON, strong VGLUT-1-ir was visible (Figs 4a,c and 7). Although distribution of VGLUT-1 staining was noticeable within the SON, it was much less pronounced than that of VGLUT-2. VGLUT-1 labelling was slightly stronger in the dendritic zone and was completely absent in the optic chiasm. Similar to the pattern seen with the VGLUT-2 antibodies, little or no VGLUT-1-ir was present in the VGL, in the astrocytes therein, along the meninges or along blood vessels (Fig. 7). Colocalisation experiments revealed the presence of VGLUT-1 outside the VGLUT-3 positive OT- or VP-labelled SON neurones (Fig. 7). This is consistent with VGLUT-1 residing mainly within glutamatergic terminals in the SON.
Fig. 7.
Vesicular glutamate transporter (VGLUT)-1 expression in the supraoptic nucleus (SON). (a–c) Though faint labelling is seen inside the SON proper, strong VGLUT-1-immunoreactivity consistent with a terminal distribution occurs in the cell sparse region lateral to the nucleus (arrows in a and c). (d) Colocalisation of oxytocin and vasopressin with VGLUT-3 but not VGLUT-1 is seen. (a1,b1,c1) Expansions from boxes in the respective panels. (e) is the same as (d1) with the addition of the nuclear stain Hoechst.
RT-PCR of VGLUTs in the SON
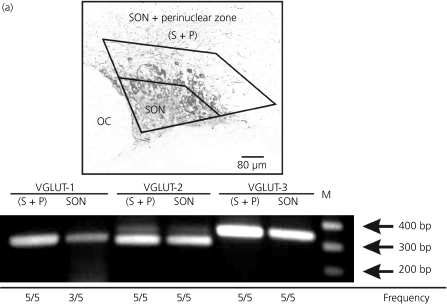
To corroborate the immunocytochemical experiments, we performed RT-PCR using mRNA isolated from SON and SON plus the perinuclear zone of five adult rats, the latter serving as a positive control for all three VGLUTs (Fig. 8, top). Whereas PCR products for VGLUTs-2 and -3 were found in all five of the isolated SONs, PCR products for VGLUT-1 were found in only three of five cases (Fig. 8, bottom). Consistent with the immunocytochemical results, PCR products representing the mRNA for all three VGLUT isoforms were found in SON plus perinuclear zone from all five rats. These results agree with the idea that VGLUTs 2 and 3 are heavily expressed in the SON, whereas VGLUT-1 is only sparsely expressed. Additionally, these results are also consistent with the known presence of mRNAs in terminals.
Fig. 8.
Detection of vesicular glutamate transporter (VGLUT) mRNA in the supraoptic nucleus (SON). Top: Micrograph showing from where the tissue was collected, either the SON only (n = 5) or the SON + perinuclear zone (S + P, n = 5). OC, Optic chiasm. Bottom: Reverse transcriptase-polymerase chain reaction analysis from SON and S + P tissue reveals the presence of mRNA for all three VGLUT isoforms: VGLUT-1(311 bp), VGLUT-2(294 bp) and VGLUT-3(322 bp). M, DNA ladder marker. Detection frequencies for the mRNAs are given at the bottom.
Discussion
For several years, glutamate has been known to be the main excitatory transmitter involved in neuroendocrine regulation (22, 23). In addition to general excitation, glutamate-implicated phenomena occurring in the magnocellular neuroendocrine system include osmoresponsiveness (24, 25) and bursting rhythmogenesis (26). Here, we present evidence that an additional source for that glutamate may be somatodendritically released glutamate from magnocellular neurones themselves.
