Abstract
Objective
To evaluate the multicenter application of intraoperative lymphatic mapping, sentinel lymphadenectomy, and selective complete lymph node dissection (LM/SL/SCLND) for the management of early-stage melanoma.
Summary Background Data
The multidisciplinary technique of LM/SL/SCLND has been widely adopted, but not validated in a multicenter trial. The authors began the international Multicenter Selective Lymphadenectomy Trial (MSLT) 5 years ago to evaluate the survival of patients with early-stage primary melanoma after wide excision alone versus wide excision plus LM/SL/SCLND. This study examined the accuracy of LM/SL/SCLND in the MSLT, using the experience of the organizing center (John Wayne Cancer Institute [JWCI]) as a standard for comparison.
Methods
Before entering patients into the randomization phase, each center in the MSLT was required to finish a 30-case learning phase with complete nuclear medicine, pathology, and surgical review. Selection of MSLT patients in the LM/SL/SCLND treatment arm was based on complete pathologic and surgical data. The comparison group of JWCI patients was selected using these criteria: primary cutaneous melanoma having a thickness ≥1 mm with a Clark level ≥III, or a thickness <1 mm with a Clark level ≥IV (MSLT criterion); LM/SL performed between June 1, 1985, and December 30, 1998; and patient not entered in the MSLT. The accuracy of LM/SL/SCLND was determined by comparing the rates of sentinel node (SN) identification and the incidence of SN metastases in the MSLT and JWCI groups.
Results
There were 551 patients in the MSLT group and 584 patients in the JWCI group. In both groups, LM performed with blue dye plus a radiocolloid was more successful (99.1%) than LM performed with blue dye alone (95.2%) (p = 0.014). After a center had completed the 30-case learning phase, the success of SN identification in the MSLT group was independent of the center’s case volume or experience in the MSLT.
Conclusions
Lymphatic mapping and sentinel lymphadenectomy can be successfully learned and applied in a standardized fashion with high accuracy by centers worldwide. Successful SN identification rates of 97% can be achieved, and the incidence of nodal metastases approaches that of the organizing center. A multidisciplinary approach (surgery, nuclear medicine, and pathology) and a learning phase of ≥30 consecutive cases per center are sufficient for mastery of LM/SL in cutaneous melanoma. Lymphatic mapping performed using blue dye plus radiocolloid is superior to LM using blue dye alone.
Management of the regional lymph nodes in early-stage melanoma has remained controversial since 1892, when Snow 1 first recommended routine complete lymph node dissection in patients without clinical evidence of regional metastasis. If metastatic melanoma progresses sequentially from primary site to the regional lymph nodes and then to more distant sites, early removal of regional nodes should interrupt the metastatic cascade. 2 Multiple retrospective single-institution studies support Snow’s approach, reporting a survival advantage when patients without evidence of nodal metastasis undergo routine elective complete lymph node dissection (ECLND). 2–4
If metastatic melanoma does not invariably spread first to the regional nodes, then ECLND may not be justified. Three randomized prospective clinical trials have failed to demonstrate an overall survival benefit for patients undergoing ECLND instead of observation after wide excision. 5–7 These findings raise the possibility that regional metastasis may be a marker of systemic disease rather than the primary metastatic pathway, because melanoma may spread to distant sites without involvement of regional nodes. The recent Intergroup Melanoma Trial raised further questions by demonstrating an unexpected survival advantage for ECLND in a subset of patients who had relatively thin primaries. 8
Part of the controversy about the value of ECLND centers around its potential complications and cost. Because only approximately 20% of patients with an intermediate-thickness primary are expected to have metastases in the regional nodes, 80% of patients undergoing ECLND are at risk for acute wound problems, chronic lymphedema, nerve injury, and anesthetic complications, without actual benefit. 9 The costs of ECLND increase the total cost of care for melanoma patients at least fivefold compared with wide excision alone. 10
The single most important prognostic factor for patients with early-stage melanoma is the tumor status of the regional nodes draining the primary tumor. 2 Until recently, the only method to identify regional nodal metastasis was complete lymph node dissection, with pathologic examination of each excised node using hematoxylin and eosin (H&E) staining. This technique samples only a small percentage of each node and therefore might underestimate the true frequency of nodal metastasis. 11
In 1977, we described the use of cutaneous lymphoscintigraphy to identify the lymph basins at risk for metastasis from truncal primary melanomas and introduced the concept of selective lymphadenectomy of the deep iliac/obturator lymph nodes directed by the pathologic status of Cloquet’s node and the superficial inguinal nodes. 12 Our mapping studies with various radiopharmaceuticals were the impetus for the intraoperative use of vital dyes to identify the sentinel node (SN)—that is, the first lymph nodes within the lymphatic basin reached by lymph draining the primary lesion. A feline model demonstrated the feasibility of intraoperative lymphatic mapping (LM) of the SN, 13 and in 1985 we began our first clinical trials of LM and sentinel lymphadenectomy (SL). 14
Improvements in pathologic evaluation were also necessary to develop a better approach to the regional nodes. An important step was the ability to detect single melanoma cells. This followed our demonstration that S-100 protein could be used as an exquisitely sensitive marker for melanoma. 15,16 Immunohistochemical staining using antibodies to S-100 upstaged regional lymph nodes relative to H&E findings and identified occult tumor cells that were not detected in H&E-stained sections of regional lymph nodes. 11,17,18 We reported the prognostic value of the number of melanoma-containing lymph nodes 2,19 and later showed that morphometric evaluation of tumor in individual nodes could predict outcome with even greater accuracy. 20 The realization that early stages of regional metastasis affected a comparatively small number of lymph nodes followed a series of studies in which we demonstrated that the nodes anatomically closest to a primary melanoma were immunosuppressed and were the site of early metastases. 21–28 This stimulated a search for techniques to detect these critical nodes in vivo. Accurate pathologic evaluation of lymph nodes to which a primary tumor drains directly is essential to the success of any staging technique in patients with early-stage melanoma.
In 1990, we reported our initial series of 223 patients with early-stage cutaneous melanoma, who underwent LM/SL using isosulfan blue or patent blue V dye. 14,29 Although cutaneous lymphoscintigraphy was used only in selected cases with potentially ambiguous drainage patterns, blue-stained SNs were identified in 194 of 237 (82%) basins. Complete lymph node dissection was performed after all LM/SL procedures, so that the tumor status of non-SNs could be compared with SN status. In essentially all cases, SN histology predicted the tumor status of the entire nodal basin; only 2 of 194 (1%) lymph node basins had metastases confined to (allegedly) non-SNs. Complete nodal staging can thus be obtained by examination of the SN alone. After extensive phase II trials, 30–32 we abandoned routine ECLND, performing complete lymph node dissection selectively (SCLND) in patients with tumor-positive SNs.
Because of the technical difficulty of LM/SL, we were concerned about its accuracy outside high-volume melanoma centers. 29 Since our initial report, 29 LM/SL has been validated by several large single-institution studies, 33,34 but there are no data on its accuracy in a multicenter trial using uniform entry criteria and standardized surgical, pathologic, and nuclear medicine techniques. In 1994, we initiated the Multicenter Selective Lymphadenectomy Trial (MSLT) to evaluate LM/SL/SCLND for staging the regional lymph nodes in patients with early-stage primary cutaneous melanoma. The primary aim of this randomized trial is to compare survival after wide excision alone versus wide excision plus LM/SL/SCLND (Fig. 1). The MSLT is a multidisciplinary protocol combining surgery, nuclear medicine, and pathology. Sixteen centers joined the trial after demonstrating 85% accuracy for ≥30 cases. Thus far, >1100 patients have entered the MSLT, which is in its fifth year of accrual. This report examines the accuracy of standardized LM/SL/SCLND and its transferability worldwide.

Figure 1. Study design. Patients are assigned in a 60:40 distribution to wide excision (WEX) plus lymphatic mapping, sentinel lymphadenectomy, and selective complete lymph node dissection (LM/SL/SCLND) or to WEX alone. All patients are followed for overall survival as the primary endpoint. F/U, follow up.
