Abstract
Cryptococcus neoformans is a ubiquitous fungus that can cause life-threatening infections during immunosuppressive states such as AIDS and after bone marrow transplantation. In this study we investigated the antifungal efficacy of an agonist antibody to CD40, an important costimulator of immune function, in combination with interleukin 2 (IL-2) in a murine model of disseminated cryptococcosis. Only the combination of anti-CD40 and IL-2 significantly prolonged the survival time of infected mice. This protection was correlated with decreased yeast burdens in the brain and kidney. Increased immune cell populations in the spleens, as well as increased serum gamma interferon (IFN-γ) and tumor necrosis factor alpha levels were observed in infected mice treated with anti-CD40 and IL-2. Further experiments with IFN-γ knockout mice demonstrated that the protection induced by anti-CD40 and IL-2 treatment was dependent on IFN-γ. Depletion of CD4+ T cells did not affect the increased serum IFN-γ levels induced by anti-CD40 and IL-2 treatment and, importantly, did not affect the antifungal effect of combination therapy. These studies indicate that immunotherapy using anti-CD40 and IL-2 has therapeutic potential in augmenting host resistance to disseminated cryptococcosis and that IFN-γ is essential for efficacy.
Cryptococcus neoformans is an encapsulated yeast that can cause significant disease in immunocompromised hosts. Although the pulmonary tract is considered to be the major infection route, meningitis and meningoencephalitis caused by dissemination of C. neoformans to the brain are the most common manifestations and the main reason for high mortality of cryptococcosis. In murine models, C. neoformans infection through the intravenous route has been used widely to study the pathology as well as treatment of disseminated cryptococcosis (1, 5, 10, 31, 35).
CD40 is a member of the tumor necrosis factor alpha (TNF-α) receptor family and is expressed on numerous cell types, including B cells, dendritic cells, and monocytes. CD40 ligand (CD154) is expressed on activated T cells and NK cells (44). The interaction of CD40 and its ligand is important for optimal T-cell responses and for inducing inflammatory cytokine production by monocytes and dendritic cells (3). CD40 stimulation is also critical for dendritic cell differentiation and function (9, 44). CD40 signaling plays an important role in various pathogenic processes, such as chronic inflammation, autoimmune disorders, graft-versus-host disease, and resistance to tumors (4, 16, 36). Agonist antibodies to CD40 have been shown to facilitate antibody responses to T-independent antigens by bypassing the need for CD4-mediated “help” (14). We have shown that the immune-potentiating effects of anti-CD40 are further augmented by coadministration of interleukin-2 (IL-2) (36). The importance of the CD40/CD40L interaction in host immune defense has been demonstrated for infections such as Leishmania, mycobacteria, and human immunodeficiency virus (16, 39, 45). Deficiencies in either CD40 or CD154 caused an increased susceptibility to infections, including lung infections caused by C. neoformans (37). Studies have also demonstrated a role for CD40/CD40L interactions in the immune response to C. neoformans both in vitro (39, 46, 47) and in vivo (37).
The goal of this study was to assess the effect of an agonist antibody to CD40 given in combination with IL-2 on host resistance in a murine model of disseminated cryptococcosis. Our recent studies demonstrating that anti-CD40 in combination with IL-2 resulted in synergistic antitumor effects in mice by promoting type 1 cytokine responses (36) suggested that this combination may enhance host resistance to C. neoformans. Our results demonstrate that CD40 stimulation and IL-2 prolonged the survival time of mice previously infected with C. neoformans. We found that therapeutic effects of anti-CD40 and IL-2 were correlated with increased dendritic cell and CD8 T-cell expansion in the spleens of mice and that gamma interferon (IFN-γ) is a pivotal immune mediator for the efficacy of anti-CD40 and IL-2 in this model.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were obtained from the Charles River Laboratory of the National Cancer Institute (Frederick, MD). B6.129S7-Ifngtm1Ts/J (IFN-γ knockout [GKO]) and C57BL/6J wild-type (WT) control mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were maintained in an animal facility at University of Nevada, Reno, and all studies were approved by the Institutional Animal Care and Use Committee. All mice were between 8 and 12 weeks of age.
Murine model of disseminated cryptococcosis.
CN6 is a virulent encapsulated strain of serotype A that produces cells of a uniform size and capsule width (15). C. neoformans cells were cultured for 3 days at 30°C with 5% CO2 on Sabouraud dextrose agar (SAB) plates (Becton, Dickinson and Company, Sparks, MD). Yeast cells were harvested from the 3-day culture, washed, counted, and diluted in Dulbecco's phosphate-buffered saline (DPBS) (Mediatech, Inc., Herndon, VA). Mice were infected with C. neoformans (1 × 105 total yeast cells) via the intravenous (i.v.) route. The viability of the inoculum was determined by quantitative culturing on SAB plates. Viability was typically 55% to 65%. Infected mice were observed for morbidity, primarily hydrocephalus and lethargy with partial paralysis. Morbid mice were euthanatized by CO2 based on clinical signs of meningitis and weight loss.
Reagents.
Recombinant human IL-2 (TECIN [Teceleukin]); Roche) was provided by the National Cancer Institute (Frederick, MD). Agonist rat anti-mouse CD40 (1.77 endotoxin units/mg antibody; clone FGK115B3, a subclone of FGK115, which was a kind gift from Bruce Blazar, University of Minnesota) was produced as ascites fluid in CB.17 SCID mice. The monoclonal antibody (MAb) was isolated by differential precipitation with caprylic acid and ammonium sulfate and was dialyzed against DPBS. Antibody concentration was determined by enzyme-linked immunosorbent assay (ELISA), and endotoxin content was determined by quantitative Limulus amoebocyte lysate assay (QCL-1000) (Biowhittaker, Walkersville, MD).