A precendent already exists for intranuclear release of substances from magnocellular neurones. The release of OT and VP from SON neuronal somata and dendrites under different conditions can influence presynaptic activity (1, 27). The presence of glutamate-containing MVs in magnocellular neuronal terminals in the NL has also been described (3). The same group found glutamate-ir both in afferent terminals in the SON and, more relevant here, in magnocellular somata and dendrites (15, 28). This glutamate was postulated to serve mainly a metabolic function and the possibility for somatodendritic exocytosis of glutamate largely remained uninvestigated. In the present study, we offer strong evidence that the somatodendritic compartment of SON neurones contains the principle machinery that defines a glutamatergic phenotype. This is consistent with the reported presence of VGLUT-2-ir in the NL (4). Although earlier investigations of VGLUTs have described a complementary expression pattern of the proteins, we present evidence for both VGLUT-3 and VGLUT-2 expression by OT- or VP-positive SON neurones. The possibility of multiple VGLUTs in a given neurone presents some obvious and interesting questions regarding the specific roles for the different VGLUTs found within the same neurone.
Potential functions of VGLUT-3 in the SON
Cloned and described by three independent groups in 2002, VGLUT-3 was shown to exist in serotonergic, cholinergic, and GABAergic neurones (8, 9, 21). Unlike VGLUTs-1 and -2, VGLUT-3-ir has been found in somata and dendrites in addition to synaptic terminals. Here, we report the presence of VGLUT-3 in the somata of magnocellular neurones, astrocytes, along the endothelium and along meningeal cells. One commonality shared by these various cell types in this particular region is a high level of metabolic activity.
Similar to serotonergic, cholinergic and many GABAergic interneurones, SON cells are especially active. In the resting, nonactivated state (e.g. hydrated animal), OT and VP cells continue to discharge action potentials spontaneously both in vivo and in vitro, resulting in basal circulating concentrations of these hormones. Under these conditions, the cells continue to synthesise the peptide hormones and electron micrographs display magnocellular neurones densely populated with peptide-containing DCVs.
When presented with a physiological challenge (e.g. dehydration or lactation/suckling), magnocellular neurones of the SON and PVN become highly activated. A number of plastic anatomical and electrophysiological changes occur throughout the nucleus. Some examples include: (i) the size of magnocellular nucleoli increasing significantly (29); (ii) the OT cells bursting synchronously and the VP cells exhibiting a stereotypic phasic firing pattern (30); and (iii) astrocytic processes in the neural lobe and SON retracting, allowing for increases in neurone-neurone appositions and synapse formation (31). With these changes, elevated levels of circulating hormones ensue.
The blood supply to the SON is also distinctive. Along with the PVN, the SON is enriched with the highest capillary density in the brain, at least twice as high as observed in other brain nuclei (32). Doubtless, the cells of this especially active and plastic nucleus require extraordinary levels of factors garnered from an enriched blood supply and additionally need a readily accessible pool of glutamate to maintain their high metabolic and protein-synthetic activity. In addition to VGLUT involvement in exocytosis, the presence of VGLUT-3-ir in this case possibly suggests a metabolic function similar to that proposed by Meeker et al. (15). Consistent with this hypothesis is the strong VGLUT-3-ir observed in neurones with multiple nucleoli (Fig. 2c).
VGLUT-2 expression and a glutamatergic phenotype
VGLUT-2 expression is strongly linked to glutamatergic transmission. It is known to exist at many well-characterised glutamatergic synapses and is restricted to sites of known release. VGLUT-2-ir in OT and VP neurones is in agreement with a previous study demonstrating VGLUT-2 mRNA in the magnocellular hypothalamic nuclei (10). However, mRNA expression is not an infallible indication of protein expression and such studies reveal no information regarding the specific location of the molecules of interest. Here, we add to that work by demonstrating that VGLUT-2-ir is not only expressed in local glutamatergic neurones and terminals contacting SON neurones, but also in OT/VP-positive SON neurones themselves. Colocalisation of the transporter within the magnocellular neurones is now established, suggesting that these cells may release both glutamate and either OT or VP from their somata and dendrites.
The suggestion of dendritic glutamate release from magnocellular neurones presents an interesting puzzle concerning its function. The clustering of microvesicles in SON magnocellular dendrites or somata has not been described, suggesting that glutamate is probably not released at specific sites in high concentration (i.e. synapses), but is instead released from a more general area (i.e. a somatic or dendritic zone). Because these cells and many of their afferent terminals express glutamate receptors, the released glutamate may function in autocrine, paracrine or retrograde transmission and, in some cases, perhaps contribute to a feed-forward activation system. A similar autocrine feed-forward system exists for OT acting on OT neurones during the episodic firing of milk ejection bursts. There, somatodendritic released OT results in enhanced local concentrations of OT which lead to an increased frequency and amplitude of subsequent milk ejection bursts (33–35). Furthermore, autocrine signalling of VP-cell derived dynorphin has been shown to affect VP neurone bursting (36, 37).