PATIENTS AND METHODS
MSLT Group
The MSLT group was drawn from patients enrolled in the MSLT, which is still open for accrual. Eligible patients have invasive primary cutaneous melanoma of the head and neck, trunk, extremities, sole of the foot, palm of the hand, or a subungual site. Primary melanomas are Clark level III and Breslow thickness ≥1 mm, or Clark level IV/V with any Breslow thickness (see Fig. 1). Patients enter the trial no more than 10 weeks after biopsy, and the definitive surgical procedure must be within 12 weeks of diagnosis. The age range is 18 to 75 years. Patients are ineligible after any surgical procedure that could have disrupted lymphatic drainage patterns from the primary site, including prior wide excision of the primary with a shortest margin ≥1.5 cm. They are also ineligible if they have a history of prior melanoma or other invasive malignancy within 5 years of the diagnosis of melanoma, and/or if their life expectancy (excluding the diagnosis of melanoma) is <10 years. Other exclusion criteria are primary or secondary immune deficiency, and pregnancy. All patients provide informed consent based on the approved protocol of each institution’s review board.
MSLT Treatment Arms
Patients are randomly assigned to wide excision alone or wide excision plus LM/SL/SCLND (see Fig. 1). Wide excision is performed with surgical margins ≥2 cm; the technique of LM/SL/SCLND is described below. Randomization assigns patients in a 60:40 distribution to wide excision plus LM/SL/SCLND or to wide excision alone.
JWCI Group
We reviewed our melanoma database of 9500 patients to identify all patients undergoing LM/SL at JWCI for early-stage melanoma between June 1, 1985, and December 30, 1998 (Fig. 2). Patients who met MSLT inclusion criteria but were not entered in the MSLT because they were treated before the trial or chose not to participate made up the JWCI group against which the MSLT group was compared. All patients in the JWCI group underwent LM/SL and either routine complete lymph node dissection or SCLND after wide excision. The surgical techniques were identical to those described below for patients enrolled in the MSLT.
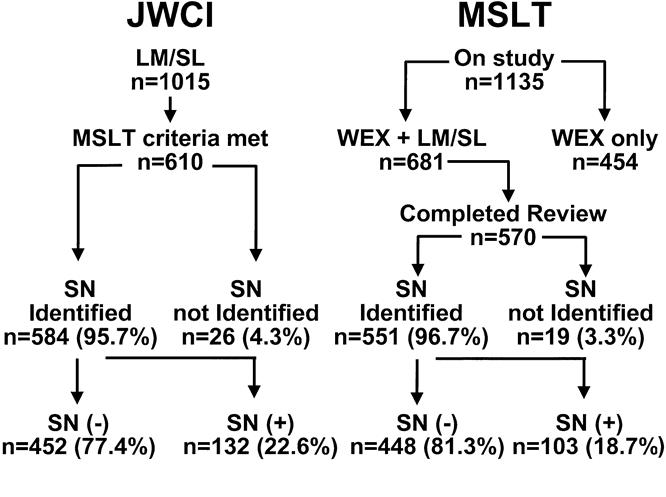
Figure 2. Rates of sentinel node (SN) identification and SN metastasis in the Multicenter Selective Lymphadenectomy Trial (MSLT) and John Wayne Cancer Institute (JWCI) groups. Numbers (n) shown are numbers of patients. Some patients had more than one drainage basin (see Table 2). WEX, wide excision.
Technique of LM/SL/SCLND
The mapping technique used at JWCI and in the MSLT is based on the technique that we first described in 1990 before the Society of Surgical Oncology 14 and that was first published in 1992. 29 The procedure is performed under general or regional anesthesia with a single intradermal injection of 1 to 2 cc of vital blue dye (patent blue or isosulfan blue) around the primary tumor or excisional biopsy wound. The injection site is gently massaged for several minutes before incising the regional lymph basin. The location and orientation of the incision are based on the anatomy of the regional basin, allowing for subsequent SCLND if indicated. Skin flaps made in the lymph node basin allow visual identification of the blue-stained lymphatics from the edge of the wound to the SN(s). If an SN is not identified within 20 minutes, a second injection of blue dye is made. All blue-stained SNs are excised; if H&E staining or immunohistochemistry (see below) reveals metastases in these nodes, complete lymph node dissection is performed.
Preoperative cutaneous lymphoscintigraphy is required and is performed in the United States with technetium-99m (99mTc)-labeled albumin colloid (CIS-US, Inc., Bedford, MA), 99mTc sulfur colloid (CIS-US), or 99mTc human serum albumin (Amersham Mediphysics, Arlington Heights, IL) 35; colloidal antimony sulfide is used in Australia, and human albumin nanocolloid is commonly used in European centers. Approximately 18.5 to 30 MBq (0.5 to 0.8 mCi) of radiopharmaceutical is injected at the primary site. A scintillation camera documents the drainage pattern from the primary through the dermal lymphatics to the regional lymph nodes. The skin overlying the SN is marked. Because of variation in the transit speed of various radiopharmaceuticals and the distance from the primary to the regional basin, dynamic imaging is essential to differentiate SNs from secondary non-SNs. In our experience, SNs are identified by 30 minutes; often by 4 hours SNs and non-SNs can no longer be differentiated because of migration of the radiocolloid to nodes beyond the SN. 36,37
Based on the concept of cutaneous lymphoscintigraphy and the dynamic nature of the radiopharmaceuticals used for these studies, in 1993 we devised the technique of intraoperative radiolymphoscintigraphy using one of three radiopharmaceuticals, and in 1994 we reported our experience with 99mTc-labeled albumin before the Society of Surgical Oncology. 38 During our initial studies, we injected the radiopharmaceutical during surgery with isosulfan blue dye at the primary site. We then used the hand-held gamma probe to determine the radioactive count over a background site, over the afferent lymph channels, over the skin site marked during preoperative lymphoscintigraphy, and over each blue-stained node before and after its excision. From these counts we established a relative count ratio of blue-stained SNs and non-SNs. This relative count ratio allowed us to develop a strategy for intraoperative use of the hand-held gamma probe to help identify SNs and to create a baseline level of radioactivity to help identify all blue-stained lymph nodes. 32,37,39 The technique has been refined with experience and is now routinely performed with same-day cutaneous lymphoscintigraphy: 1 to 4 hours before surgery, the patient is brought to the nuclear medicine suite, where 99mTc-labeled filtered sulfur colloid is injected at the primary site. This avoids intraoperative injection of the radiocolloid. Although we accept a radioactive count two times or more greater than background as corroborative evidence of an SN, the blue coloration of a node remains the gold standard for SN identification in the MSLT. Centers initially used blue dye to identify SNs but now combine radiocolloid and dye.
MSLT Learning Phase
During the learning phase, each participating center was required to complete ≥30 consecutive cases with an SN identification rate of ≥85%. Each individual surgeon documented ≥15 consecutive cases. Documentation included a detailed surgical note and a description of how the SN was identified (surgical visualization and/or pathologic confirmation of a blue-stained node). Complete lymph node dissection was performed in all 30 learning cases to confirm the absence of metastases in non-SNs when the reported SN was free of tumor. No center entered the trial until all learning-phase cases were reviewed by the trial coordinating board (surgeons, nuclear medicine physician, and pathologist). Over the last few years, radiolymphoscintigraphy has been introduced as an adjunct for SN identification during LM, 32,37,39–43 and centers have incorporated the hand-held gamma probe into the mapping procedure.
MSLT Trial Phase
Each participating center must perform LM/SL/SCLND by the same technique used during the learning phase. Complete lymph node dissection is recommended if no SN is identified during LM and must be performed if the SN contains tumor. Patients in both treatment groups are monitored after surgery with routine clinical examination, blood tests, and chest x-rays. Follow-up is calculated from initial diagnosis to last follow-up examination or death.