Mice were treated with the core regimen 1 day after infection for all of the survival studies. The agonist anti-CD40 or isotype control rat immunoglobulin G (IgG) (Jackson ImmunoResearch Laboratories, West Grove, PA) was administered intraperitoneally (i.p.) once a day for 4 days (100 μg/dose). IL-2 was given at 500,000 IU/dose i.p. twice a day twice a week, for a total of eight injections. DPBS (0.2 ml/dose) was administered i.p. as vehicle control for IL-2 according to the same injection schedule as for IL-2. The core regimen was completed 10 days after C. neoformans infection. Six to eight mice per group were used in survival studies, and each survival study was repeated two to five times.
Organ CFU assay.
Three mice per group were sacrificed and various organs were harvested 11 days after C. neoformans infection (1 day after the completion of the core regimen). CFU assay was performed as previously described (41). Briefly, brain and kidney cell suspensions were prepared and diluted in sterile distilled water. Cell suspensions were then plated on the SAB plates, and CFU were calculated as colonies per organ. Experiments were repeated twice.
In vivo CD4+ T-cell depletion in mice.
CD4+ T cells were depleted with an anti-CD4 antibody (clone GK1.5, a kind gift from Garry B. Huffnagle, University of Michigan). Mice were injected i.p. with either anti-CD4 antibody or isotype control rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at 200 μg/dose starting 1 day prior to C. neoformans infection and every 4 days, for a total of three injections during the full course of anti-CD40 and IL-2 treatments. Cell depletion efficiency (>99%) was determined by flow cytometric analysis of the splenocytes.
Anti-GXM IgG ELISA.
Sera were collected from three mice per group 11 days after C. neoformans infection (1 day after completion of the core regimen). Antiglucoronoxylomannan (anti-GXM) IgG ELISA was performed as described previously (28), using a horseradish peroxidase-labeled secondary antibody specific for mouse IgG heavy chains (Southern Biotechnology Associates, Inc., Birmingham, AL). A monoclonal anti-GXM antibody (MAb clone 3C2, described previously [25] was used as a positive control) Data are presented as absorbance at 450 nm at a 1:40 dilution of each sample. Experiments were conducted twice.
Flow cytometric analysis.
For flow cytometric analysis, three mice per group were sacrificed 1 day after the completion of the core regimen. Spleens were harvested, minced, and incubated with 1 mg/ml type D collagenase (Sigma-Aldrich Co., St. Louis, MO) for 45 min at 37°C. The resulting slurries were filtered through a nylon filter, and red blood cells were lysed with ACT buffer containing 0.74% ammonium chloride (Sigma-Aldrich Co., St. Louis, MO) and 17 mM Tris-HCl (FisherBiotech, Fair Lawn, NJ). Leukocyte suspensions were centrifuged and resuspended in blocking buffer containing 1% human AB serum, 1% fetal bovine serum, and 1% penicillin-streptomycin in DPBS.
For identification of various leukocyte populations, 1 × 106 cells from spleen suspensions were labeled for 30 min at 4°C with the following antibodies: fluorescein isothiocyanate-conjugated rat anti-mouse CD3, phycoerythrin-conjugated rat anti-mouse CD4, Cy-Chrome-conjugated rat anti-mouse CD8, fluorescein isothiocyanate-conjugated anti-mouse CD11c, or phycoerythrin-conjugated anti-mouse IAb (PharMingen, San Diego, CA). Nonspecific binding was corrected with isotype-matched controls. Cells were then washed with DPBS and fixed in 1% paraformaldehyde. All results were obtained using a FACScan (Becton-Dickinson, San Diego, CA). Forward- and side-scatter settings were gated to exclude red cells and debris. Ten thousand to 20,000 cells were analyzed for each determination. Four replicate experiments were conducted, and the results are reported as numbers of each cell population in the spleens.
IFN-γ and TNF-α ELISA.
Blood was collected from three mice per group 1 day after completion of the core regimen, and serum was prepared. Serum samples were assayed by ELISA using a Quantikine M mouse IFN-γ and TNF-α immunoassay kit (R&D Systems, Minneapolis, MN). The assay was performed according to the manufacturer's instructions. Five replicate experiments were conducted, and the results are reported as IFN-γ or TNF-α concentration (picograms per milliliter) in the sera.
Statistics.
Survival data were plotted by the Kaplan-Meier method and analyzed by the log rank test. Comparisons of cell populations and cytokine production were made by one-way analysis of variance (ANOVA) with post hoc analysis by the Tukey or Bonferroni test. A P value of less than 0.05 was considered significant.
RESULTS
Anti-CD40 and IL-2 protect mice with disseminated cryptococcosis.