Whereas activation of pre- or post-synaptic ionotropic glutamate receptors would result in direct membrane depolarisation and/or release of presynaptic transmitter, the activation of pre- and post synaptic metabotropic glutamate receptors (mGluRs) results in more subtle forms of modulation. Evidence exists for the presence of mGluRs on magnocellular neurones, and on glutamatergic and GABAergic terminals in the SON (38, 39). Activation of terminally located group III mGluRs causes a reduction in the frequency of spontaneous glutamatergic and GABAergic release. Alternatively, activation of postsynaptic group I mGluRs induces excitation through activation of inward currents carried by K+ or Ca2+ (38, 39). Therefore, the qualitative result of somatodendritic released glutamate will depend upon which receptors are subsequently activated.
Consistent with the immunocytochemical evidence presented in a recent study, VGLUT-2 is also probably involved in afferent glutamatergic transmission in the SON (40). Larger VGLUT-2 immunoreactive puncta are seen along dendrites and somata of SON neurones, which may represent glutamatergic terminals forming synapses within the nucleus.
VGLUT-1 immunoreactive input to the SON
Although VGLUT-1-ir in the SON was not as intense as that seen for either VGLUT-2 or VGLUT-3, strong labelling was evident lateral to the nucleus. This area is enriched with mitral cell axons and terminals that form the glutamatergic lateral olfactory tract (41–43). Mitral cell and glomerular layers of the olfactory bulb are immunopositive for VGLUT-1 (40). Because mitral cells form dendrodendritic synapses in the glomerular layer, this offers the possibility of VGLUT-1 being the transporter used by this cell type.
The detection of VGLUT-1 mRNA-containing SON tissue in three of five cases along with five of five cases using SON tissue plus the surrounding perinuclear region is consistent with a previous report showing only faint labelling for VGLUT-1 mRNA in the SON and perinuclear area (11). However, neither our study nor theirs addresses the precise localisation of VGLUT-1 mRNA within specific cell types of the perinuclear zone. A recent anatomical study addressed the possibility of glutamatergic perinuclear neurones by exploring the origin of afferent glutamatergic terminals in the SON (13). These results, together with a previous physiological study (44), suggest that many perinuclear neurones are indeed glutamatergic.
Is Dale's principle upheld?
Magnocellular neurones have two well-documented peptide release sites: (i) the NL terminals and (ii) somatodendritically within nuclei. However, somatodendritic release of peptides does not necessarily mirror peripheral release (1). Little is known concerning MV exocytosis in the NL or from the dendrites. The intranuclear colocalisation of VGLUT-2 with OT and VP agrees with evidence first offered in a recent review showing that the neural lobe was immunoreactive for VGLUT-2 (4). In agreement with Dale's principle (45), this suggests that the somata and dendrites, similar to the terminals, release the same transmitters: peptides and glutamate. However, the assumption that the VGLUT-2 labelling seen in the NL is in magnocellular terminals may not be a safe one. First, it has long been known that there are terminals in the NL originating from neurones other than magnocellular neurones (46). Second, neocortical astrocytes, and thus possibly those in the NL (pituicytes), can express VGLUT-2 (16). In the absence of a positive magnocellular terminal marker, it remains unclear which cell type in the NL (pituicyte, OT, VP, non-neurosecretory neurone, endothelial, etc.) expresses VGLUT-2.
Furthermore, ultrastructural experiments are still lacking investigating the subcellular localisation of specific VGLUT isoforms to particular classes of somatic and dendritic MVs. Information addressing these issues could shed further light on alternative functions of VGLUTs and better clarify whether SON neurones express VGLUT-1 at levels not detectible at the light microscopic level. Additionally, physiological experiments monitoring VGLUT expression and somatodendritic release of glutamate under different conditions could address whether or not triggers for glutamate release are distinctive or the same as those for peptide release.