Histopathologic Examination of the Sentinel Node
Sentinel node specimens are reviewed as permanent sections. Although frozen-section analysis was routinely used during our development of the SL technique, 29 we have moved to permanent sections to minimize loss of diagnostic material during “facing up.” Interpretation of permanent material is always more accurate, and this policy allows the preparation and evaluation of optimal immunohistochemical sections. 11,36,44 Most MSLT centers now use permanent section alone.
Theoretically, each SN should be serially sectioned to extinction, but this is impossibly expensive and clearly impractical. As a compromise between the ideal and the possible, we recommend cutting the lymph node into two halves through its longest circumference. These two halves are placed cut-face down in cassettes and fixed for ≥24 hours. The technician is instructed to minimize “facing up.” As soon as a full-faced section can be obtained, 10 serial sections are removed. Sections 1, 3, 5, and possibly 10 are stained by H&E; section 2 is stained for S-100 protein; and section 4 is stained for HMB-45. Sections 6 and 7 are negative controls for the immunoperoxidase studies, and sections 8 and 9 are used to repeat any study that is technically unsatisfactory or for additional immunohistochemistry. If suspicious or anomalous appearances are seen within the first 10 sections, additional groups of 10 sections can be examined. Immunohistochemistry is essential because H&E histology misses up to 12% of positive nodes.
When SCLND is performed, non-SNs are examined by conventional H&E staining alone.
Statistics
Descriptive statistics for quantitative variables included means, standard deviation, minimum, and maximum; frequencies (absolute and relative) were obtained for categorical or ordinal data. Descriptive statistics for each variable were presented in side-by-side columns for MSLT and JWCI groups. For quantitative variables such as age, t tests for comparison of means were carried out; given that the numbers of patients in each study were quite close, the t test is robust to heterogeneous variances. For ordinal or categorical variables, the initial comparisons between the MSLT and JWCI groups were carried out using the chi-square p value for 2×2 tables. Supplemental analysis compared MSLT and JWCI groups by level, using 2 × 2 table analysis by the standard chi-square statistic. Exact probability values were reported based on exact analytic methods when the sample sizes were too small for the standard large-sample–based chi-square analysis. Probability values were not adjusted for multiple testing.
RESULTS
Each participating center fulfilled learning-phase criteria before entering the MSLT. Most centers had some prior experience with LM/SL, and this may have contributed to the high success rate of SN identification. Figure 2 shows the relation between successful LM/SL and the presence of SN metastases in the MSLT and JWCI groups. By December 30, 1998, 1135 patients had entered the MSLT; 315 were from centers in North America, 175 from centers in Europe, and 645 from centers in Australia. Complete data for review (nuclear medicine, surgery, pathology) were available for 570 patients randomized to the LM/SL arm. Of 1015 patients undergoing LM/SL for early-stage melanoma at JWCI between June 1, 1985, and December 30, 1998, 610 fulfilled MSLT criteria. An SN was identified in at least one basin in 584 patients (95.7%) from the JWCI group and in 551 (96.7%) patients from the MSLT group.
The 551 MSLT patients were remarkably similar to the 584 JWCI patients with regard to gender and Breslow thickness of the primary melanoma (Table 1). There were more extremity primaries in the MSLT group than the JWCI group (52% vs. 44%), whereas the JWCI group had more truncal primaries (45% vs. 37%; p = 0.018). Although the mean primary thickness was the same, tumors in the JWCI group had a higher Clark level (p = 0.011), and JWCI patients were younger (p = 0.001).
Table 1. CHARACTERISTICS OF MSLT AND JWCI GROUPS

JWCI, John Wayne Cancer Institute; MSLT, Multicenter Selective Lymphadenectomy Trial.
* Number of patients and their percentage distribution within each prognostic factor.
† Compares the overall proportion of patients within each prognostic factor between the JWCI and MSLT groups.
‡ Chi square test.
§ t test.
Accuracy of SN identification was based on the visualization of a blue lymphatic channel leading to a blue node. The relation between the site of the mapped basin and the rate of SN identification is shown in Table 2. Because some patients had drainage to more than one basin, LM/SL was attempted in 676 basins in the JWCI group and 623 basins in the MSLT group. The overall rate of SN identification was 95.3% for the JWCI group and 93.3% for the MSLT group (p = 0.122). There was no difference in axillary success rates (95.7% for the MSLT group vs. 95.0% for the JWCI group); however, inguinal mapping was more successful in the MSLT group than the JWCI group (99.0% vs. 92.5%; p = 0.001), whereas cervical mapping was more successful in the JWCI group than the MSLT group (99.0% vs. 86%; p < 0.01). The overall lower incidence of successful SN identification in the neck may reflect the relatively small number of head and neck primaries (13%) and the complex anatomy of this region. Numbers were too small to compare the success of mapping for other (ectopic) lymphatic basins.
Table 2. RELATION BETWEEN SITE OF MAPPED BASINS AND SUCCESS OF LM
JWCI, John Wayne Cancer Institute; MSLT, Multicenter Selective Lymphadenectomy Trial.
* Popliteal, epitrochlear, and ectopic sites such as parascapular triangular space.
Learning Curve in the MSLT Group
Because we observed a shallow learning curve among JWCI surgeons, 29 a detailed analysis of technical aspects of LM/SL was undertaken in the MSLT group to determine whether the mandatory 30-case learning phase had affected the learning curve. When we compared the success rates of initial versus more recent procedures, there was no difference in performance between low-volume and high-volume centers (Table 3). There was no difference in the rate of SN identification between low-volume and high-volume centers (Table 4), which indicates that the performance of 30 cases is sufficient to learn the procedure. 45,46 Because LM/SL was performed without radiocolloid in 353 early cases, it was possible to compare the technical success of LM/SL performed using blue dye alone (95.2%, or 336/353) versus blue dye plus radiocolloid (99.1%, or 215/217; p = 0.014) (Fig. 3).
Table 3. LM/SL LEARNING CURVE*

LM/SL, lymphatic mapping/sentinel lymphadenectomy.
* Comparing first 50% with second 50% of each center’s experience in relation to case volume.
Table 4. LM SUCCESS IN RELATION TO CASE VOLUME AT MSLT CENTERS

LM, lymphatic mapping; MSLT, Multicenter Selective Lymphadenectomy Trial; SN, sentinel node.
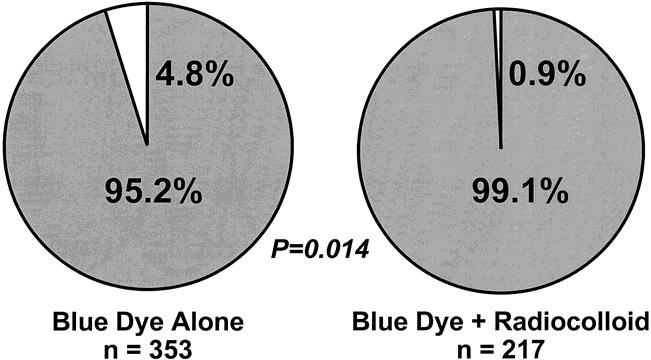
Figure 3. Success of intraoperative lymphatic mapping in relation to mapping agent(s).
We could not use the entire JWCI experience with LM/SL to assess radiolymphoscintigraphy in the MSLT because the technique was not routine at JWCI until recently. However, we have reported 100 LM/SL cases using blue dye and surgical radiolymphoscintigraphy, demonstrating a 98% rate of SN identification (94% with blue dye alone and 98% with the combined technique). The routine use of radiolymphoscintigraphy in addition to blue dye has not significantly increased the number of SNs removed during the MSLT. On average, 1.65 SNs and 1 non-SN were excised per case. This distribution did not vary from center to center or by the basin site (Table 5); this reflects the high level of standardization and compliance with details of the LM/SL.