CD40 and CD40 ligand interactions have been demonstrated to play a critical role in the host immune defense against C. neoformans infection (37, 46). CD40 stimulation using the agonist MAb (anti-CD40) can augment an anticryptococcal response and IL-12 secretion by human monocytes in vitro (46). We therefore examined CD40 stimulation and IL-2 in a murine model of disseminated cryptococcosis in which C57BL/6 mice were challenged i.v. with C. neoformans. In this disseminated model, multiple organs, notably the brain and kidneys, are targeted by the yeast. Mice were then treated 1 day later with anti-CD40 with or without IL-2. Anti-CD40 or isotype control rat IgG was administered at 100 μg/dose i.p. once a day for 4 days. IL-2 was administered at 500,000 IU/dose i.p. twice a day twice a week, for a total of eight injections. DPBS (IL-2 vehicle control, 0.2 ml/dose) was given on the same schedule as IL-2. This regimen is similar to the regimen used when synergistic antitumor effects were observed (36). The results (Fig. 1) showed that either anti-CD40 or IL-2 given alone failed to significantly prolong the survival of C. neoformans-infected mice. In marked contrast, anti-CD40 in combination with IL-2 significantly (P < 0.005) prolonged the survival of mice, where the mice survived an additional 15 to 20 days. Mice treated with rat IgG-PBS, IL-2 alone, or anti-CD40 alone also showed evidence of brain involvement at a much earlier time than mice receiving combination treatments. Although all mice eventually succumbed to the infection, increasing the amount or time of either anti-CD40 or IL-2 did not result in increased protection (data not shown). These data indicate that administration of anti-CD40 and IL-2 results in protection of mice previously infected with disseminated C. neoformans.
FIG. 1.
Anti-CD40 and IL-2 provide antifungal effects in mice previously infected with C. neoformans. One day after infection, C57BL/6 mice (n = 6 to 8/group) were treated with anti-CD40 (▴) or recombinant human IL-2 alone (▾) or in combination (×) or were given rat IgG and PBS as controls (▪). Anti-CD40 or isotype control rat IgG was administered at 100 μg/dose i.p. once a day for 4 days. IL-2 was administered at 500,000 IU/dose i.p. twice a day twice a week, for a total of eight injections. DPBS (vehicle control for IL-2, 0.2 ml/dose) was given on the same schedule as IL-2. The regimen was completed 10 days after C. neoformans infection. The combination of anti-CD40 and IL-2 resulted in significantly (by log rank test; *, P < 0.005) prolonged survival time compared with that for control mice or mice receiving anti-CD40 or IL-2 alone. Survival analysis was plotted according to the Kaplan-Meier method. Representative data from one of five similar experiments are shown, and statistical differences were determined with the log rank test.
Treatment with anti-CD40 and IL-2 decreases C. neoformans burdens in the organs of infected mice.
Cryptococcal meningitis represents the most frequent clinical manifestation of disseminated cryptococcosis (37, 38). To study whether the prolonged survival of mice treated with anti-CD40 and IL-2 is correlated with decreased organ fungal burdens, mice were sacrificed 1 day after completion of the treatment schedule, and fungal burdens in the brain and kidney were assessed. Mice treated with IL-2 alone showed no reduction in fungal burdens in those organs compared to the control mice. Mice treated with anti-CD40 alone had lower cryptococcal burdens in the brains (P < 0.05) but not in the kidneys compared to the controls (Fig. 2). However, mice treated with both anti-CD40 and IL-2 showed significantly reduced cryptococcal burdens in both the brains (P < 0.001) and the kidneys (P < 0.05) compared to the control mice or mice treated with either anti-CD40 or IL-2 alone (Fig. 2). At a higher challenge dose of C. neoformans (106, compared to the lower challenge dose of 105), mice treated with anti-CD40 in combination with IL-2 showed significantly (P < 0.05) reduced cryptococcal burdens in the livers and the lungs as well as in the brains and kidneys (data not shown). These results demonstrate that the increased survival obtained by administering anti-CD40 and IL-2 can be correlated with reduced fungal burden in the tissues of infected mice.
FIG. 2.
Anti-CD40 and IL-2 treatment decreases the cryptococcal burdens in the brains and kidneys of mice previously infected with C. neoformans. One day following completion of treatment (day 11), three mice per group were sacrificed and quantitative organ cultures of brains (A) and kidneys (B) were prepared. Statistical differences were determined by one-way ANOVA with post hoc comparisons with the Tukey test. A P value of <0.05 is considered significant. Representative data from one of three similar experiments are shown. Error bars indicate standard deviations.
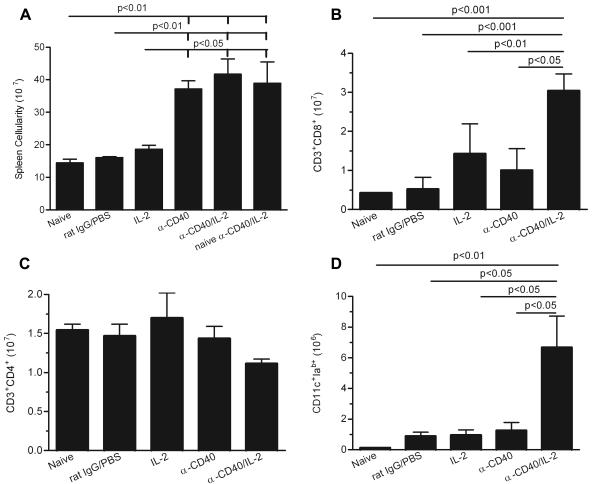
Treatment with anti-CD40 and IL-2 increases dendritic cell and CD8+ T-cell numbers in the spleens of infected mice.