Summary
In the present study, we investigate the presence of VGLUT-1, 2 and 3 protein in the SON. Immunoreactivity for VGLUT-1 was observed mainly lateral to the SON, whereas VGLUT-2-ir was apparent in positively identified magnocellular neuronal somata and dendrites. Expression of VGLUT-3 was seen in SON neurones as well as local SON astrocytes, in addition to being along endothelial and meningeal cells. Magnocellular neuronal expression of VGLUT-3 was mainly restricted to the perinuclear area of the soma. From these data, we suggest that magnocellular SON neurones are both peptidergic and glutamatergic.
Acknowledgments
This research was supported by NIH grant NS009140.
References
- Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 2.Sladek CD, Kapoor JR. Neurotransmitter/neuropeptide interactions in the regulation of neurohypophyseal hormone release. Exp Neurol. 2001;171:200–209. doi: 10.1006/exnr.2001.7779. [DOI] [PubMed] [Google Scholar]
- 3.Meeker RB, Swanson DJ, Greenwood RS, Hayward JN. Ultrastructural distribution of glutamate immunoreactivity within neurosecretory endings and pituicytes of the rat neurohypophysis. Brain Res. 1991;564:181–193. doi: 10.1016/0006-8993(91)91454-9. [DOI] [PubMed] [Google Scholar]
- 4.Hisano S, Nogami H. Transporters in the neurohypophysial neuroendocrine system, with special reference to vesicular glutamate transporters (BNPI and DNPI): a review. Microsc Res Tech. 2002;56:122–131. doi: 10.1002/jemt.10014. [DOI] [PubMed] [Google Scholar]
- 5.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 6.Moos F, Gouzenes L, Brown D, Dayanithi G, Sabatier N, Boissin L, Rabie A, Richard P. New aspects of firing pattern autocontrol in oxytocin and vasopressin neurones. Adv Exp Med Biol. 1998;449:153–162. doi: 10.1007/978-1-4615-4871-3_18. [DOI] [PubMed] [Google Scholar]
- 7.Fremeau RT, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisano S, Hoshi K, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Nogami H. Regional expression of a gene encoding a neuron-specific Na(+)-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Brain Res Mol Brain Res. 2000;83:34–43. doi: 10.1016/s0169-328x(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- 12.Lin W, McKinney K, Liu L, Lakhlani S, Jennes L. Distribution of vesicular glutamate transporter-2 messenger ribonucleic acid and protein in the septum-hypothalamus of the rat. Endocrinology. 2003;144:662–670. doi: 10.1210/en.2002-220908. [DOI] [PubMed] [Google Scholar]
- 13.Csaki A, Kocsis K, Kiss J, Halasz B. Localization of putative glutamatergic/aspartatergic neurons projecting to the supraoptic nucleus area of the rat hypothalamus. Eur J Neurosci. 2002;16:55–68. doi: 10.1046/j.1460-9568.2002.02059.x. [DOI] [PubMed] [Google Scholar]
- 14.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 15.Meeker RB, Swanson DJ, Hayward JN. Light and electron microscopic localization of glutamate immunoreactivity in the supraoptic nucleus of the rat hypothalamus. Neuroscience. 1989;33:157–167. doi: 10.1016/0306-4522(89)90318-7. [DOI] [PubMed] [Google Scholar]
- 16.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy KD, deVellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- 19.Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 21.Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 22.van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- 23.Meeker RB, Greenwood RS, Hayward JN. Glutamate is the major excitatory transmitter in the supraoptic nuclei. Ann NY Acad Sci. 1993;689:636–639. doi: 10.1111/j.1749-6632.1993.tb55614.x. [DOI] [PubMed] [Google Scholar]
- 24.Inenaga K, Cui LN, Nagatomo T, Honda E, Ueta Y, Yamashita H. Osmotic modulation in glutamatergic excitatory synaptic inputs to neurons in the supraoptic nucleus of rat hypothalamus in vitro. J Neuroendocrinol. 1997;9:63–68. doi: 10.1046/j.1365-2826.1997.00597.x. [DOI] [PubMed] [Google Scholar]