Table 5. MEAN NUMBER OF LYMPH NODES EXCISED PER BASIN
Incidence of SN Metastases in Relation to Other Prognostic Factors
Table 6 summarizes the frequency of positive SNs in the MSLT and JWCI groups. Positivity was highly correlated with younger age (p = 0.003), thicker primary (p = 0.001), higher Clark level (p = 0.001), and truncal location (p = 0.001) in both groups. There was no significant difference in the frequency of positive nodes (18.7% for MSLT vs. 22.6% for JWCI; p = 0.104). The slightly higher nodal positivity in the JWCI group can be explained by the somewhat greater proportion of patients with adverse risk factors for nodal metastases (i.e., younger age, truncal primary, and higher Clark level; see Table 1).
Table 6. SENTINEL NODE POSITIVITY IN RELATION TO OTHER PROGNOSTIC FACTORS
JWCI, John Wayne Cancer Institute, MSLT, Multicenter Selective Lymphadenectomy Trial; SN, sentinel node.
* Chi square test.
† t test.
There was a trend toward more tumor-positive SNs with head and neck primaries (JWCI 21% vs. MSLT 9%; p = 0.06), which may indicate the difficulty experienced by some centers in identifying SNs in the cervical area. Tumor-positive dissections in patients with Clark level IV primaries were more frequent in the JWCI group than the MSLT group, but this difference was not significant. The incidence of tumor-positive dissections increased with increasing thickness of the primaries. No MSLT or JWCI patients with primaries <0.75 mm had metastases in the SN.
Among patients who had positive SNs and therefore underwent complete lymph node dissection, the distribution of involved nodes was remarkably similar: 71% of MSLT and 72% of JWCI patients had only one involved node, whereas 29% of MSLT and 28% of JWCI patients had two or more involved nodes (see Table 6). This distribution is very similar to that reported in our original description of the technique. 29 We have not yet identified any patient characteristics or pathologic features of the primaries or SNs that identify with complete accuracy patients who will have more than one positive node.
The best method to determine the predictive accuracy of LM/SL is to assess the incidence of same-basin recurrence in patients who have tumor-negative SNs. Our experience at JWCI shows a 4.8% incidence of same-basin recurrence at median follow-up of 45 months. Some of these cases could be explained by SN micrometastases that were initially missed by the pathologists, often when the initial review did not include immunoperoxidase. Other cases may reflect surgical misidentification of the true SN. Recurrences were as early as 12 months and as late as 5 years after LM/SL alone. The relatively short follow-up of the MSLT group (median 21 months), with only six recurrences, prevents us from determining the true rate of recurrence in dissected basins at this time.
DISCUSSION
In 1990, we presented our initial results with LM/SL to the Society of Surgical Oncology. 14 Despite our demonstrated success with this technique, many of our colleagues doubted its accuracy and utility. As a result, the paper reporting our findings was initially rejected by prominent journals and then subjected to multiple revisions before its ultimate publication 2 years later. 29 In the spring of 1991, we invited a distinguished group of melanoma surgeons and pathologists to visit, learn the technique, and consider joining a multicenter trial to evaluate its therapeutic utility. Surgeons from Roswell Park, the University of South Florida, M.D. Anderson, Memorial Sloan-Kettering, and the University of Pennsylvania visited us to learn the technique, and many joined the MSLT. Studies in melanoma and breast cancer, by our group and other investigators, have confirmed the SN hypothesis, 33,34,39,47–50 and the present analysis of MSLT data confirms its applicability in a multicenter setting. A learning phase of 30 cases clearly is adequate for mastery of LM/SL/SCLND by a multidisciplinary team of surgeons, nuclear medicine physicians, and pathologists. 45
The success of SN identification in the MSLT is largely the result of the strict standardization of the technique, which is conceptually simple but technically challenging. Our approach has been to identify and eliminate those critical areas of variability that may lead to error, such as the definition of an SN when LM is performed using a radioactive tracer alone. There are at least five definitions of a radioactive SN. 39,43,51 The number of radioactive hot lymph nodes will depend on the radiocolloid agent used and the time between its injection and subsequent surgery. As the radiolabeled colloid flows along the lymphatic chain, the SN should be the first draining node and the only hot node—if the mapping procedure is performed promptly after radiocolloid injection. As additional time elapses, the radiolabeled colloid flows further up the chain of nodes and additional nodes become hot; therefore, not all hot nodes are necessarily SNs. Moreover, the hottest node is not always the SN. 43,51 Thus, the surgeon using radiocolloid alone may not be able to determine the true SN among the many hot nodes that are removed. The accuracy of radiopharmaceuticals alone for identifying the SN in melanoma has never been demonstrated in a multicenter trial, because most groups use both blue dye and radiocolloids. In the MSLT, we define the hot node by a radioactive count at least twofold higher than that of a background site; this definition has not changed. In the MSLT, the radiocolloid is considered an adjunct to the blue dye for identification of the SN. At present, the dye must remain the gold standard because approximately 8% of blue nodes are not hot, and some of these will be the only site of metastasis. 39
Another key area of standardization is the technique for histopathologic analysis of the SN. Because an SL specimen contains a much smaller number of nodes than a complete lymph node dissection specimen, pathologists can cost-effectively examine SNs at multiple levels using immunohistochemical techniques. 11 The most common targets for immunohistochemical staining for melanoma are S-100 protein 17,52,53 and HMB-45 antigen. 44 Antibodies binding to these epitopes are visualized with an enzymatic reaction to a chromogen aminoethyl carbazol, which gives a red reaction product that contrasts with brown melanin, thus allowing identification of melanoma cells in formalin-fixed paraffin-embedded sections. There is no question that immunohistochemistry is more sensitive than conventional H&E staining: it can identify up to 12% more positive SNs. Gershenwald et al 54 in 1998 reported that in 10 of 243 patients with stage I or II melanoma, a first recurrence developed in a previously mapped nodal basin that was negative by H&E staining. Reevaluation of the SNs by immunohistochemistry with anti–S-100 and anti–HMB-45 demonstrated occult disease in 7 (70%) of the 10 patients. Molecular techniques may further upstage patients whose SNs are tumor-free by H&E and immunohistochemical staining, 53 but their standardized use is considerably more troublesome. Also, results are difficult to interpret because of variations in the methods used for reverse transcriptase–polymerase chain reaction and the presence of m-tyrosinase in capsular nevi and Schwann cells in nerves that occur in many normal lymph nodes.
Standardization is essential to maintain quality control in any multicenter trial, particularly when expertise is required from different disciplines such as nuclear medicine, surgery, and pathology. The nuclear medicine physician identifies the nodal basin(s) at risk, determines the number of lymphatic channels leading to separate SNs, and accurately marks the cutaneous location overlying each SN to direct the surgeon. Each physician must be familiar with the common lymphatic drainage patterns for different areas of the body, aberrant routes of the lymphatics, and aberrant locations of the SN. 32,55–57 Imaging must be timed to avoid missing true SNs or identifying non-SNs as SNs. 35,42,58
Two criteria can be used to compare the accuracy of LM/SL/SCLND among centers participating in the MSLT:
1. The incidence of SN metastases, which is remarkably similar between the JWCI and MSLT groups after adjusting for the slightly higher incidence of risk factors for nodal metastases in the JWCI study group (higher Clark level, truncal primary site, and young age)
2. The incidence of same-basin recurrence in patients whose SNs were reported as histopathologically negative.
The latter criterion is important because it assesses the accuracy of the three different disciplines in correctly identifying and evaluating the SN, and it determines whether histology and immunohistochemical techniques can detect all clinically significant occult metastases. However, the follow-up period of the MSLT is still too short to judge with accuracy the incidence of recurrence in reportedly tumor-negative nodal basins, with only six recurrences after a median follow-up of 21 months.
An important inference from MSLT data is that the natural history of cutaneous melanoma is similar in the United States, Australia, and Europe when standard and precise surgical and pathology techniques are used to stage the primary site and regional nodes. After correcting for prognostic factors, the incidence of nodal metastases was remarkably similar in the JWCI and MSLT groups.