Studies using anti-CD40 and IL-2 in tumor models found significant increases in the numbers of dendritic cells and CD8+ T cells in the spleens of treated mice (36). To determine whether a similar effect occurred in a cryptococcosis model, we examined dendritic cell and T-cell populations in the spleens of C. neoformans-infected mice treated with anti-CD40 or IL-2 alone, or in combination. Mice were assessed 1 day after completion of the treatment schedule. The results (Fig. 3) showed a significant increase in the total number of splenocytes (P < 0.05), CD8+ T cells (P < 0.01), and CD11c+ Iab+ dendritic cells (P < 0.01) in mice receiving both anti-CD40 and IL-2 compared with either control mice or mice receiving either reagent alone. In contrast, the total number of CD4+ T cells remained unchanged despite a significant increase in the total number of splenocytes (Fig. 3C). Thus, protection by the combination of anti-CD40 and IL-2 is correlated with significantly increased splenic CD8+ T-cell and dendritic cell populations but not with the number of CD4+ T cells. We further determined whether splenocyte expansion induced by anti-CD40 and IL-2 depends on the presence of C. neoformans. Without C. neoformans infection, anti-CD40 and IL-2 significantly increased splenocyte numbers (Fig. 3A) (P < 0.01), indicating that this is not an antigen-dependent event. This observation is consistent with previous tumor studies (36).
FIG. 3.
Effects of anti-CD40 and IL-2 treatment on immune cell parameters in the spleens of mice. Mice were i.v. injected with C. neoformans or PBS. One day following completion of treatments (day 11), three mice/group were sacrificed and flow cytometry was used to assess the effect of anti-CD40 and IL-2 on numbers of whole splenocytes (A), CD8+ T cells (B), CD4+ T cells (C), and dendritic cells (D) in the spleens. Three mice were analyzed per group in each experiment. The results are representative of three independent experiments. Statistical differences are indicated (one-way ANOVA with post hoc comparisons with Tukey test; P < 0.05). Error bars indicate standard deviations.
Effect of anti-CD40 and IL-2 treatment on serum anti-GXM IgG levels.
Treatment of mice with CD40 agonist antibodies leads to a markedly enhanced antibody response to T-independent antigens (14). GXM, the major capsular antigen of C. neoformans, is a T-independent antigen (42), and we have found that immunization of mice with GXM in combination with a high dose of anti-CD40 (400 μg) leads to a very rapid IgG response (unpublished results). Since antibodies to GXM can alter the course of cryptococcosis (13), we examined sera of infected mice that were treated with anti-CD40 and IL-2 for the production of anti-GXM IgG. Serum anti-GXM IgG levels in the sera collected 1 day after completion of anti-CD40 and IL-2 treatment (day 11) were tested by ELISA. The results showed that administration of anti-CD40 alone slightly increased anti-GXM IgG levels in the treated mice (P < 0.05) (Fig. 4). However, there were no significant differences in the levels of anti-GXM IgG in the sera of mice treated with IL-2 alone or in combination with anti-CD40 compared to the controls, even though only the combination group showed significantly prolonged survival. These data suggest that induction of GXM-specific antibodies most likely plays a marginal role in the anticryptococcal efficacy of combined anti-CD40 and IL-2 treatment.
FIG. 4.
Effects of anti-CD40 and IL-2 treatment on anti-GXM-specific IgG levels in the sera of C. neoformans infected mice. One day following completion of treatments (day 11), mice were bled for sera. Anti-GXM IgG was assessed by ELISA. Optical densities (O.D.) at a 1/40 dilution of sera are shown. Three mice per group were analyzed in each experiment. The results are a compilation of two independent experiments (experiment 1, open symbols; experiment 2, closed symbols). Statistical differences are indicated (one-way ANOVA with post hoc comparison with Bonferroni test; *, P < 0.05).
Treatment with anti-CD40 and IL-2 increases serum IFN-γ and TNF-α levels in treated mice.
The Th1-Th2 cytokine balance influences host immune responses to C. neoformans, with Th1-type cytokines (i.e., IFN-γ) being associated with a protective immune response against C. neoformans (18, 24). To determine if a beneficial Th1 response can be induced by anti-CD40 and IL-2, mice were bled for serum the day after completion of the treatment regimen. IFN-γ levels in sera were then tested by ELISA. Mice receiving either IL-2 or anti-CD40 alone showed no significant difference in serum IFN-γ levels compared to the control mice (Fig. 5A). Only mice receiving the combination treatment showed a significant increase (P < 0.01) in serum IFN-γ levels compared to rat IgG-PBS-treated control mice (Fig. 5A). TNF-α is a pleiotropic inflammatory cytokine that has a large variety of functions in immune responses. Several studies have shown that TNF-α is required for development of protective immunity to C. neoformans infections (17, 20). Therefore, serum was collected 1 day after the completion of the treatment regimen, and TNF-α levels were assessed by ELISA. Significantly increased levels of TNF-α were found in the sera from mice treated with the combination of anti-CD40 and IL-2 compared to either the control mice (P < 0.01), mice treated with IL-2 alone (P < 0.01), or mice treated with anti-CD40 alone (P < 0.05) (Fig. 5B). Thus, both IFN-γ and TNF-α levels were increased by anti-CD40 and IL-2 combination therapy, and the results could be correlated with effects on survival. To further investigate whether IFN-γ and TNF-α production induced by anti-CD40 and IL-2 is dependent on the presence of antigen, mice were treated with anti-CD40 and IL-2 without C. neoformans infection (Fig. 5). Serum IFN-γ and TNF-α levels were increased despite of the absence of C. neoformans (P < 0.01), suggesting that antigen is not required for anti-CD40- and IL-2-induced cytokine release, which is also in agreement with previous tumor models using this regimen (36).