- 25.Dyball RE, McKenzie DN, Thomas GP. Osmoresponsiveness of the rat supraoptic nucleus in vivo depends on glutamatergic inputs. Neurobiology (Bp) 1995;3:351–362. [PubMed] [Google Scholar]
- 26.Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol. 1992;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kombian SB, Hirasawa M, Mouginot D, Pittman QJ. Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus. Prog Brain Res. 2002;139:235–246. doi: 10.1016/s0079-6123(02)39020-4. [DOI] [PubMed] [Google Scholar]
- 28.Meeker RB, Swanson DJ, Greenwood RS, Hayward JN. Quantitative mapping of glutamate presynaptic terminals in the supraoptic nucleus and surrounding hypothalamus. Brain Res. 1993;600:112–122. doi: 10.1016/0006-8993(93)90408-f. [DOI] [PubMed] [Google Scholar]
- 29.Lafarga M, Andres MA, Berciano MT, Maquiera E. Organization of nucleoli and nuclear bodies in osmotically stimulated supraoptic neurons of the rat. J Comp Neurol. 1991;308:329–339. doi: 10.1002/cne.903080302. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. [PubMed] [Google Scholar]
- 31.Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- 32.Palkovits M. Micro- and macroscopic structure, innervation, and vasculature of the hypothalamus. In: Conn PM, Freeman ME, editors. Neuroendocrinology in Physiology and Medicine. Totowa NJ: Humana Press; 2000. pp. 23–40. [Google Scholar]
- 33.Freund-Mercier MJ, Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moos F, Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YF, Hatton GI. Burst firing of oxytocin neurons in male rat hypothalamic slices. Brain Res. 2005;1032:36–43. doi: 10.1016/j.brainres.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 36.Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roper P, Callaway J, Armstrong W. Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study. J Neurosci. 2004;24:4818–4831. doi: 10.1523/JNEUROSCI.4203-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrader LA, Tasker JG. Modulation of multiple potassium currents by metabotropic glutamate receptors in neurons of the hypothalamic supraoptic nucleus. J Neurophysiol. 1997;78:3428–3437. doi: 10.1152/jn.1997.78.6.3428. [DOI] [PubMed] [Google Scholar]
- 39.Schrader LA, Tasker JG. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J Neurophysiol. 1997;77:527–536. doi: 10.1152/jn.1997.77.2.527. [DOI] [PubMed] [Google Scholar]
- 40.Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- 41.Smithson KG, Weiss ML, Hatton GI. Supraoptic nucleus afferents from the main olfactory bulb – I. Anatomical evidence from anterograde and retrograde tracers in rat. Neuroscience. 1989;31:277–287. doi: 10.1016/0306-4522(89)90373-4. [DOI] [PubMed] [Google Scholar]
- 42.Hatton GI, Yang QZ. Supraoptic nucleus afferents from the main olfactory bulb – II. Intracellularly recorded responses to lateral olfactory tract stimulation in rat brain slices. Neuroscience. 1989;31:289–297. doi: 10.1016/0306-4522(89)90374-6. [DOI] [PubMed] [Google Scholar]
- 43.Smithson KG, Weiss ML, Hatton GI. Supraoptic nucleus afferents from the accessory olfactory bulb. evidence from anterograde and retrograde tract tracing in the rat. Brain Res Bull. 1992;29:209–220. doi: 10.1016/0361-9230(92)90028-v. [DOI] [PubMed] [Google Scholar]
- 44.Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- 45.Feldman RS, Meyer JS, Quenzer LF. Principles of Neuropsychopharmacology. 1. Sunderland MA: Sinauer; 1997. p. 909. [Google Scholar]
- 46.Boersma CJ, Van Leeuwen FW. Neuron–glia interactions in the release of oxytocin and vasopressin from the rat neural lobe: the role of opioids, other neuropeptides and their receptors. Neuroscience. 1994;62:1003–1020. doi: 10.1016/0306-4522(94)90339-5. [DOI] [PubMed] [Google Scholar]