JWCI’s melanoma database, which contains data for >1000 patients undergoing LM/SL, shows that almost 30% of lymphatic basins containing a tumor-positive SN have more than one tumor-involved node. 29 MSLT data confirm this observation (see Table 1). The retrospective detection of occult melanoma cells in some ostensibly negative SNs from patients in whom nodal recurrence developed indicates the critical importance of meticulous pathologic evaluation (including immunohistochemistry) of all SNs. At present, SCLND is considered necessary in all patients with tumor-positive SNs. The fact that not all nodes reexamined after ipsilateral regional failure contain tumor emphasizes the burden of responsibility for accurate SN identification placed on nuclear medicine personnel and surgeons.
Sentinel nodes are tumor-positive in 15% to 26% of patients in various series, with a false-negative rate of 0% to 2%. 29,39–41 These numerous studies and the MSLT experience make clear that LM/SL performed by an experienced nuclear medicine, surgical oncology, and pathology team is a safe, accurate, and reproducible method of identifying patients with lymph node metastases.
An important but neglected (or ignored) aspect of standardization is terminology. The term “sentinel node” is generally accepted, but our original procedure—intraoperative lymphatic mapping and selective lymphadenectomy 29 —has variously been called selective lymphadenectomy, 59 sentinel lymph node dissection, 60 sentinel lymphadenectomy, 61 sentinel node biopsy, 33,62 sentinel node harvest, 63 minimal-access surgery, 41 and gamma probe-guided lymph node localization. 64 None of these more recent terms is wrong, but none describes the technique of identification and excision of the SN as accurately as our original term “intraoperative lymphatic mapping and sentinel lymphadenectomy.” We now introduce a third term—SCLND—in an effort to convey the selective use of completion lymphadenectomy in patients with a tumor-positive SN.
This preliminary report examining the accuracy of MSLT data confirms the crucial importance of a learning period for LM/SL. 29,45 Experienced investigators have achieved high rates of SN identification and accurate pathologic evaluation only after ascending a learning curve to technical expertise. Published reports of LM/SL indicate that the learning curve is shallow and that experience with many cases is required for acceptable skill in identifying the SN (≥90% accuracy). The MSLT’s standard of 30 cases for melanoma is in keeping with a recent consensus statement 46 and suggests that immediate application of LM/SL/SCLND may not be appropriate among community-based surgeons who treat only a few patients with melanoma each year.
Experience at JWCI and other cancer centers clearly shows that successful lymphatic mapping is directly related to the surgeon’s experience. While ascending the learning curve, the surgeon must perform complete lymph node dissection after LM/SL to monitor the rate of false-negative SNs identified by dye, probe, or preferably dye plus probe. Unless each surgeon can develop and validate his or her own accuracy, the patient may undergo an expensive procedure that does not determine the true tumor status of the SN and nodal basin. This may deprive the patient of the potential therapeutic benefit of early complete lymph node dissection for micrometastatic disease. 2,8,29
CONCLUSIONS
LM/SL/SCLND shows much promise in the management of patients with early-stage melanoma. Our SN hypothesis has been validated beyond doubt in this and other clinical studies. The technical details of preoperative mapping and surgical identification have been refined. Pathologic detection of occult tumor cells has been improved by the examination of multiple levels and the use of immunohistochemical techniques. Molecular techniques may further refine nodal evaluation. The MSLT may produce data indicating the therapeutic utility of LM/SL/SCLND and will certainly indicate its accuracy as a staging procedure.
The standardized approach to LM/SL/SCLND used in the MSLT will eventually be transferred to the community hospital setting. In the meantime, its use should be confined to clinical trials conducted by centers that have validated results for the identification and pathologic evaluation of SNs. In other settings, LM/SL should be considered a means to procure tissue for accurate pathologic staging, and for the present it should be followed by complete lymph node dissection. Concerted efforts to ensure multidisciplinary quality and to validate results will facilitate the universal application of LM/SL/SCLND to solid tumors of the breast, vulva, colon, thyroid, and other anatomic sites. 47
Appendix
Multicenter Selective Lymphadenectomy Trial Group
John Wayne Cancer Institute, Santa Monica, CA: Donald L. Morton, MD; Sydney Melanoma Unit, Sydney, Australia: John F. Thompson, MD; Netherlands Cancer Institute, Amsterdam, The Netherlands: Omgo E. Nieweg, MD, PhD; New York University, New York, NY: Daniel F. Roses, MD; Millard Fillmore Hospital, Buffalo, NY: Constantine P. Karakousis, MD, PhD; Istituto Nazionale dei Tumori de Napoli, Naples, Italy: Nicola Mozzillo, MD; H. Lee Moffit Cancer Center, Tampa, FL: Douglas Reintgen, MD; University of California, San Francisco, Mt. Zion Medical Center: Stanley P.L. Leong, MD; Royal Adelaide Hospital, Adelaide, Australia: Brendon J. Coventry, BM, BS, PhD; Roswell Park Cancer Institute, Buffalo, NY: William Kraybill, MD; Princess Alexandra Hospital, Brisbane, Australia: Mark Smithers, MB, BS; Academisch Ziekenhuis Groningen, Groningen, The Netherlands: H. Schraffordt Koops, MD, PhD; Henry Ford Hospital, Detroit, MI: S. David Nathanson, MD; University of Texas Southwestern Medical Center at Dallas: James F. Huth, MD; University of Hawaii at Manoa, Honolulu: Jan H. Wong, MD; University of Pennsylvania, Philadelphia, PA: Douglas L. Fraker, MD; Tom Baker Cancer Centre, Calgary, Alberta, Canada: Walley Temple, MD
Discussion
Dr. Charles M. Balch (Pasadena, California): I want to congratulate Dr. Morton on his presentation and for his continued series of substantial contributions in the management of melanoma, especially as they relate to lymph node metastasis. I also want to thank him for the opportunity of reviewing the manuscript beforehand.
The surgeon’s role is greatly diminishing in the management of lymph node metastases, both for staging and for curative treatment, because of a prevailing view that all of these patients have systemic disease anyway. More recently, the pendulum has begun to swing, I think, around to a more selective approach, targeting patients for lymph node dissection based upon the natural history of the disease as defined by prognostic factors and, importantly, using the available tools for defining which lymph node basins and which specific lymph nodes are at risk for harboring clinically occult metastases.
Dr. Morton has made singular contributions in this area, first by pioneering the use of continuous lymphoscintigraphy, which tells us which nodal basin are at risk. Numerous studies have shown that 10% to 15% of patients have more than one nodal basin at risk that wouldn’t be predicted based upon anatomic drawings. More recently, Dr. Morton developed the technique of intraoperative mapping and sentinel lymphadenectomy that is based upon a hypothesis that there is a single node within each nodal basin that is representative of the rest of the nodal basin—that is, if the sentinel node is positive, there may be metastases elsewhere in the nodal basin, but if it is negative, then the rest of the nodal basin will be free of metastases as well. This is a very substantial biological contribution to our understanding of melanoma metastases.
This paper focuses on the technology transfer of sentinel lymphadenectomy. It is made more important, I think, by the results of the Intergroup Melanoma Trial which we reported to the Association 4 years ago as an interim analysis and 2 weeks ago at the Society of Surgical Oncology as an update with more definitive data after a median 10 years of follow-up.
This trial, as you know, had 760 patients with intermediate thickness melanoma, with 97% follow-up and 98% evaluability, who were randomized to receive elective node dissection or observation of their lymph nodes. The results, as yet unpublished, show that in prospectively defined and individually randomized groups of patients, there are highly significant differences based upon the Cox regression or multifactorial analysis. That is, there was a 30% reduction in mortality for melanomas measuring 1.0 to 4.0 mm that were not ulcerated (p = 0.03). This is a more definitive conclusion than we have made in the past and it puts some pressure to bear on those surgeons to minimize their false-negative rates if they substitute sentinel lymphadenectomy for elective node dissection in the subgroups of patients with intermediate thickness.