FIG. 5.
Effects of anti-CD40 and IL-2 treatment on serum IFN-γ and TNF-α levels. Mice were injected i.v. with C. neoformans or PBS (Naive α-CD40/IL-2). One day following completion of anti-CD40 and IL-2 treatments (day 11), three mice per group were bled for sera. Serum IFN-γ (A) and TNF-α (B) levels were then tested by mouse IFN-γ and TNF-α ELISA. Representative data from four similar experiments are shown. Statistical differences are indicated (by one-way ANOVA with post hoc comparisons with Tukey test; P < 0.05). Error bars indicate standard deviations.
IFN-γ knockout mice are more susceptible to C. neoformans infection and show no protection induced by anti-CD40 and IL-2 treatment.
To confirm that IFN-γ is an important mediator of anti-CD40 and IL-2 therapy, the efficacy of anti-CD40 and IL-2 therapy in GKO mice was compared to that in wild-type mice. GKO mice were found to be much more susceptible to C. neoformans infection than WT controls (Fig. 6) (P < 0.0002). Moreover, treated GKO mice showed no protection by anti-CD40 and IL-2 against disseminated cryptococcosis, while in the WT controls, the combination therapy significantly enhanced host resistance to C. neoformans infection (Fig. 6) (P < 0.0001). These data suggest that IFN-γ plays an essential role in both host immune defense and anti-CD40 and IL-2 immunotherapy.
FIG. 6.
Requirement for IFN-γ in the antifungal effects of anti-CD40 and IL-2 treatment. C57BL/6J WT and GKO mice (eight mice per group) received either control injections or anti-CD40 and IL-2. The combination of anti-CD40 and IL-2 had no protective effects in GKO mice (▵ and ▴), while anti-CD40 and IL-2 prolonged the survival of WT mice (□ versus ▪, P < 0.0002). GKO mice were more susceptible to C. neoformans infection than were WT controls (▵ versus □, P < 0.0002). Survival analysis was plotted according to the Kaplan-Meier method, and statistical differences were determined with the log rank test.
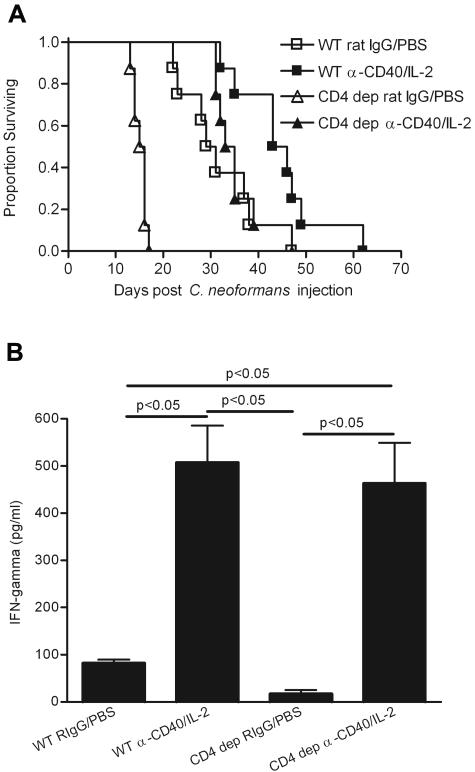
CD4+ T cells are not necessary for the protection provided by anti-CD40 and IL-2 immunotherapy.
It was surprising that the CD4+ T-cell population was not expanding in either the brains or the spleens of treated mice in this model, given the fact that CD4+ T cells have been shown to play a role in the host immune defense against C. neoformans (19, 21, 30) and also in several immunotherapies for cryptococcosis (6, 22, 34). To study whether CD4+ T cells were important for the efficacy of this immunotherapy and also to mimic the clinical condition where there are low CD4+ T-cell counts (i.e., AIDS), CD4+ T cells in mice were depleted by a specific monoclonal anti-CD4 antibody 1 day prior to the C. neoformans infection and during the full course of anti-CD40 and IL-2 treatment. Our results showed that despite the absence of CD4+ T cells, anti-CD40 and IL-2 treatments significantly prolonged the survival time of CD4+ T-cell-depleted mice previously infected with C. neoformans (Fig. 7A) (P < 0.0001) at a level comparable to that for the WT (Fig. 7A) (P < 0.002). Although WT treated mice survived slightly longer than CD4+ T-cell-depleted and treated mice (Fig. 7A) (P < 0.05), the extent of protection induced by anti-CD40 and IL-2 was comparable, taking into consideration the difference in the mean survival time of CD4+ T-cell rat IgG-PBS controls versus WT rat IgG-PBS controls. This suggests that CD4+ T cells are not necessary for the antifungal efficacy of this immunotherapy. Our results also confirm the importance of CD4+ T cells for host immunity to cryptococcosis (Fig. 7A) (P < 0.0001). Furthermore, similar levels of increased serum IFN-γ were observed in either CD4+ T-cell-depleted or WT mice (Fig. 7B) (P < 0.05). This indicates that CD4+ T cells are not the dominant IFN-γ-producing cells in anti-CD40 and IL-2 immunotherapy, and therefore protection is not adversely affected in their absence.
FIG. 7.
CD4+ T cells are not required for the antifungal effect of anti-CD40 and IL-2 treatment. (A) C57BL/6 mice (eight mice per group) received either isotype control rat IgG or anti-CD4 antibody starting 1 day prior to C. neoformans injection and every 4 days during the anti-CD40 and IL-2 treatment. The combination of anti-CD40 and IL-2 treatments significantly prolonged the survival time of CD4+ T-cell-depleted mice with disseminated cryptococcosis (▵versus▴, P < 0.0001), as well as WT mice (□ versus ▪, P < 0.002), although WT treated mice survived significantly longer than CD4+ T-cell-depleted and treated mice (▪ versus ▴, P < 0.05). CD4+ T-cell-depleted mice were significantly more susceptible to disseminated cryptococcosis (▵ and □, P < 0.0001). Survival analysis was plotted according to the Kaplan-Meier method, and statistical differences were determined with the log rank test. Representative data from one of two similar experiments are shown. (B) Sera were taken 1 day after completion of anti-CD40 and IL-2 treatments (day 11), and IFN-γ levels were determined by ELISA. Anti-CD40 and IL-2 were able to increase serum IFN-γ levels with or without the presence of CD4+ T cells. Representative data from one of two similar experiments are shown, and statistical differences are indicated (one-way ANOVA with post hoc comparisons with Tukey test; P < 0.05). Error bars indicate standard deviations.
DISCUSSION
The incidence of invasive fungal infections, including candidiasis, cryptococcosis, and aspergillosis, has increased in recent years (40). Options for preventing and treating invasive fungal infections consist of several classes of antifungal agents, including polyenes, azoles, nucleoside analogs, and echinocandins. Although many of these drugs have improved the outcomes of fungal infections, failure rates remain high due to emergence of resistant fungal strains and serious side effects of antifungal agents (40). Therefore, new strategies for treating fungal infections are needed to substitute for these agents or compensate for primary treatment failure. Immunotherapy has tremendous potential in treating both infectious diseases and cancer. This is the first report that demonstrates a potent antifungal activity of anti-CD40 and IL-2 in mice previously infected with C. neoformans. Neither agent alone was therapeutic, but the combination provides significant prolongation of survival time. This protection was dependent on IFN-γ and associated with CD8+ T-cell expansion. CD4+ T cells were not necessary for the efficacy of this immunotherapy, for absence of CD4+ T cells did not abrogate either the protective effect or the increase of serum IFN-γ levels induced by anti-CD40 and IL-2. These results suggest the potential for applying anti-CD40 and IL-2 immunotherapy to a clinical situation where there are low CD4+ T-cell counts.
Our results did not identify a specific mechanism for the synergistic efficacy of anti-CD40 and IL-2. Immunomodulation through CD40 can promote dendritic cell differentiation and function and enhance production of cytokines such as IL-12, which induces IFN-γ (2). IL-2 is a T-cell growth factor that could enhance Th1-type immune responses. Therefore, combining anti-CD40 with IL-2 could strengthen the interaction between innate and adaptive immunities, induce optimal T-cell responses, and enhance Th1-associated inflammatory cytokine production, such as that of IFN-γ. This interaction might prove beneficial for host resistance to C. neoformans infection.
It is not yet determined which cells are the primary producers of IFN-γ in this model. T cells and NK cells are the major IFN-γ-producing cells in mice (7). Cell depletion studies or knockout mice are needed to answer the question of which cell type is the primary IFN-γ-producing cell type in anti-CD40/IL-2 therapy for disseminated cryptococcosis. IFN-γ has been demonstrated to be critical in host immune defense in experimental models of cryptococcosis (41, 43), and expression of the IFN-γ receptor is necessary for protective inflammatory responses to C. neoformans (11). The role of IFN-γ in anti-CD40 and IL-2 therapy is likely to be indirect through activating other cells. CD8+T cells, NK cells, macrophages, and neutrophils have all been found to possess the ability to kill C. neoformans (12, 23, 32, 33, 37), and the cytotoxicity of these cell types could be affected by cytokines, including IFN-γ (8, 27, 29). Therefore, these cell types could potentially be the effector cells in anti-CD40 and IL-2 therapy for disseminated cryptococcosis.
CD40 stimulation has been shown to bypass CD4+ T-cell help to facilitate a strong antibody response to T-independent antigens (14, 26). As a consequence, induction of an antibody response to GXM is a possible mechanism for the therapeutic action of the CD40 agonist MAb. Our results showed that there was no protective anti-GXM IgG antibody production observed in the sera of mice treated with the combination of anti-CD40 and IL-2 compared to mice receiving anti-CD40 alone. This may be caused by a more profound Th1 type of immune response induced by using IL-2 that potentially suppressed antibody responses. We did observe a limited antibody response to GXM in mice that received the agonist MAb alone. We do not believe that induction of anti-GXM was a component of protection mediated by the anti-CD40/IL-2 combination. First, the level of antibody observed following administration of the agonist MAb was relatively low. Second, there was no therapeutic effect induced by the agonist MAb alone. Third, the antibody response was observed well after challenge. It is unlikely that the limited antibody response could rescue mice well into the course of disseminated cryptococcosis. Finally, an antibody response was not observed in the case of the therapeutically successful anti-CD40/IL-2 combination. While our studies do not rule out the possibility that antibodies contribute to the treatment effect, our results suggest that this is not a dominant mediator in this model.