In my melanoma practice, I am comfortable about leaving the nodal basin behind with an accurately performed sentinel node biopsy. The issue, then, is the false-negative rate, because if the dye does not identify the biological event of metastasis, we may have a false-negative result and the patient might otherwise miss an opportunity for cure with a complete lymph node dissection.
I have two questions for Dr. Morton. First, why did you choose 30 patients as a threshold initially for surgeons entering patients into this trial? And what do you think is the long-term acceptable false-negative rate in these patients?
My second question focuses on the maturity of the data supporting the technique of mapping and sentinel lymphadenectomy. Is the data sufficiently mature at the present time for sentinel lymphadenectomy to be considered as standard of care in the community? In other words, do surgeons treating melanoma have to do this? Are they liable if they do not use this as part of their treatment approach?
Thank you very much for the opportunity to discuss this paper and congratulations on an excellent presentation.
Presenter Dr. Donald L. Morton (Santa Monica, California): Thank you, Dr. Balch. Why 30 patients? The initial report was based on our developmental phase of intraoperative lymphatic mapping and sentinel lymphadenectomy (LM/SL); during this time we were essentially starting from scratch to establish initial guidelines for this technique. After we had established these guidelines, we found that new fellows and new surgical staff were able to ascend the LM/SL learning curve faster. Thus, although I performed about 60 cases during the initial development of LM/SL before reaching a 90% success rate, surgeons learning the established technique at the John Wayne Cancer Institute can reach the top of the learning curve after 30 cases. It must be remembered that unlike breast cancer, where regional nodal metastases are confined to the axilla and the internal mammary chain, melanoma can also involve regional inguinal and cervical nodal basins. Each lymph basin is a little different and its mapping requires experience for consistent success.
Is LM/SL the standard of care? In my opinion, absolutely not. Only 20% of patients with primary melanoma have nodal metastases and therefore can benefit from elective lymph node dissection; unless the Multicenter Selective Lymphadenectomy Trial (MSLT) demonstrates that the use of LM/SL to identify these patients has a significant impact on survival, then this technique has no therapeutic value. If LM/SL has no therapeutic utility, why incur the approximately $2000 of additional expenses associated with lymphoscintigraphy, surgeon’s fees, and special pathologic studies of the sentinel node? Formerly, it was thought that this technique would be useful in identifying patients who might benefit from high-dose interferon therapy; however, recent results of the ECOG 1690 trial demonstrate that high-dose interferon provides no overall survival benefit and cannot be considered as standard postoperative therapy for patients with high-risk melanoma.
Dr. Kirby I. Bland (Providence, Rhode Island): I, too, wish to congratulate Dr. Morton and his colleagues at the John Wayne Cancer Institute for bringing this important analysis to our attention. This study confirms that lymphatic mapping/sentinel lymphadenectomy can be successfully learned and applied in a standardized fashion with a high degree of accuracy.
There are several important messages to be taken from this study. First are the strong results. The outcomes send a clear message that the combination—again I emphasize the combination—of intraoperative radiolymphoscintigraphy with gamma probe and blue dye are highly accurate in diagnosis, highly reproducible even in low-volume centers, and superior to the original technique of using blue dye alone. As you saw from this analysis, lymphatic mapping and sentinel lymphadenectomy, with blue dye alone, had reproducibility of 95%; adding radiocolloid increased the accuracy to 99.1% (p = 0.014).
The use of these new techniques for tissue analysis will prevent about 80% of the morbidity of the traditional, nonselective, complete node dissection and also cost less than the traditional procedures. These are important savings in this era of obsessive concerns about costs, which sometimes seem to override any concern for patient benefit. Thus, this new technique is superior and appears to be more cost-effective.
Second, Dr. Morton, I congratulate you and your colleagues on the rapidity with which the results of this study have been conveyed to the medical profession. Seven short years after the first published clinical outcomes, we have the statistics from a multicenter trial of over 1,100 patients. Particularly noteworthy is that the excellent and similar results across all institutions were products of the mandatory training to ensure that all members perform the exact procedures at the same level of competency as that practiced at the John Wayne Cancer Institute. I think that sends an important message for planning of future trials that compliance training for such techniques should be guided by the investigatory institution.
A few questions, Don.
Perhaps the general surgeons who do not perform enough procedures to achieve competency may think that they should refer all such cases to high-volume academic medical centers. However, this policy of across-the-board referrals is improbable, impractical, and it is obviously unenforceable. So, for the surgeons who cannot perform significant annual volumes, should there be methods of compliance to ensure performance using assisted learning techniques such as video, animal laboratories, or other techniques? I would be interested to learn if you have already used some of these.
Secondly, once an operator or a center reaches the mandatory 30 cases necessary to achieve competency, have you found that additional improvement occurs? What rates of success have the most experienced surgeons at JWCI achieved? And how long did that take?
Finally, if the analyses confirm the absence of therapeutic advantage using mapping, sentinel lymphadenectomy, as well as the selective complete excision of these nodes, can you justify the cost of a procedure which includes lymphoscintigraphy (in the Northeast that is somewhere between $500 to $800—if you get reimbursed for it, that is), tissue histochemistry (between $400 and $600), the surgeon’s fee, and the cost of operating time? This procedure is currently treated as a lymph-node biopsy. Therefore, if you analyze this, it is going to be clearly a negative impact on cost recovery.
Would you please address those questions? I enjoyed this very much and congratulate you.
Dr. Morton: Thank you, Dr. Bland. I firmly believe that unless an institution has a committed and dedicated multidisciplinary team (nuclear medicine physician, surgeon, pathologist) that has established a high rate of accurate, reproducible results for identification, excision, and examination of the sentinel node, then the patient should undergo wide excision and complete node dissection, or be referred to a high-volume melanoma center that has significant experience with LM/SL. I do not believe that the 2-day courses promoted by various medical instrument companies are adequate because they cannot provide the comprehensive training in all technical aspects to be mastered by the multidisciplinary team at each center. In addition, the learning phase of LM/SL requires experience, and each surgeon must perform enough cases to ascend his/her learning curve.
If the MSLT demonstrates a therapeutic advantage for early removal of tumor-positive lymph nodes, then every community hospital that has a significant number of melanoma patients should establish and fund the training of an LM/SL team. However, at the present time, we cannot justify the additional costs of this multidisciplinary training. In my opinion, LM/SL is still an investigational procedure rather than the standard of care. Moreover, even if the sentinel node contains tumor cells, there is no postoperative adjuvant therapy that is the universally accepted standard of care.
Dr. Harold J. Wanebo (Providence, Rhode Island): Dr. Morton, you and your colleagues are to be congratulated, not only for pioneering this technique which has revolutionized the management of melanoma and breast cancer, but also for conducting the multicenter trial which will ultimately establish some rules about selected node dissection. You have also achieved validated results of the findings at your own center.
I have several questions. First, do you detect occult metastases in the sentinel node by using multiple step section, immunohistochemistry, or the RTPCR, and have you found that this has an impact on management and actually alters outcome? Should this technique be used in practice outside of a clinical trial?
Secondly, are there subgroups of patients in your study with intermediate thickness melanomas, 1 to 4 mm, who are sentinel node-negative and perhaps have such a good prognosis that they probably are not candidates for adjuvant therapy? Often these patients are included in cooperative study group trials that have a lot of toxicity, i.e., interferon-based trials. From your large database can you make that kind of determination?
Lastly, have you had the opportunity to go back and look at primary melanoma thickness and node status to see if there are correlations between thickness, node status, and metastatic potential as determined by the sentinel node data?
Dr. Morton: Thank you, Dr. Wanebo. Although the risk of nodal metastasis increases with the thickness of a primary melanoma, even thin melanomas (<1.5 mm) are associated with about a 10% incidence of nodal involvement, and we have seen sentinel node metastases in patients with lesions as thin as 0.6 mm. If early excision of tumor-involved nodes confers a survival benefit, then lymphadenectomy may be justified even in patients with thin melanomas.