There are several murine models of cryptococcosis, including infecting the mice via intravenous, intratracheal/nasal, and intracerebral routes. In the intravenous infection model, the yeast disseminates throughout the animal, and morbidity and mortality are primarily due to infection of the brain. This model contrasts with intratracheal/nasal infection, in which cryptococcosis is primarily a pulmonary disease that may disseminate at later stages. In the intravenous infection model used in this study, mice treated with anti-CD40 and IL-2 eventually succumbed to cryptococcosis, probably because of an inadequate immune response in the brain. It is uncertain whether the anticryptococcal effect of anti-CD40/IL-2 immunotherapy is due to controlling the Cryptococcus infection at the brain itself or to reducing the fungal load in the body and subsequent dissemination to the brain. Future experiments applying anti-CD40 and IL-2 therapy to mice with intracerebral infection with Cryptococcus are needed to gain a better understanding of the mechanism of this immunotherapy. Further, it will also be of interest to combine anti-CD40 and IL-2 immunotherapy with conventional antifungal agents to achieve greater effects in the control of the infection, as well as apply anti-CD40 and IL-2 immunotherapy to other fungal pathogens, such as Candida and Aspergillus.
Acknowledgments
This work was supported in part by Public Health Service grants CA95572 and AI14209.
We thank Bruce R. Blazar (University of Minnesota Hospital and Cancer Center, Minneapolis) for the kind gift of FGK115 hybridoma. We thank Gary B. Huffnagle (University of Michigan Medical School, Ann Arbor) for the kind gift of GK1.5 hybridoma. We thank Weihong Ma, William H. D. Hallett, and Danice E. C. Wilkins for their great help.
Editor: A. Casadevall
REFERENCES
- 1.Aguirre, K., E. A. Havell, G. W. Gibson, and L. L. Johnson. 1995. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect. Immun. 63:1725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderson, M. R., R. J. Armitage, T. W. Tough, L. Strockbine, W. C. Fanslow, and M. K. Spriggs. 1993. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J. Exp. Med. 178:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage, R. J., C. R. Maliszewski, M. R. Alderson, K. H. Grabstein, M. K. Spriggs, and W. C. Fanslow. 1993. CD40L: a multi-functional ligand. Semin. Immunol. 5:401-412. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Bazan, D. Blanchard, F. Briere, J. P. Galizzi, C. Van Kooten, Y. J. Liu, F. Rousset, and S. Saeland. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12:881-922. [DOI] [PubMed] [Google Scholar]
- 5.Barchiesi, F., E. Spreghini, A. M. Schimizzi, M. Maracci, D. Giannini, F. Carle, and G. Scalise. 2004. Posaconazole and amphotericin B combination therapy against Cryptococcus neoformans infection. Antimicrob. Agents Chemother. 48:3312-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauman, S. K., G. B. Huffnagle, and J. W. Murphy. 2003. Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect. Immun. 71:68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billiau, A., H. Heremans, K. Vermeire, and P. Matthys. 1998. Immunomodulatory properties of interferon-gamma. An update. Ann. N. Y. Acad. Sci. 856:22-32. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan, C. 2000. The function of type I interferons in antimicrobial immunity. Curr. Opin. Immunol. 12:419-424. [DOI] [PubMed] [Google Scholar]
- 9.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlier, C., F. Chretien, M. Baudrimont, E. Mordelet, O. Lortholary, and F. Dromer. 2005. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am. J. Pathol. 166:421-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, G. H., R. A. McDonald, J. C. Wells, G. B. Huffnagle, N. W. Lukacs, and G. B. Toews. 2005. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect. Immun. 73:1788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2002. Effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on polymorphonuclear neutrophils, monocytes or monocyte-derived macrophages combined with voriconazole against Cryptococcus neoformans. Med. Mycol. 40:21-26. [DOI] [PubMed] [Google Scholar]
- 13.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dullforce, P., D. C. Sutton, and A. W. Heath. 1998. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nat. Med. 4:88-91. [DOI] [PubMed] [Google Scholar]
- 15.Gates, M. A., P. Thorkildson, and T. R. Kozel. 2004. Molecular architecture of the Cryptococcus neoformans capsule. Mol. Microbiol. 52:13-24. [DOI] [PubMed] [Google Scholar]
- 16.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 17.Herring, A. C., J. Lee, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2002. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 70:2959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huffnagle, G. B. 1996. Role of cytokines in T cell immunity to a pulmonary Cryptococcus neoformans infection. Biol. Signals 5:215-222. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35-42. [DOI] [PubMed] [Google Scholar]
- 20.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 21.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 173:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami, K., Y. Kinjo, S. Yara, K. Uezu, Y. Koguchi, M. Tohyama, M. Azuma, K. Takeda, S. Akira, and A. Saito. 2001. Enhanced gamma interferon production through activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide in interleukin-18-deficient mice with systemic cryptococcosis. Infect. Immun. 69:6643-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami, K., Y. Koguchi, M. H. Qureshi, S. Yara, Y. Kinjo, K. Uezu, and A. Saito. 2000. NK cells eliminate Cryptococcus neoformans by potentiating the fungicidal activity of macrophages rather than by directly killing them upon stimulation with IL-12 and IL-18. Microbiol. Immunol. 44:1043-1050. [DOI] [PubMed] [Google Scholar]
- 24.Koguchi, Y., and K. Kawakami. 2002. Cryptococcal infection and Th1-Th2 cytokine balance. Int. Rev. Immunol. 21:423-438. [DOI] [PubMed] [Google Scholar]
- 25.Kozel, T. R., R. S. MacGill, and K. K. Wall. 1998. Bivalency is required for anticapsular monoclonal antibodies to optimally suppress activation of the alternative complement pathway by the Cryptococcus neoformans capsule. Infect. Immun. 66:1547-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel, T. R., W. J. Murphy, S. Brandt, B. R. Blazar, J. A. Lovchik, P. Thorkildson, A. Percival, and C. R. Lyons. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. USA 101:5042-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumaratilake, L. M., A. Ferrante, and C. Rzepczyk. 1991. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J. Immunol. 146:762-767. [PubMed] [Google Scholar]
- 28.Leinonen, M., and C. E. Frasch. 1982. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect. Immun. 38:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Page, C., P. Genin, M. G. Baines, and J. Hiscott. 2000. Interferon activation and innate immunity. Rev. Immunogenet. 2:374-386. [PubMed] [Google Scholar]
- 30.Lipscomb, M. F., G. B. Huffnagle, J. A. Lovchik, C. R. Lyons, A. M. Pollard, and J. L. Yates. 1993. The role of T lymphocytes in pulmonary microbial defense mechanisms. Arch. Pathol. Lab Med. 117:1225-1232. [PubMed] [Google Scholar]
- 31.Lutz, J. E., K. V. Clemons, and D. A. Stevens. 2000. Enhancement of antifungal chemotherapy by interferon-gamma in experimental systemic cryptococcosis. J. Antimicrob. Chemother. 46:437-442. [DOI] [PubMed] [Google Scholar]
- 32.Ma, L. L., J. C. Spurrell, J. F. Wang, G. G. Neely, S. Epelman, A. M. Krensky, and C. H. Mody. 2002. CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J. Immunol. 169:5787-5795. [DOI] [PubMed] [Google Scholar]
- 33.Mambula, S. S., E. R. Simons, R. Hastey, M. E. Selsted, and S. M. Levitz. 2000. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect. Immun. 68:6257-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyagi, K., K. Kawakami, Y. Kinjo, K. Uezu, T. Kinjo, K. Nakamura, and A. Saito. 2005. CpG oligodeoxynucleotides promote the host protective response against infection with Cryptococcus neoformans through induction of interferon-gamma production by CD4+ T cells. Clin. Exp. Immunol. 140:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee, J., L. S. Zuckier, M. D. Scharff, and A. Casadevall. 1994. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob. Agents Chemother. 38:580-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, W. J., L. Welniak, T. Back, J. Hixon, J. Subleski, N. Seki, J. M. Wigginton, S. E. Wilson, B. R. Blazar, A. M. Malyguine, T. J. Sayers, and R. H. Wiltrout. 2003. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J. Immunol. 170:2727-2733. [DOI] [PubMed] [Google Scholar]
- 37.Pietrella, D., P. Lupo, S. Perito, P. Mosci, F. Bistoni, and A. Vecchiarelli. 2004. Disruption of CD40/CD40L interaction influences the course of Cryptococcus neoformans infection. FEMS Immunol. Med. Microbiol. 40:63-70. [DOI] [PubMed] [Google Scholar]
- 38.Ross, J. J., and J. D. Katz. 2002. Cryptococcal meningitis and sarcoidosis. Scand. J. Infect. Dis. 34:937-939. [DOI] [PubMed] [Google Scholar]
- 39.Sabeti, P., S. Usen, S. Farhadian, M. Jallow, T. Doherty, M. Newport, M. Pinder, R. Ward, and D. Kwiatkowski. 2002. CD40L association with protection from severe malaria. Genes Immun. 3:286-291. [DOI] [PubMed] [Google Scholar]
- 40.Shoham, S., and S. M. Levitz. 2005. The immune response to fungal infections. Br. J. Haematol. 129:569-582. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui, A. A., A. E. Brouwer, V. Wuthiekanun, S. Jaffar, R. Shattock, D. Irving, J. Sheldon, W. Chierakul, S. Peacock, N. Day, N. J. White, and T. S. Harrison. 2005. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J. Immunol. 174:1746-1750. [DOI] [PubMed] [Google Scholar]
- 42.Sundstrom, J. B., and R. Cherniak. 1992. The glucuronoxylomannan of Cryptococcus neoformans serotype A is a type 2 T-cell-independent antigen. Infect. Immun. 60:4080-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uicker, W. C., H. A. Doyle, J. P. McCracken, M. Langlois, and K. L. Buchanan. 2005. Cytokine and chemokine expression in the central nervous system associated with protective cell-mediated immunity against Cryptococcus neoformans. Med. Mycol. 43:27-38. [DOI] [PubMed] [Google Scholar]
- 44.Van Kooten, C., and J. Banchereau. 1997. Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 9:330-337. [DOI] [PubMed] [Google Scholar]
- 45.Van Kooten, C., and J. Banchereau. 1997. Functional role of CD40 and its ligand. Int. Arch. Allergy Immunol. 113:393-399. [DOI] [PubMed] [Google Scholar]
- 46.Vecchiarelli, A., C. Retini, D. Pietrella, C. Monari, and T. R. Kozel. 2000. T lymphocyte and monocyte interaction by CD40/CD40 ligand facilitates a lymphoproliferative response and killing of Cryptococcus neoformans in vitro. Eur. J. Immunol. 30:1385-1393. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi, P. S., J. R. Bleharski, K. Uyemura, J. Kim, P. A. Sieling, A. Miller, H. Brightbill, K. Schlienger, T. H. Rea, and R. L. Modlin. 2000. A role for CD40-CD40 ligand interactions in the generation of type 1 cytokine responses in human leprosy. J. Immunol. 165:1506-1512. [DOI] [PubMed] [Google Scholar]