Dr. Reintgen at the H. Lee Moffit Cancer Center and Dr. Hoon at the John Wayne Cancer Institute have used reverse transcriptase-polymerase chain reaction (RT-PCR) to document the presence of regional micrometastases by molecular techniques in a substantial number of patients who have no evidence of regional involvement by hematoxylin and eosin staining (H&E) or immunohistochemistry (IHC). Although RT-PCR results can be falsely positive due to the presence of tyrosinase genes in capsular nevi and Schwann cells (the nerve cells going through the nodes), serial sectioning of some RT-PCR–positive nodes reveals occult tumor cells missed by routine methods. Furthermore, we have found that most early recurrences in patients with negative H&E/IHC results are associated with positive RT-PCR findings. The clinical significance of this observation is unclear; if indeed the rate of nodal metastasis is much higher than indicated by conventional pathology techniques, then routine elective lymph node dissection should produce a more marked survival benefit for a larger proportion of patients undergoing this procedure.
We cannot predict which patients with regional metastasis will have more than one tumor-involved node. Others have suggested that more than one nodal metastasis is unlikely in patients with primaries ≤1.8 or ≤2.8 mm. This suggestion cannot be confirmed by MSLT data or by data from the John Wayne Cancer Institute, possibly because of the rigorous histopathological examination of nonsentinel nodes required during the learning phase for LM/SL.
In closing, I would like to add a cautionary note. LM/SL is of potential diagnostic value for breast cancer and other solid tumors that metastasize to the regional lymph nodes, but unless we can demonstrate a survival benefit for early excision of tumor-involved nodes, then LM/SL will not have direct therapeutic relevance. In other words, unless a tumor metastasizes to the regional nodes before distant sites, then a tumor-positive sentinel node is merely a marker for the metastatic phenotype. Hopefully, blood and tissue analyses using molecular and immunologic markers will confirm the sequential passage of tumor cells to regional lymph nodes and then to distant sites, thereby creating a therapeutic window of opportunity for lymphadenectomy while the tumor cells are confined to the sentinel nodes. However appealing the theory underlying LM/SL, until the long-term results of the MSLT are available, it remains a hypothesis with little scientific support from randomized trials. I thank the American Surgical Association for the honor of presenting this paper.
Footnotes
Correspondence: Donald L. Morton, MD, John Wayne Cancer Institute, 2200 Santa Monica Blvd., Santa Monica, CA 90404.
Presented at the 119th Annual Meeting of the American Surgical Association, April 15–17, 1999, Hyatt Regency Hotel, San Diego, California.
The members of the Multicenter Selective Lymphadenectomy Trial Group are listed in the Appendix.
Supported by grant CA 29605 from the National Cancer Institute. Dr. Essner is a recipient of an American Cancer Society Career Development Award.
Accepted for publication April 1999.
References
- 1.Snow H. Melanotic cancerous disease. Lancet 1892; 2: 872. [Google Scholar]
- 2.Morton DL, Wanek L, Nizze JA, et al. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg 1991; 214: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Murad TM, et al. A multifactorial analysis of melanoma:: III. Prognostic factors in melanoma patients with lymph node metastases (stage II). Ann Surg 1981; 193: 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milton GW, Shaw HM, McCarthy WH, et al. Prophylactic lymph node dissection in clinical stage I cutaneous malignant melanoma:: results of surgical treatment in 1,319 patients. Br J Surg 1982; 69: 108–111. [DOI] [PubMed] [Google Scholar]
- 5.Sim FH, Taylor WF, Ivins JC, et al. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Cancer 1978; 41: 948–956. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Adamus J, Bandiera DC, et al. Inefficacy of immediate node dissection in stage I melanoma of the limbs. N Engl J Med 1977; 297: 627–630. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Adamus J, Bandiera DC, et al. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer 1982; 49: 2420–2430. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Soong SJ, Bartolucci AA, et al. Efficacy of an elective regional lymph node dissection of 1- to 4-mm-thick melanomas for patients 60 years of age and younger. Ann Surg 1996; 224: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urist MM, Maddox WA, Kennedy JE, Balch CM. Patient risk factors and surgical morbidity after regional lymphadenectomy in 204 melanoma patients. Cancer 1983; 51: 2152–2156. [DOI] [PubMed] [Google Scholar]
- 10.Essner R, Conforti A, Kelley MC, et al. Cost-conscious management of the inguinal nodes in early-stage melanoma. Melanoma Res 1997; 7(suppl 1): S29. [Google Scholar]
- 11.Cochran AJ, Wen DR, Morton DL. Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol 1988; 12: 612–618. [DOI] [PubMed] [Google Scholar]
- 12.Robinson DS, Sample WF, Fee HJ, et al. Regional lymphatic drainage in primary malignant melanoma of the trunk determined by colloidal gold scanning. Surg Forum 1977; 28: 147–148. [PubMed] [Google Scholar]
- 13.Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg 1991; 214: 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton D, Cagle L, Wong J, et al. Intraoperative lymphatic mapping and selective lymphadenectomy: technical details of a new procedure for clinical stage I melanoma. Presented at the Annual Meeting of the Society of Surgical Oncology. Washington DC, 1990.
- 15.Gaynor R, Herschman HR, Irie R, et al. S-100 protein:: a marker for human malignant melanomas? Lancet 1981; 1: 869–871. [DOI] [PubMed] [Google Scholar]
- 16.Cochran AJ, Wen DR, Herschman HR, Gaynor RB. Detection of S-100 protein as an aid to the identification of melanocytic tumors. Int J Cancer 1982; 30: 295–297. [DOI] [PubMed] [Google Scholar]
- 17.Cochran AJ, Wen DR, Herschman HR. Occult melanoma in lymph nodes detected by antiserum to S-100 protein. Int J Cancer 1984; 34: 159–163. [DOI] [PubMed] [Google Scholar]
- 18.Chen ZL, Perez S, Holmes EC, et al. Frequency and distribution of occult micrometastases in lymph nodes of patients with non-small cell lung carcinoma. J Natl Cancer Inst 1993; 85: 493–498. [DOI] [PubMed] [Google Scholar]
- 19.Callery C, Cochran AJ, Roe DJ, et al. Factors prognostic for survival in patients with malignant melanoma spread to the regional lymph nodes. Ann Surg 1982; 196: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran AJ, Lana AM, Wen DR. Histomorphometry in the assessment of prognosis in stage II malignant melanoma. Am J Surg Pathol 1989; 13: 600–604. [DOI] [PubMed] [Google Scholar]
- 21.Cochran AJ, Pihl E, Wen DR, et al. Zoned immune suppression of lymph nodes draining malignant melanoma: : histologic and immunohistologic studies. J Natl Cancer Inst 1987; 78: 399–405. [PubMed] [Google Scholar]
- 22.Hoon DS, Korn EL, Cochran AJ. Variations in functional immunocompetence of individual tumor-draining lymph nodes in humans. Cancer Res 1987; 47: 1740–1744. [PubMed] [Google Scholar]
- 23.Wen DR, Hoon DS, Chang C, Cochran AJ. Variations in lymphokine generation by individual lymph nodes draining human malignant tumors. Cancer Immunol Immunother 1989; 30: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farzad Z, McBride WH, Ogbechi H, et al. Lymphocytes from lymph nodes at different distances from human melanoma vary in their capacity to inhibit/enhance tumor cell growth in vitro. Melanoma Res 1997; 7(suppl 2): S59–S65. [PubMed] [Google Scholar]
- 25.Hoon DS, Jung T, Naungayan J, et al. Modulation of human macrophage functions by gangliosides. Immunol Lett 1989; 20: 269–276. [DOI] [PubMed] [Google Scholar]
- 26.Cochran AJ, Wen DR, Farzad Z, et al. Immunosuppression by melanoma cells as a factor in the generation of metastatic disease. Anticancer Res 1989; 9: 859–864. [PubMed] [Google Scholar]
- 27.Hoon DS, Bowker R, Cochran AJ. Suppressor cell activity in breast cancer-draining lymph nodes. Surg Res Commun 1990; 9: 167–176. [PubMed] [Google Scholar]
- 28.Hoon DS, Bowker R, Cochran AJ. Suppressor cell activity in melanoma-draining lymph nodes. Cancer Res 1987; 47: 1529–1533. [PubMed] [Google Scholar]
- 29.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early-stage melanoma. Arch Surg 1992; 127: 392–399. [DOI] [PubMed] [Google Scholar]
- 30.Morton DL, Wen DR, Foshag LJ, et al. Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early-stage melanomas of the head and neck. J Clin Oncol 1993; 11: 1751–1756. [DOI] [PubMed] [Google Scholar]
- 31.Morton DL. Sentinel lymphadenectomy for patients with clinical stage I melanoma. J Surg Oncol 1997; 66: 267–269. [DOI] [PubMed] [Google Scholar]
- 32.Bostick P, Essner R, Sarantou T, et al. Intraoperative lymphatic mapping for early-stage melanoma of the head and neck. Am J Surg 1997; 174: 536–539. [DOI] [PubMed] [Google Scholar]
- 33.Thompson JF, McCarthy WH, Bosch CM, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res 1995; 5: 255–260. [DOI] [PubMed] [Google Scholar]
- 34.Reintgen D, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg 1994; 220: 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass EC, Essner R, Morton DL. Kinetics of three lymphoscintigraphic agents in patients with cutaneous melanoma. J Nucl Med 1998; 39: 1185–1190. [PubMed] [Google Scholar]
- 36.Kelley MC, Ollila DW, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for melanoma. Semin Surg Oncol 1998; 14: 283–290. [DOI] [PubMed] [Google Scholar]
- 37.Essner R. The role of lymphoscintigraphy and sentinel node mapping in assessing patient risk in melanoma. Semin Oncol 1997; 24: S8–10. [PubMed] [Google Scholar]
- 38.Essner R, Foshag L, Morton DL. Intraoperative radiolymphoscintigraphy: a useful adjuvant to intraoperative lymphatic mapping and selective lymphadenectomy in patients with clinical stage I melanoma. Presented at the Annual Meeting of the Society of Surgical Oncology, March 1994, Houston, TX.
- 39.Bostick P, Essner R, Glass E, et al. Comparison of blue dye and probe-assisted intraoperative lymphatic mapping in melanoma to identify sentinel nodes in 100 lymphatic basins. Arch Surg 1999; 134: 43–49. [DOI] [PubMed] [Google Scholar]
- 40.Albertini JJ, Cruse CW, Rapaport D, et al. Intraoperative radio-lympho-scintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg 1996; 223: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krag DN, Meijer SJ, Weaver DL, et al. Minimal-access surgery for staging of malignant melanoma. Arch Surg 1995; 130: 654–658. [DOI] [PubMed] [Google Scholar]
- 42.Pijpers R, Collet GJ, Meijer S, et al. The impact of dynamic lymphoscintigraphy and gamma probe guidance on sentinel node biopsy in melanoma. Eur J Nucl Med 1995; 22: 1238–1241. [DOI] [PubMed] [Google Scholar]
- 43.Thompson JF, Niewind P, Uren RF, et al. Single-dose isotope injection for both preoperative lymphoscintigraphy and intraoperative sentinel lymph node identification in melanoma patients. Melanoma Res 1997; 7: 500–506. [DOI] [PubMed] [Google Scholar]
- 44.Cochran AJ, Lu HF, Li PX, et al. S-100 protein remains a practical marker for melanocytic and other tumours. Melanoma Res 1993; 3: 325–330. [DOI] [PubMed] [Google Scholar]
- 45.Morton DL. Intraoperative lymphatic mapping and sentinel lymphadenectomy: : community standard care or clinical investigation? Cancer J Sci Am 1997; 3: 328–330. [PubMed] [Google Scholar]
- 46.Morton DL, Giuliano AE, Reintgen DS, et al. Symposium: Lymphatic mapping and sentinel node biopsy in patients with breast cancer and melanoma. Contemp Surg 1998; 53:281–298 (part 1) and 353–361 (part 2).
- 47.Bilchik AJ, Giuliano A, Essner R, et al. Universal application of intraoperative lymphatic mapping and sentinel lymphadenectomy in solid neoplasms. Cancer J Sci Am 1998; 4: 351–358. [PubMed] [Google Scholar]
- 48.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 1996; 276: 1818–1822. [PubMed] [Google Scholar]
- 49.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: : the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol 1999; 17: 976–983. [DOI] [PubMed] [Google Scholar]
- 50.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994; 220: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morton DL, Bostick PJ. Will the true sentinel node please stand? [editorial]. Ann Surg Oncol 1999; 6: 12–14. [DOI] [PubMed] [Google Scholar]
- 52.Morton D, Wen D, Cochran A. Management of early-stage melanoma by intraoperative lymphatic mapping and selective lymphadenectomy. Surg Oncol Clin North Am 1992; 1: 247–259. [Google Scholar]
- 53.Shivers SC, Wang X, Li W, et al. Molecular staging of malignant melanoma:: correlation with clinical outcome. JAMA 1998; 280: 1410–1415. [DOI] [PubMed] [Google Scholar]
- 54.Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol 1998; 16: 2253–2260. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien CJ, Uren RF, Thompson JF, et al. Prediction of potential metastatic sites in cutaneous head and neck melanoma using lymphoscintigraphy. Am J Surg 1995; 170: 461–466. [DOI] [PubMed] [Google Scholar]
- 56.Mudun A, Murray DR, Herda SC, et al. Early-stage melanoma:: lymphoscintigraphy, reproducibility of sentinel node detection, and effectiveness of the intraoperative gamma probe. Radiology 1996; 199: 171–175. [DOI] [PubMed] [Google Scholar]
- 57.Thompson JF, Uren RF, Shaw HM, et al. The location of sentinel lymph nodes in patients with cutaneous melanoma: new insights into lymphatic anatomy. J Am Coll Surg (in press). [DOI] [PubMed]
- 58.Uren RF, Thompson JF, Howman-Giles RB. Lymphatic drainage of the skin and breast: locating the sentinel nodes. Amsterdam: Harwood Academic Publishers; 1999.
- 59.Ross MI, Reintgen D, Balch CM. Selective lymphadenectomy: : emerging role for lymphatic mapping and sentinel node biopsy in the management of early-stage melanoma. Semin Surg Oncol 1993; 9: 219–223. [PubMed] [Google Scholar]
- 60.Leong SP, Steinmetz I, Habib FA, et al. Optimal selective sentinel lymph node dissection in primary malignant melanoma. Arch Surg 1997; 132: 666–672. [DOI] [PubMed] [Google Scholar]
- 61.Bachter D, Balda BR, Vogt H, et al. Primary therapy of malignant melanomas:: sentinel lymphadenectomy. Int J Dermatol 1998; 37: 278–282. [DOI] [PubMed] [Google Scholar]
- 62.van der Veen H, Hoekstra OS, Paul MA, et al. Gamma probe-guided sentinel node biopsy to select patients with melanoma for lymphadenectomy. Br J Surg 1994; 81: 1769–1770. [DOI] [PubMed] [Google Scholar]
- 63.Reintgen D. Lymphatic mapping and sentinel node harvest for malignant melanoma. J Surg Oncol 1997; 66: 277–281. [DOI] [PubMed] [Google Scholar]
- 64.Alex JC, Weaver DL, Fairbank JT, et al. Gamma probe-guided lymph node localization in malignant melanoma. Surg Oncol 1993; 2: 303–308. [DOI] [PubMed] [Google Scholar]





